Effects of Repetitive Peripheral Magnetic Stimulation through Hand Splint Materials on Induced Movement and Corticospinal Excitability in Healthy Participants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
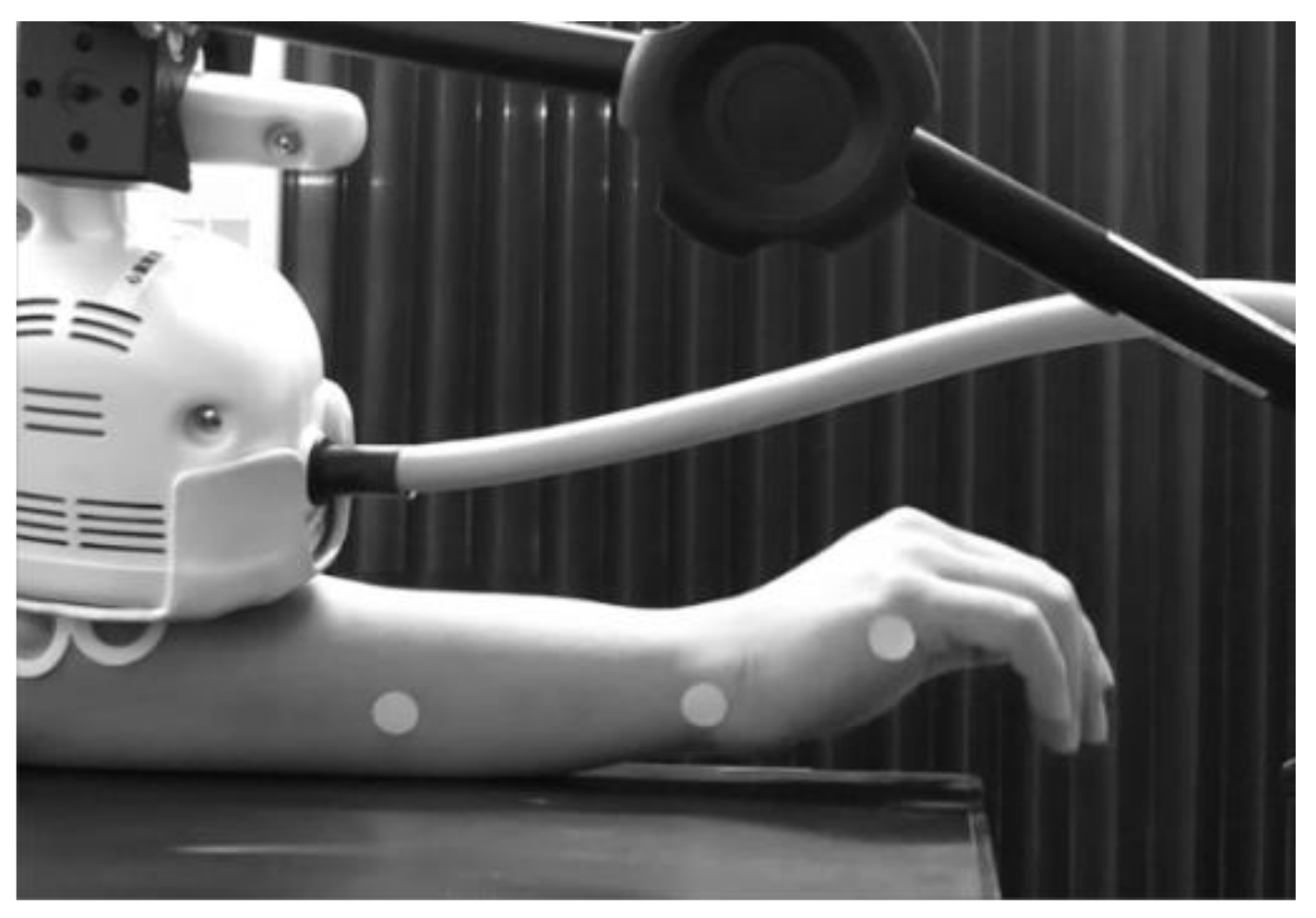
2.2. Measurement of Induced Movement
2.3. Measurement of Corticospinal Excitability
2.4. rPMS
2.5. Data and Statistical Analysis
3. Results
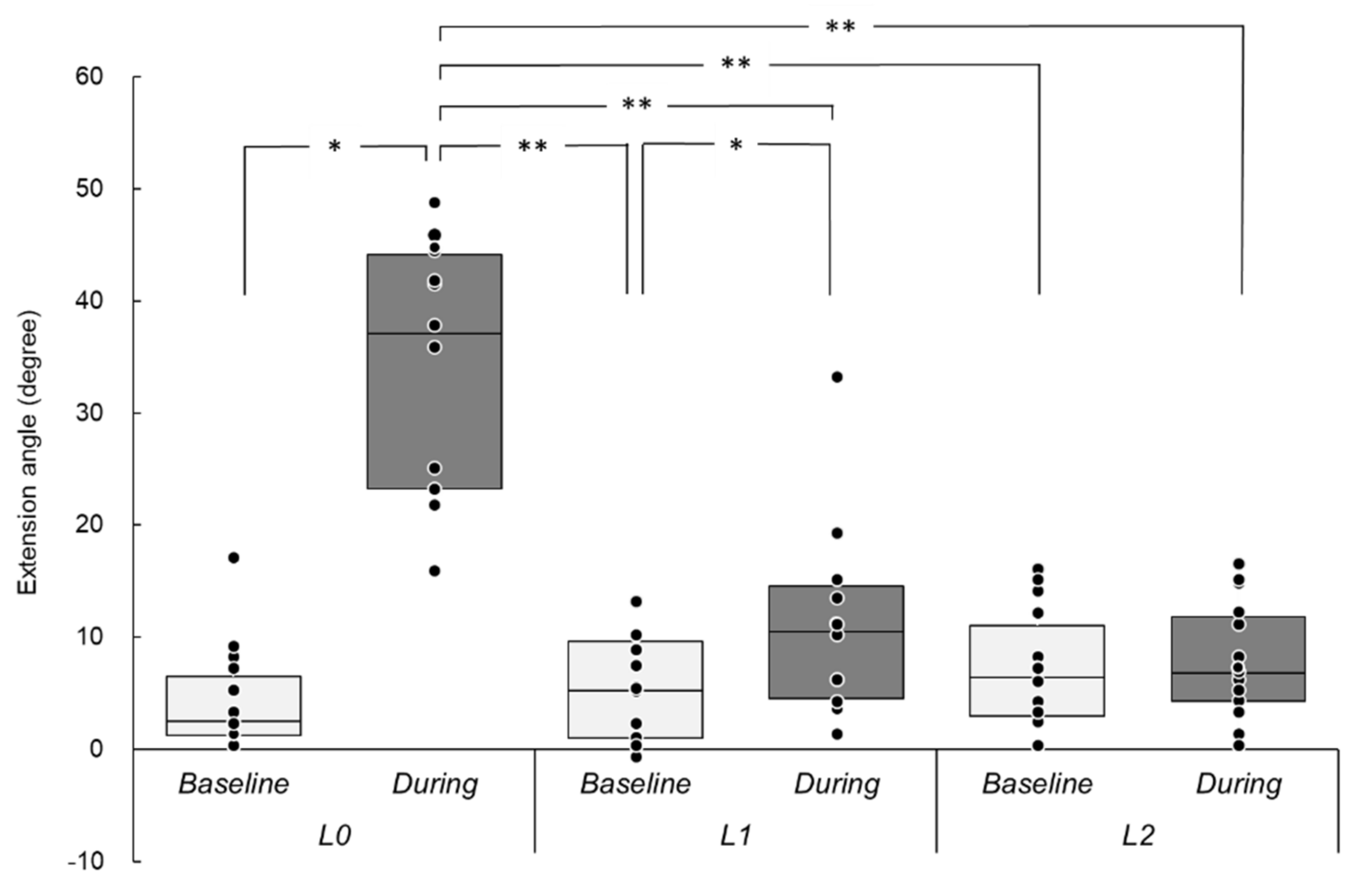
3.1. Induced Wrist Movements
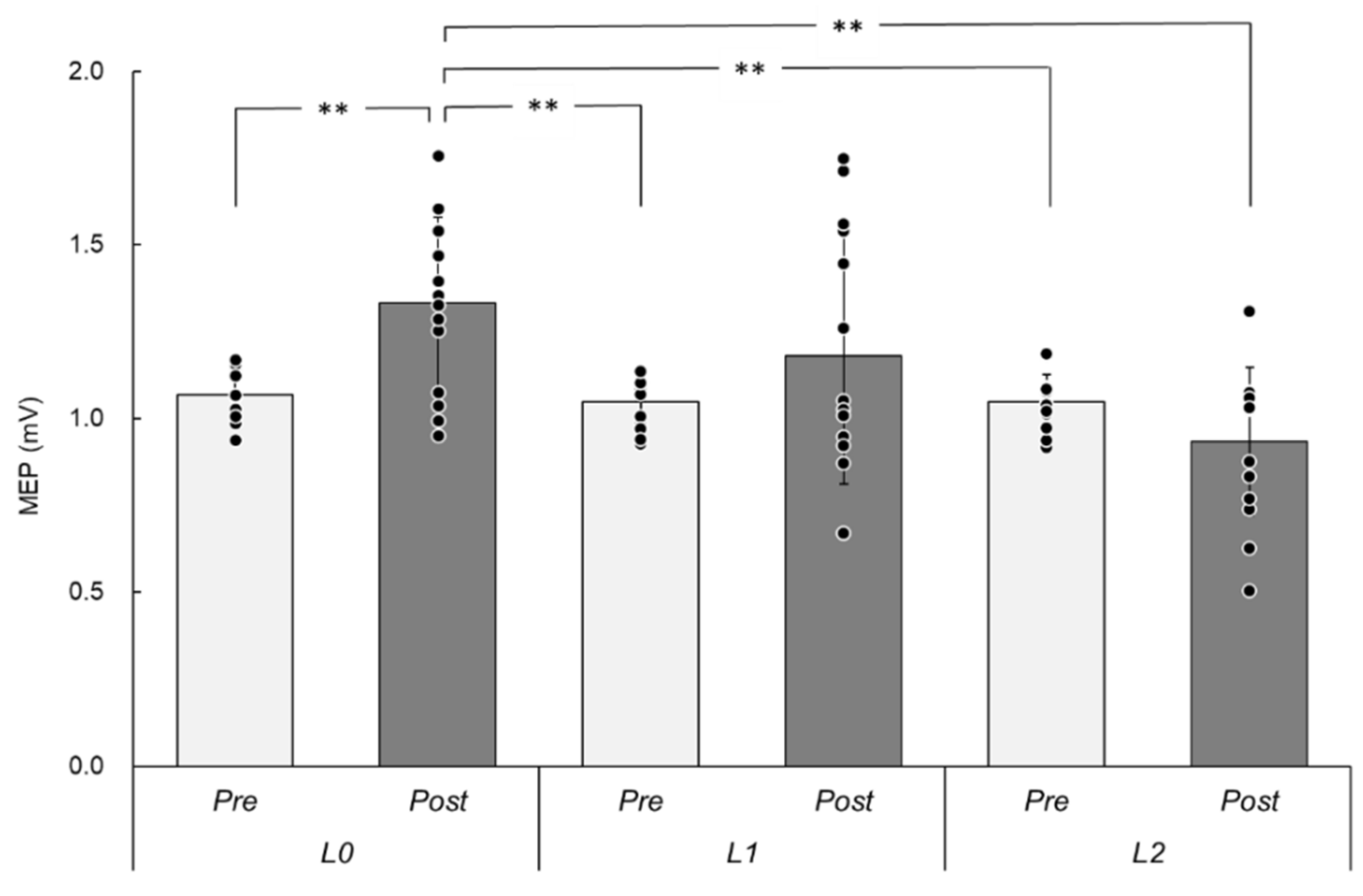
3.2. MEPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Struppler, A.; Binkofski, F.; Angerer, B.; Bernhardt, M.; Spiegel, S.; Drzezga, A.; Bartenstein, P. A fronto-parietal network is mediating improvement of motor function related to repetitive peripheral magnetic stimulation: A PET-H2O15 study. Neuroimage 2007, 36, T174–T186. [Google Scholar] [CrossRef] [PubMed]
- Gallasch, E.; Christova, M.; Kunz, A.; Rafolt, D.; Golaszewski, S.; Nelson, A.J. Modulation of sensorimotor cortex by repetitive peripheral magnetic stimulation. Front. Hum. Neurosci. 2015, 9, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, P.; Staube, A. Peripheral repetitive magnetic stimulation induces intracortical inhibition in healthy subjects. Neurol. Res. 2008, 30, 690–694. [Google Scholar] [CrossRef]
- Nito, M.; Katagiri, N.; Yoshida, K.; Koseki, T.; Kudo, D.; Nanba, S.; Tanabe, S.; Yamaguchi, T. Repetitive Peripheral Magnetic Stimulation of Wrist Extensors Enhances Cortical Excitability and Motor Performance in Healthy Individuals. Front. Neurosci. 2021, 15, 632716. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, X.; Wei, J.; Li, D.; Wang, C.; Wang, X.; Liu, H. Modulation of the corticomotor excitability by repetitive peripheral magnetic stimulation on the median nerve in healthy subjects. Front. Neural. Circuits 2021, 15, 616084. [Google Scholar] [CrossRef]
