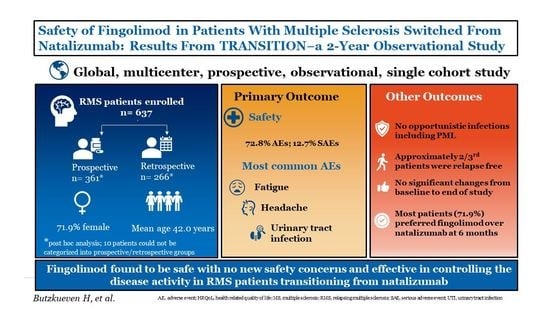
Safety of Fingolimod in Patients with Multiple Sclerosis Switched from Natalizumab: Results from TRANSITION―A 2-Year, Multicenter, Observational, Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Patient Population
2.3. Study Assessments
2.4. Other Assessments
2.5. Sample Size and Statistical Analysis
3. Results
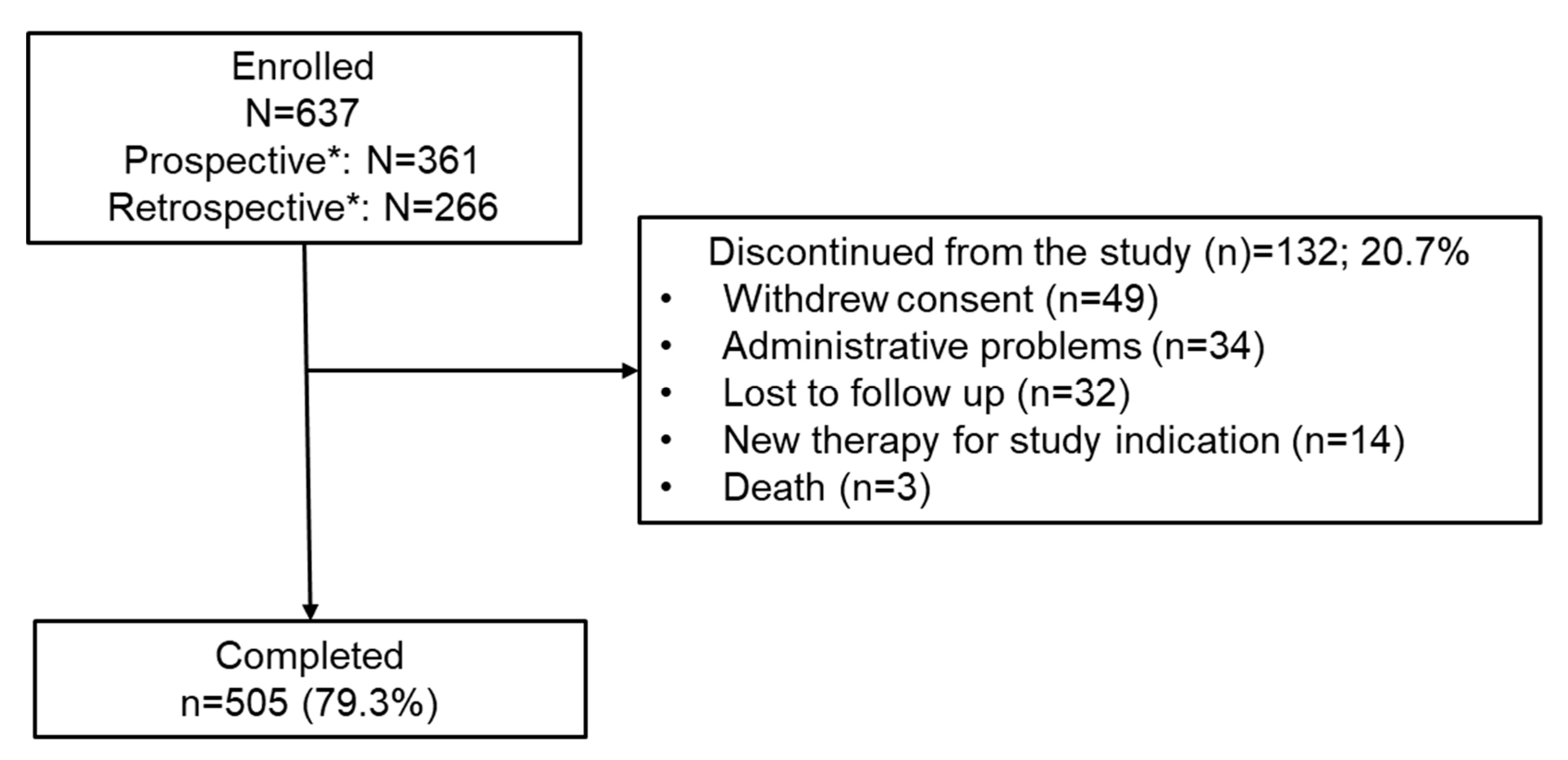
3.1. Patient Disposition and Baseline Characteristics
3.2. Safety Outcomes
3.2.1. Vital Signs
3.2.2. Selected Safety Outcomes
3.2.3. First-Dose Observation
3.2.4. Laboratory Abnormalities
3.2.5. Safety Outcomes in Subgroups
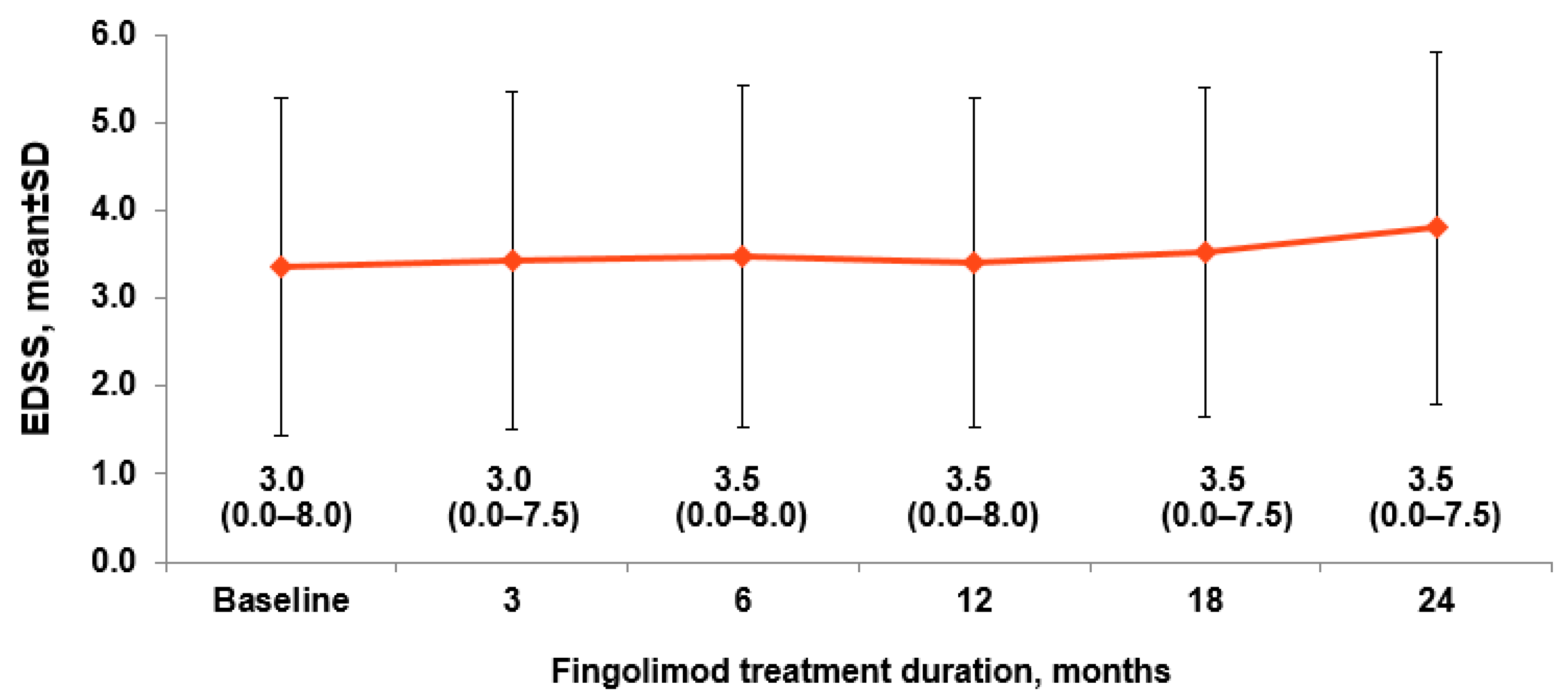
3.3. Effectiveness Outcomes
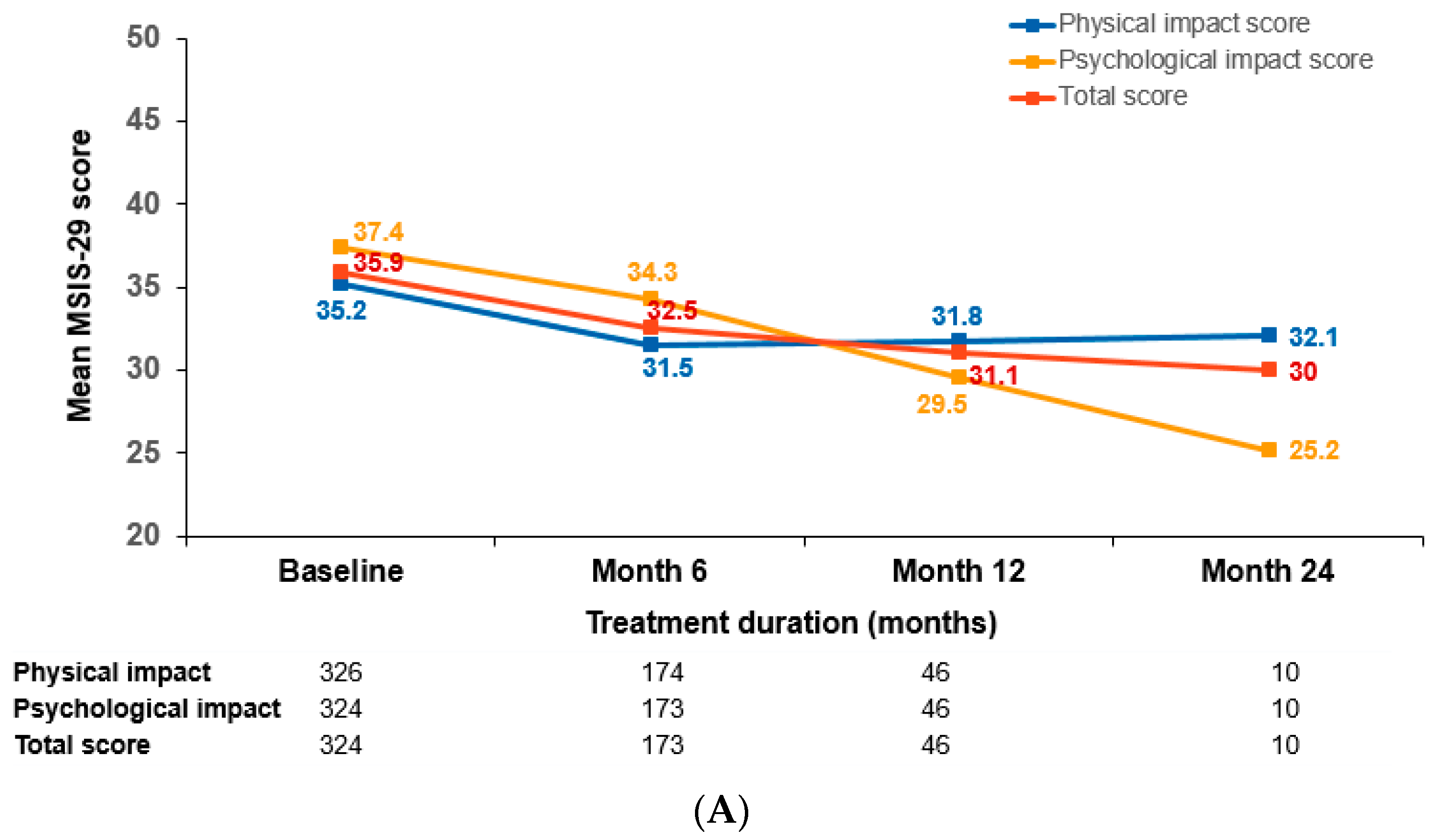
