The Clinical Concept of LTDpathy: Is Dysregulated LTD Responsible for Prodromal Cerebellar Symptoms?
Abstract
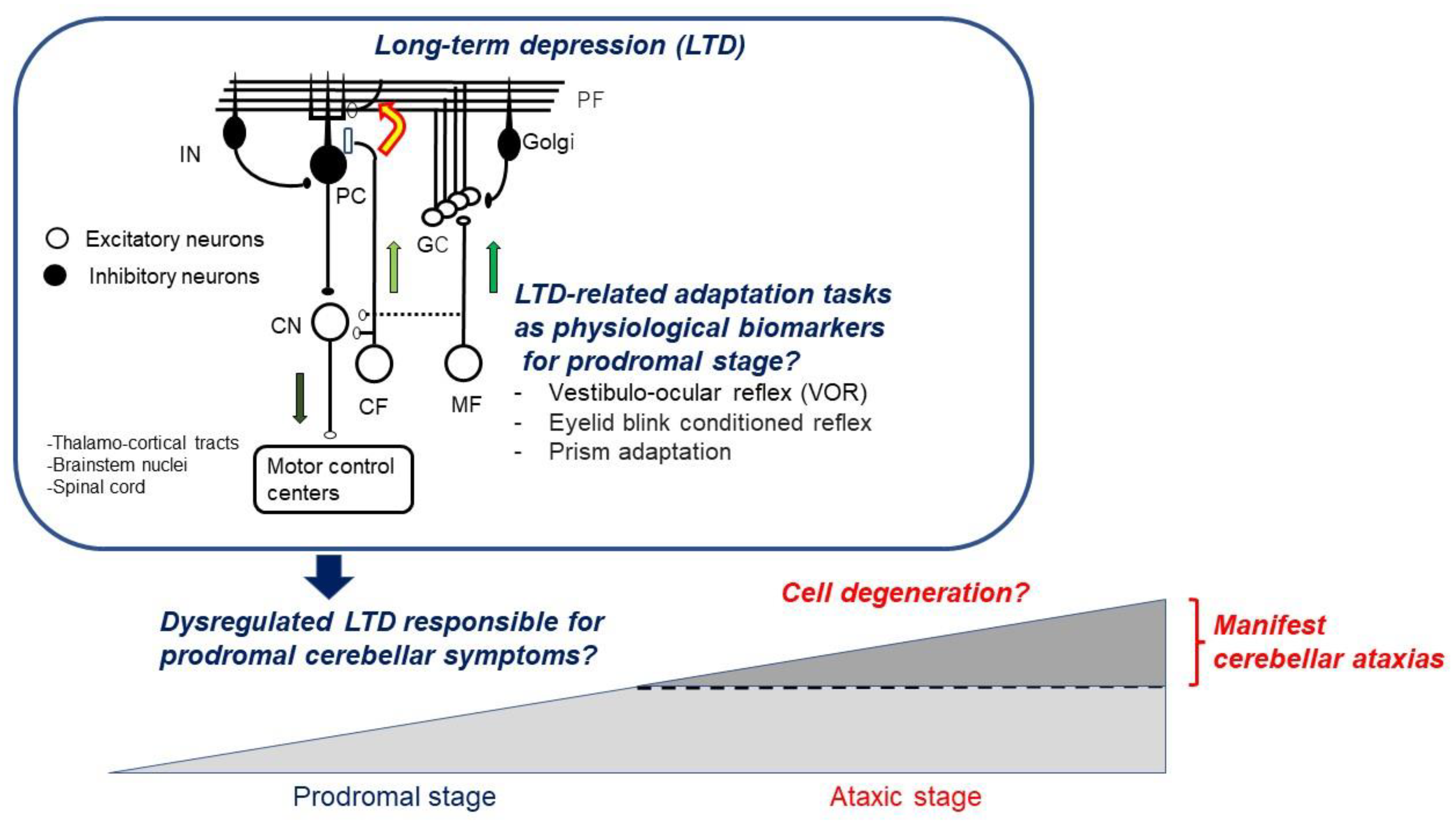
:1. Introduction
2. Dysregulated LTD and Immune-Mediated Cerebellar Ataxia
3. Dysregulated LTD and Degenerative Cerebellar Ataxia
3.1. Prodromal Stage in SCA
3.2. SCA1
3.3. SCA3
3.4. SCA6
3.5. Dysregulated LTD Preceding Cell Death
4. Conclusions: Definition of LTDpathy and its Clinical Significance
- ⬤
- LTDpathy is a clinical spectrum comprising etiologies characterized by impairments of the PF-PC LTD and related to adaptative behaviors such as VOR, blink reflex, and prism adaptation;
- ⬤
- LTDpathy includes CA of immune origin associated with anti-VGCC, anti-mGluR1, and anti-GluR delta Abs as well as of genetic origins such as SCA1, 3, and 6.