- Struppler, A.; Havel, P.; Müller-Barna, P. Facilitation of skilled finger movements by repetitive peripheral magnetic stimulation (RPMS)—A new approach in central paresis. NeuroRehabilitation 2003, 18, 69–82. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Massé-Alarie, H.; Brouwer, B.; Schneider, C. Noninvasive neurostimulation in chronic stroke: A double-blind randomized sham-controlled testing of clinical and corticomotor effects. Top. Stroke Rehabil. 2015, 22, 8–17. [Google Scholar] [CrossRef]
- Beaulieu, L.D.; Massé-Alarie, H.; Camiré-Bernier, S.; Ribot-Ciscar, É.; Schneider, C. After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: The role of afferents recruited. Neurophysiol. Clin. 2017, 47, 275–291. [Google Scholar] [CrossRef]
- Obayashi, S.; Takahashi, R. Repetitive peripheral magnetic stimulation improves severe upper limb paresis in early acute phase stroke survivors. NeuroRehabilitation 2020, 46, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Ikeda, K.; Hama, M.; Suzuki, S.; Abo, M. Repetitive peripheral magnetic stimulation combined with intensive physical therapy for gait disturbance after hemorrhagic stroke: An open-label case series. Int. J. Rehabil. Res. 2020, 43, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Flamand, V.H.; Moffet, H.; Schneider, C. Peripheral neurostimulation and specific motor training of deep abdominal muscles improve posturomotor control in chronic low back pain. Clin. J. Pain 2013, 29, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Massé-Alarie, H.; Beaulieu, L.D.; Preuss, R.; Schneider, C. Repetitive peripheral magnetic neurostimulation of multifidus muscles combined with motor training influences spine motor control and chronic low back pain. Clin. Neurophysiol. 2017, 128, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.D.; Schneider, C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: A literature review on parameters of application and afferents recruitment. Neurophysiol. Clin. 2015, 45, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.T. An introduction to the basic principles of magnetic nerve stimulation. J. Clin. Neurophysiol. 1991, 8, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ruohonen, J.; Ilmoniemi, R.J. Basic physics and design of TMS device and coils. In Magnetic Stimulation in Clinical Neurophysiology, 2nd ed.; Hallet, M., Chokroverty, S., Eds.; Butterworth–Heinemann: Oxford, UK, 2005; pp. 17–30. [Google Scholar]