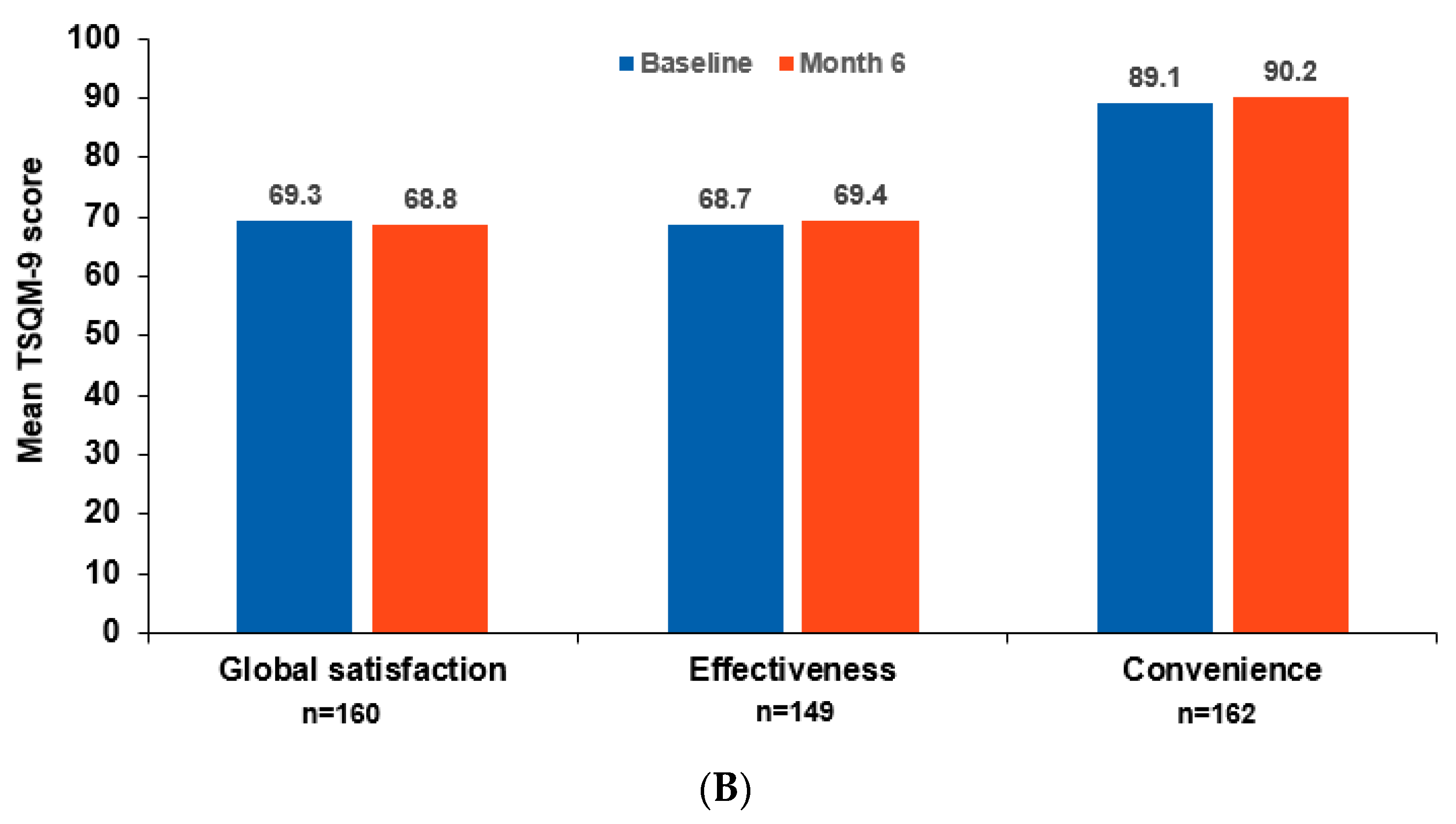
3.4. Patient-Reported Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, J.A.; Barkhof, F.; Comi, G.; Hartung, H.P.; Khatri, B.O.; Montalban, X.; Pelletier, J.; Capra, R.; Gallo, P.; Izquierdo, G.; et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.A.; Radue, E.W.; Goodin, D.; Jeffery, D.; Rammohan, K.W.; Reder, A.T.; Vollmer, T.; Agius, M.A.; Kappos, L.; Stites, T.; et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014, 13, 545–556. [Google Scholar] [CrossRef]
- Kappos, L.; Radue, E.W.; O’Connor, P.; Polman, C.; Hohlfeld, R.; Calabresi, P.; Selmaj, K.; Agoropoulou, C.; Leyk, M.; Zhang-Auberson, L.; et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 2010, 362, 387–401. [Google Scholar] [CrossRef]