- ⬤
- In paraneoplastic IMCAs or SCA characterized by persistent impairments of a wide range of molecular mechanisms, these functional disorders are followed by degenerative cell processes;
- ⬤
- In such cases, PF-PC LTD-related maladaptive symptoms are one of the prodromal symptoms. However, these adaptive behavior disorders are difficult to notice for clinicians (subtle symptoms) in routine neurological examinations;
- ⬤
- In such conditions, impairment of PF-PC LTD and related adaptative behaviors can be promising biomarkers for early intervention. Early intervention is necessary during the period when cerebellar reserve, defined as the capacity for compensation and restoration pathologies, is preserved [43].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ito, M.; Sakurai, M.; Tongroach, P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J. Physiol. 1982, 324, 113–134. [Google Scholar] [CrossRef]
- Hirano, T.; Ohmori, H. Voltage-gated and synaptic currents in rat Purkinje cells in dissociated cell cultures. Proc. Natl. Acad. Sci. USA 1986, 83, 1945–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, M. Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J. Physiol. 1987, 394, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Ito, M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol. Rev. 2001, 81, 1143–1195. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; van Beugen, B.J.; de Zeeuw, C.I. Distributed synergistic plasticity and cerebellar learning. Nat. Rev. Neurosci. 2012, 13, 619–635. [Google Scholar] [CrossRef]
- Galliano, E.; de Zeeuw, C.I. Questioning the cerebellar doctrine. Prog. Brain Res. 2014, 210, 59–77. [Google Scholar]
- De Zeeuw, C.I.; Lisberger, S.G.; Raymond, J.L. Diversity and dynamics in the cerebellum. Nat. Neurosci. 2021, 24, 160–167. [Google Scholar] [CrossRef]
- Streng, M.L.; Popa, L.S.; Ebner, T.J. Complex spike wars: A new hope. Cerebellum 2018, 17, 735–746. [Google Scholar] [CrossRef] [Green Version]
- Mitoma, H.; Honnorat, J.; Yanmaguchi, K.; Manto, M. Fundamental mechanisms of autoantibody-induced impairments on ion channels and synapses in immune-mediated cerebellar ataxias. Int. J. Mol Sci. 2020, 21, 4936. [Google Scholar] [CrossRef]
- Mitoma, H.; Honnorat, J.; Yanmaguchi, K.; Manto, M. Cerebellar long-term depression and auto-immune target of auto-antibodies: The concept of LTDpathies. Mol. Biomed. 2021, 2, 2. [Google Scholar] [CrossRef]
- Mitoma, H.; Honnorat, J.; Yanmaguchi, K.; Manto, M. LTDpathies: A novel clinical concept. Cerebellum 2021, 20, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Dueñas, A.; Ashizawa, T.; Brice, A.; Magri, S.; McFarland, K.N.; Pandolfo, M.; Pulst, S.M.; Riess, O.; Rubinsztein, D.C.; Schmidt, J.; et al. Consensus paper: Pathological mechanisms underlying neurodegeneration in spinocerebellar ataxia. Cerebellum 2014, 13, 269–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoxha, E.; Tempia, F.; Lippiello, P.; Miniaci, M.C. Modulation, plasticity and pathophysiology of the parallel fiber-Purkinje cell synapse. Front. Synaptic. Neurosci. 2016, 8, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasumu, A.; Bezprozvanny, I. Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias. Cerebellum 2012, 11, 630–639. [Google Scholar] [CrossRef] [Green Version]