- Aoyagi, Y.; Tsubahara, A. Therapeutic orthosis and electrical stimulation for upper extremity hemiplegia after stroke: A review of effectiveness based on evidence. Top. Stroke Rehabil. 2004, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Giang, T.A.; Ong, A.W.G.; Krishnamurthy, K.; Fong, K.N.K. Rehabilitation interventions for poststroke hand oedema: A systematic review. Hong Kong J. Occup. Ther. 2016, 27, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lannin, N.A.; Herbert, R.D. Is hand splinting effective for adults following stroke? A systematic review and methodological critique of published research. Clin. Rehabil. 2003, 17, 807–816. [Google Scholar] [CrossRef]
- Pritchard, K.; Edelstein, J.; Zubrenic, E.; Tsao, L.; Berendsen, M.; Wafford, E. Systematic review of orthoses for stroke-induced upper extremity deficits. Top. Stroke Rehabil. 2019, 26, 389–398. [Google Scholar] [CrossRef]
- Fujiwara, T.; Kawakami, M.; Honaga, K.; Tochikura, M.; Abe, K. Hybrid assistive neuromuscular dynamic stimulation therapy: A new strategy for improving upper extremity function in patients with hemiparesis following stroke. Neural. Plast. 2017, 2017, 2350137. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Miyaguchi, S.; Onishi, H.; Kojima, S.; Sugawara, K.; Tsubaki, A.; Kirimoto, H.; Tamaki, H.; Yamamoto, N. Corticomotor excitability induced by anodal transcranial direct current stimulation with and without non-exhaustive movement. Brain Res. 2013, 1529, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Sasaki, R.; Tsuiki, S.; Miyaguchi, S.; Kojima, S.; Saito, K.; Inukai, Y.; Onishi, H. Effects of passive finger movement on cortical excitability. Front. Hum. Neurosci. 