- Laroni, A.; Brogi, D.; Brescia Morra, V.; Guidi, L.; Pozzilli, C.; Comi, G.; Lugaresi, A.; Turrini, R.; Raimondi, D.; Uccelli, A.; et al. Safety and tolerability of fingolimod in patients with relapsing-remitting multiple sclerosis: Results of an open-label clinical trial in Italy. Neurol. Sci. 2017, 38, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Ticha, V.; Kodym, R.; Pocikova, Z.; Kadlecova, P. Real-World Outcomes in Fingolimod-Treated Patients with Multiple Sclerosis in the Czech Republic: Results from the 12-Month GOLEMS Study. Clin. Drug Investig. 2017, 37, 175–186. [Google Scholar] [CrossRef][Green Version]
- Alsop, J.; Medin, J.; Cornelissen, C.; Vormfelde, S.V.; Ziemssen, T. Two studies in one: A propensity-score-matched comparison of fingolimod versus interferons and glatiramer acetate using real-world data from the independent German studies, PANGAEA and PEARL. PLoS ONE 2017, 12, e0173353. [Google Scholar] [CrossRef]
- Fox, E.; Edwards, K.; Burch, G.; Wynn, D.R.; LaGanke, C.; Crayton, H.; Hunter, S.F.; Huffman, C.; Kim, E.; Pestreich, L.; et al. Outcomes of switching directly to oral fingolimod from injectable therapies: Results of the randomized, open-label, multicenter, Evaluate Patient OutComes (EPOC) study in relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 607–619. [Google Scholar] [CrossRef]
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 899–910. [Google Scholar] [CrossRef]
- Rudick, R.A.; Stuart, W.H.; Calabresi, P.A.; Confavreux, C.; Galetta, S.L.; Radue, E.W.; Lublin, F.D.; Weinstock-Guttman, B.; Wynn, D.R.; Lynn, F.; et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 911–923. [Google Scholar] [CrossRef]
- Tysabri. Prescribing Information; Biogen Inc.: Cambridge, MA, USA, December 2021. [Google Scholar]
- Iaffaldano, P.; Lucisano, G.; Pozzilli, C.; Brescia Morra, V.; Ghezzi, A.; Millefiorini, E.; Patti, F.; Lugaresi, A.; Zimatore, G.B.; Marrosu, M.G.; et al. Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain 2015, 138 (Pt 11), 3275–3286. [Google Scholar] [CrossRef]
- Data on File; Novartis Pharma AG: Basel, Switzerland, 2021.