- Prestori, F.; Moccia, F.; D’Angelo, E. Disrupted calcium signaling in animal models of human spinocerebellar ataxia (SCA). Int. J. Mol. Sci. 2019, 21, 216. [Google Scholar] [CrossRef] [Green Version]
- Shuvaev, A.N.; Hosoi, N.; Sato, Y.; Yanagihara, D.; Hirai, H. Progressive impairment of cerebellar mGluR signaling and its therapeutic potential for cerebellar ataxia in spinocerebellar type 1 model mice. J. Physiol. 2017, 595, 141–164. [Google Scholar] [CrossRef] [Green Version]
- Mark, M.D.; Krause, M.; Boele, H.J.; Kruse, W.; Pollok, S.; Kuner, T.; Dalkara, D.; Koekkoek, S.; De Zeeuw, C.I.; Herlitze, S. Spinocerebellar ataxia type 6 protein aggregates cause deficits in motor learning and cerebellar plasticity. J. Neurosci. 2015, 35, 8882–8895. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Pérez, L.; Rodriguez-Labrada, R.; González-Garcés, Y.; Arrufat-Pie, E.; Torres-Vega, R.; Medrano-Montero, J.; Ramirez-Bautista, B.; Vazquez-Mojena, Y.; Auburger, G.; Horak, F.; et al. Prodromal spinocerebellar ataxia type 2 subjects have quantifiable gait and postural sway deficits. Mov. Disord. 2021, 36, 471–480. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; Leotti, V.B.; Bolzan, G.; Cappelli, A.H.; Rocha, A.G.; Ecco, G.; Kersting, N.; Rieck, M.; Martins, A.C.; Sena, L.S.; et al. Pre-ataxic changes of clinical scales and eye movement in Machado-Joseph disease: BIGPRO study. Mov. Disord. 2021, 36, 985–994. [Google Scholar] [CrossRef]
- Ito, M. The Cerebellum: Brain for an Implicit Self; FT Press: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Coesmans, M.; Smitt, P.A.; Linden, D.J.; Shigemoto, R.; Hirano, T.; Yamakawa, Y.; van Alphen, A.M.; Luo, C.; van der Geest, J.N.; Kros, J.M.; et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann. Neurol. 2003, 53, 325–336. [Google Scholar] [CrossRef]
- Hirai, H.; Launey, T.; Mikawa, S.; Torashima, T.; Yanagihara, D.; Kasaura, T.; Miyamoto, A.; Yuzaki, M. New role of delta2-glutamate receptors in AMPA receptor trafficking and cerebellar function. Nat. Neurosci. 2003, 6, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Mitoma, H.; Manto, M. Advances in the pathogenesis of auto-antibody-induced cerebellar synaptopathies. Cerebellum 2022. [Google Scholar] [CrossRef] [PubMed]
- Muñiz-Castrillo, S.; Vogrig, A.; Ciano-Petersen, N.L.; Villagrán-García, M.; Joubert, B.; Honnorat, J. Novelities in autoimmue and paraneoplastic cerebellar ataxias: Twenty years of progress. Cerebellum 2022. [Google Scholar] [CrossRef]
- Velázquez-Pérez, L.C.; Rodríguez-Labrada, R.; Fernandez-Ruiz, J. Spinocerebellar ataxia type 2: Clinicogenetic aspects, mechanistic insights, and management approaches. Front. Neurol. 2017, 8, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, H.G.; Byam, C.E.; Lande, J.D.; Tousey, S.K.; Zoghbi, H.Y.; Orr, H.T. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum. Mol. Genet. 2004, 13, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Serra, H.G.; Duvick, L.; Zu, T.; Carlson, K.; Stevens, S.; Jorgensen, N.; Lysholm, A.; Burright, E.; Zoghbi, H.Y.; Clark, H.B.; et al. RORα-mediated Purkinje cell development determines disease severity in adult SCA1 mice. Cell 2006, 127, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, T.; Duvick, L.A.; Kaytor, M.D.; Berlinger, M.S.; Zoghbi, H.Y.; Clark, H.B.; Orr, H.T. Recovery from polyglutamine-induced neurodegeneration in conditional SCA1transgenic mice. J. Neurosci. 2004, 24, 8853–8861. [Google Scholar] [CrossRef]