2017, 11, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, T.; Miyaguchi, S.; Otsuru, N.; Onishi, H. The effect of transcranial random noise stimulation on corticospinal excitability and motor performance. Neurosci. Lett. 2019, 705, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Abe, G.; Oyama, H.; Liao, Z.; Honda, K.; Yashima, K.; Asao, A.; Izumi, S.I. Difference in pain and discomfort of comparable wrist movements induced by magnetic or electrical stimulation for peripheral nerves in the dorsal forearm. Med. Devices 2020, 13, 439–447. [Google Scholar] [CrossRef]
- Kunesch, E.; Knecht, S.; Classen, J.; Roick, H.; Tyercha, C.; Benecke, R. Somatosensory evoked potentials (SEPs) elicited by magnetic nerve stimulation. Electroencephalogr. Clin. Neurophysiol. 1993, 88, 459–467. [Google Scholar] [CrossRef]
- Zhu, Y.; Starr, A. Magnetic stimulation of muscle evokes cerebral potentials. Muscle Nerve 1991, 14, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Starr, A.; Fu, H.; Liu, J.; Wu, P. Magnetic stimulation of muscle evokes cerebral potentials by direct activation of nerve afferents: A study during muscle paralysis. Muscle Nerve 1996, 19, 1570–1575. [Google Scholar] [CrossRef]
- Chipchase, L.S.; Schabrun, S.M.; Hodges, P.W. Peripheral electrical stimulation to induce cortical plasticity: A systematic review of stimulus parameters. Clin. Neurophysiol. 2011, 122, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Pisotta, I.; Perruchoud, D.; Cesari, P.; Ionta, S. Hand-in-hand advances in biomedical engineering and sensorimotor restoration. J. Neurosci. Methods 2015, 246, 22–29. [Google Scholar] [CrossRef]
- Perruchoud, D.; Fiorio, M.; Cesari, P.; Ionta, S. Beyond variability: Subjective timing and the neurophysiology of motor cognition. Brain Stimul. 2018, 11, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Goldsworthy, M.R.; Hordacre, B.; Ridding, M.C. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience 2016, 320, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Bianani, M.; Farrell, M.; Zoghi, M.; Egan, G.; Jaberzadeh, S. The minimal number of TMS trials required for the reliable assessment of corticospinal excitability, short interval intracortical inhibition, and intracortical facilitation. Neurosci. Lett. 2018, 1, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.P. Peripheral nerve stimulation by induced electric currents: Exposure to time-varying magnetic fields. Med. Biol. Eng. Comput. 1989, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, C.; Machetanz, J.; Meyer, B.U.; Conrad, B. Repetitive magnetic nerve stimulation: Technical considerations and clinical use in the assessment of neuromuscular transmission. Electroencephalogr. Clin. Neurophysiol. 1994, 93, 15–20. [Google Scholar] [CrossRef]
- Nilsson, J.; Panizza, M.; Roth, B.J.; Basser, P.J.; Cohen, L.G.; Caruso, G.; Hallett, M. Determining the site of stimulation during magnetic stimulation of a peripheral nerve. Electroencephalogr. Clin. Neurophysiol. 1992, 85, 253–264. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asao, A.; Nomura, T.; Shibuya, K. Effects of Repetitive Peripheral Magnetic Stimulation through Hand Splint Materials on Induced Movement and Corticospinal Excitability in Healthy Participants. Brain Sci. 2022, 12, 280. https://doi.org/10.3390/brainsci12020280
Asao A, Nomura T, Shibuya K. Effects of Repetitive Peripheral Magnetic Stimulation through Hand Splint Materials on Induced Movement and Corticospinal Excitability in Healthy Participants. Brain Sciences. 2022; 12(2):280. https://doi.org/10.3390/brainsci12020280
Chicago/Turabian StyleAsao, Akihiko, Tomonori Nomura, and Kenichi Shibuya. 2022. "Effects of Repetitive Peripheral Magnetic Stimulation through Hand Splint Materials on Induced Movement and Corticospinal Excitability in Healthy Participants" Brain Sciences 12, no. 2: 280. https://doi.org/10.3390/brainsci12020280
APA StyleAsao, A., Nomura, T., & Shibuya, K. (2022). Effects of Repetitive Peripheral Magnetic Stimulation through Hand Splint Materials on Induced Movement and Corticospinal Excitability in Healthy Participants. Brain Sciences, 12(2), 280. https://doi.org/10.3390/brainsci12020280