- West, T.W.; Cree, B.A. Natalizumab dosage suspension: Are we helping or hurting? Ann Neurol. 2010, 68, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Ispe. Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol. Drug Saf. 2008, 17, 200–208. [Google Scholar]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [PubMed]
- Temple, R. Hy’s law: Predicting serious hepatotoxicity. Pharmacoepidemiol. Drug Saf. 2006, 15, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Kappos, L.; Radue, E.W.; Comi, G.; Montalban, X.; Butzkueven, H.; Wiendl, H.; Giovannoni, G.; Hartung, H.P.; Derfuss, T.; Naegelin, Y.; et al. Switching from natalizumab to fingolimod: A randomized, placebo-controlled study in RRMS. Neurology 2015, 85, 29–39. [Google Scholar] [CrossRef]
- Derfuss, T.; Kovarik, J.M.; Kappos, L.; Savelieva, M.; Chhabra, R.; Thakur, A.; Zhang, Y.; Wiendl, H.; Tomic, D. alpha4-integrin receptor desaturation and disease activity return after natalizumab cessation. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e388. [Google Scholar] [CrossRef] [PubMed]
- Plavina, T.; Muralidharan, K.K.; Kuesters, G.; Mikol, D.; Evans, K.; Subramanyam, M.; Nestorov, I.; Chen, Y.; Dong, Q.; Ho, P.-R.; et al. Reversibility of the effects of natalizumab on peripheral immune cell dynamics in MS patients. Neurology 2017, 89, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.R. Classifying PML risk with disease modifying therapies. Mult. Scler. Relat. Disord. 2017, 12, 59–63. [Google Scholar] [CrossRef]
- Cohen, M.; Maillart, E.; Tourbah, A.; De Seze, J.; Vukusic, S.; Brassat, D.; Anne, O.; Wiertlewski, S.; Camu, W.; Courtois, S.; et al. Switching from natalizumab to fingolimod in multiple sclerosis: A French prospective study. JAMA Neurol. 2014, 71, 436–441. [Google Scholar] [CrossRef]
- Leurs, C.E.; van Kempen, Z.L.; Dekker, I.; Balk, L.J.; Wattjes, M.P.; Rispens, T.; Uitdehaag, B.M.; Killestein, J. Switching natalizumab to fingolimod within 6 weeks reduces recurrence of disease activity in MS patients. Mult. Scler. 2018, 24, 1453–1460. [Google Scholar] [CrossRef]
- Guger, M.; Enzinger, C.; Leutmezer, F.; Kraus, J.; Kalcher, S.; Kvas, E.; Berger, T. Switching from natalizumab to fingolimod treatment in multiple sclerosis: Real life data from the Austrian MS Treatment Registry. J. Neurol. 2019, 266, 2672–2677. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Marta, M.; Davis, A.; Turner, B.; Gnanapavan, S.; Schmierer, K. Switching patients at high risk of PML from natalizumab to another disease-modifying therapy. Pract. Neurol. 2016, 16, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Lang, M.; Tackenberg, B.; Schmidt, S.; Albrecht, H.; Klotz, L.; Haas, J.; Lassek, C.; Cornelissen, C.; Ettle, B.; et al. Long-term real-world evidence for sustained clinical benefits of fingolimod following switch from natalizumab. Mult. Scler. Relat. Disord. 2019, 39, 101893. [Google Scholar] [CrossRef]