- Notartomaso, S.; Zappulla, C.; Biagioni, F.; Cannella, M.; Bucci, D.; Mascio, G.; Scarselli, P.; Fazio, F.; Weisz, F.; Lionetto, L.; et al. Pharmacological enhancement of mGlu1 metabotropic glutamate receptors causes a prolonged symptomatic benefit in a mouse model of spinocerebellar ataxia type1. Mol. Brain 2013, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Power, E.M.; Morales, A.; Empson, R.M. Prolonged type 1 metabotropic glutamate receptor dependent synaptic signaling contributes to spino-cerebellar ataxia type1. J. Neurosci. 2016, 36, 4910–4916. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Antal, B.; Kang, D.; Orr, H.T.; Zoghbi, H.Y. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat. Neurosci. 2000, 3, 157–163. [Google Scholar] [CrossRef]
- Kano, M.; Watanabe, T. Type-1 metabotropic glutamate receptor signaling in cerebellar Purkinje cells in health and diseases. F1000Research 2017, 6, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, A.H.; Yeh, T.H.; Ouyang, P.; Chen, Y.L.; Chen, S.Y.; Wang, H.L. Polyglutamine-expanded ataxin-3 causes cerebellar dysfunction of SCA3 transgenic mice by inducing transcriptional dysregulation. Neurobiol. Dis. 2008, 31, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Chen, Y.L.; Hu, S.H.; Chang, Y.M.; Wang, H.L. Polyglutamine-expanded ataxin-3 impairs long-term depression in Purkinje neurons of SCA3 transgenic mouse by inhibiting HAT and impairing histone acetylation. Brain Res. 2014, 1583, 220–229. [Google Scholar] [CrossRef] [PubMed]
- van Gaalen, J.; Maas, R.P.P.W.M.; Ippel, E.F.; Elting, M.W.; van Spaendonck-Zwarts, K.Y.; Vermeer, S.; Verschuuren-Bemelmans, C.; Timmann, D.; van de Warrenburg, B.P. Abnormal eyeblink conditioning is an early marker of cerebellar dysfunction in preclinical SCA3 mutation carriers. Exp. Brain Res. 2019, 237, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Solodkin, A.; Gomez, C.M. Spinocerebelalr ataxia type 6. Handb. Clin. Neurol. 2012, 103, 461–473. [Google Scholar]
- Christova, P.; Anderson, J.H.; Gomez, C.M. Impaired eye movements in presymptomatic spinocerebellar ataxia type 6. Arch. Neurol. 2008, 65, 530–536. [Google Scholar] [CrossRef]
- Tantsis, E.M.; Gill, D.; Griffiths, L.; Gupta, S.; Lawson, J.; Maksemous, N.; Ouvrier, R.; Riant, F.; Smith, R.; Troedson, C.; et al. Eye movement disorders are an early manifestation of CACNA1A mutations in children. Dev. Med. Child. Neurol. 2016, 58, 639–644. [Google Scholar] [CrossRef] [Green Version]
- Rochester, L.; Galna, B.; Lord, S.; Mhiripiri, D.; Eglon, G.; Chinnery, P.F. Gait impairment precedes clinical symptoms in spinocerebellar ataxia type 6. Mov. Disord. 2014, 29, 252–255. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Honda, T.; Matsumura, K.; Nakao, M.; Soga, K.; Katano, K.; Yokota, T.; Mizusawa, H.; Nagao, S.; Ishikawa, K. Quantitative evaluation of human cerebellum-dependent motor learning through prism adaptation of hand-reaching movement. PLoS ONE 2015, 10, e0119376. [Google Scholar] [CrossRef] [Green Version]
- Bando, K.; Honda, T.; Ishikawa, K.; Takahashi, Y.; Mizusawa, H.; Hanakawa, T. Impaired adaptive motor learning is correlated with cerebellar hemispheric gray matter atrophy in spinocerebellar ataxia patients: A voxel-based morphometry study. Front. Neurol. 2019, 10, 1183. [Google Scholar] [CrossRef]
- Honda, T.; Nagao, S.; Hashimoto, Y.; Ishikawa, K.; Yokota, T.; Mizusawa, H.; Ito, M. Tandem internal models execute motor learning in the cerebellum. Proc. Natl. Acad. Sci. USA 2018, 115, 7428–7433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitoma, H.; Buffo, A.; Gelfo, F.; Guell, X.; Fucà, E.; Kakei, S.; Lee, J.; Manto, M.; Petrosini, L.; Shaikh, A.G.; et al. Consensus paper. Cerebellar reserve: From cerebellar physiology to cerebellar disorders. Cerebellum 2019, 19, 131–153. [Google Scholar] [CrossRef] [Green Version]
- Manto, M.; Shaikh, A.G.; Mitoma, H. Identification of the prodromal symptoms and pre-ataxic stage in cerebellar disorders: The next challenge. Int. J. Environ. Res. Public Health 2021, 18, 10057. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Manto, M.; Cattaneo, Z.; Clausi, S.; Ferrari, C.; Gabrieli, J.D.E.; Guell, X.; Heleven, E.; Lupo, M.; Ma, Q.; et al. Consensus paper: Cerebellum and social cognition. Cerebellum 2020, 19, 833–868. [Google Scholar] [CrossRef] [PubMed]
| Anti-VGCC | Anti-mGluR1 | Anti-GluR Delta | |
|---|---|---|---|
| Prevalence in IMCAs | Sometimes | Rare | Rare |
| Trigger of autoimmunity | Mainly with paraneoplasia (SCLC, prostate adenocarcinoma, non-Hodgkin’s lymphoma). A few without associated cancer | Some with paraneoplasia (Hodgkin’s lymphoma, prostate adenocarcinoma). Others infrction or without paraneoplasia | Infection, vaccination |
| Median age, predominant sex | 60s, Male (>95%) | 50s, Male (57%) | Children |
| Features of CAs | Pan-cerebellar ataxias | Gait and limb ataxias | Gait ataxia, sometimes associated with limb ataxia and dysarthria |
| MRI | Variable: From none to mild atrophy | Variable: From none to mild atrophy | No atrophy |
| Therapeutic outcomes | Paraneoplasia: Variable. From good to poor response to IVIg, prednisone, and mycophenolate mofetil (Full or partial recovery 8%, stabilization 50%, and persistent worsening 42%). Without paraneoplasia: Improvement reported. | Paraneoplasia: Variable. From good to poor response to IVIg, PE. Without paraneoplasia: Generally good response to IVIg, steroid, mycophenolate, and rituximab. (Full recovery 40%, mild recovery and stabilization 52%, and persistent worsening 8%). | Generally good response to IVIg or IVMP (Full or partial recovery 67%, stabilization 33%, and persistent worsening 0%). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitoma, H.; Yamaguchi, K.; Honnorat, J.; Manto, M. The Clinical Concept of LTDpathy: Is Dysregulated LTD Responsible for Prodromal Cerebellar Symptoms? Brain Sci. 2022, 12, 303. https://doi.org/10.3390/brainsci12030303
Mitoma H, Yamaguchi K, Honnorat J, Manto M. The Clinical Concept of LTDpathy: Is Dysregulated LTD Responsible for Prodromal Cerebellar Symptoms? Brain Sciences. 2022; 12(3):303. https://doi.org/10.3390/brainsci12030303
Chicago/Turabian StyleMitoma, Hiroshi, Kazuhiko Yamaguchi, Jerome Honnorat, and Mario Manto. 2022. "The Clinical Concept of LTDpathy: Is Dysregulated LTD Responsible for Prodromal Cerebellar Symptoms?" Brain Sciences 12, no. 3: 303. https://doi.org/10.3390/brainsci12030303
APA StyleMitoma, H., Yamaguchi, K., Honnorat, J., & Manto, M. (2022). The Clinical Concept of LTDpathy: Is Dysregulated LTD Responsible for Prodromal Cerebellar Symptoms? Brain Sciences, 12(3), 303. https://doi.org/10.3390/brainsci12030303