- Vollmer, B.; Honce, J.M.; Sillau, S.; Corboy, J.R.; Vollmer, T.; Nair, K.; Alvarez, E. The impact of very short transition times on switching from Natalizumab to Fingolimod on imaging and clinical effectiveness outcomes in multiple sclerosis. J. Neurol. Sci. 2018, 390, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Naegelin, Y.; Rasenack, M.; Andelova, M.; Von Felten, S.; Fischer-Barnicol, B.; Amann, M.; Mehling, M.; Kappos, L.; Sprenger, T.; Derfuss, T. Shortening the washout to 4 weeks when switching from natalizumab to fingolimod and risk of disease reactivation in multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 25, 14–20. [Google Scholar] [CrossRef]
- Jokubaitis, V.G.; Li, V.; Kalincik, T.; Izquierdo, G.; Hodgkinson, S.; Alroughani, R.; Lechner-Scott, J.; Lugaresi, A.; Duquette, P.; Girard, M.; et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology 2014, 82, 1204–1211. [Google Scholar] [CrossRef]
| Characteristics | Fingolimod 0.5 mg N = 637 | Prospective N = 361 a | Retrospective N = 266 a |
|---|---|---|---|
| Age, years | 42.0 ± 10.4 | 42.4 ± 10.6 | 41.4 ± 10.1 |
| Women, n (%) | 458 (71.9) | 267 (74.0) | 185 (69.5) |
| Caucasian, n (%) | 580 (91.1) | 335 (92.8) | 240 (90.2) |
| MS duration since diagnosis, years | 10.5 ± 6.8 | 10.4 ± 6.9 | 10.6 ± 6.7 |
| Number of relapses in the last 12 months b | 0.5 ± 0.8 | 0.47 ± 0.76 | 0.50 ± 0.87 |
| EDSS score | 3.4 ± 1.9 | 3.43 ± 1.94 | 3.25 ± 1.88 |
| JCV status known, n (%) | 613 (96.2) | 354 (98.1) | 253 (95.1) |
| JCV-positive patients, n (%) | 512 (83.5) | 288 (81.4) | 218 (86.2) |
| Fingolimod 0.5 mg N = 628 | |||
| Any prior MS DMTs at study entry, n (%) * ^ | 626 (99.7) | 361 (100) | 265 (99.6) |
| Most common prior MS DMTs | |||
| Natalizumab | 625 (99.5) | 360 (99.7) | 265 (99.6) |
| Interferon β-1a | 334 (53.2) | 180 (49.9) | 154 (57.9) |
| Glatiramer acetate | 226 (36.0) | 138 (38.2) | 88 (33.1) |
| Interferon β-1b | 90 (14.3) | 45 (12.5) | 45 (16.9) |
| Betaseron | 58 (9.2) | 42 (11.6) | 16 (6.0) |
| Washout period | |||
| Duration, weeks | 14.5 ± 10.9 | 14.9 ± 11.3 | 13.9 ± 10.4 |
| ≤8 weeks, n (%) | 156 (24.8) | 87 (24.1) | 69 (25.9) |
| >8 weeks, n (%) | 470 (74.8) | 273 (75.6) | 197 (74.1) |
| Events | All Patients N = 628 | Prospective Patients (N = 361) | Retrospective Patients (N = 266) | |||
|---|---|---|---|---|---|---|
| n (%) | IR (95% CI) | n (%) | IR (95% CI) | n (%) | IR (95% CI) | |
| Total AEs | 457 (72.8) | 91.49 (83.29; 100.27) | 272 (75.4) | 104.13 (92.12; 117.26) | 185 (69.6) | 77.6 (66.85; 89.66) |
| Fatigue | 74 (11.8) | 6.4 (5.04; 8.06) | 45 (12.5) | 6.9 (5.01; 9.19) | 29 (10.9) | 5.8 (3.90; 8.37) |
| Headache | 61 (9.7) | 5.2 (3.97; 6.67) | 38 (10.5) | 5.7 (4.04; 7.83) | 23 (8.7) | 4.5 (2.86; 6.78) |
| Urinary tract infection | 57 (9.1) | 4.8 (3.64; 6.22) | 39 (10.8) | 5.9 (4.16; 7.99) | 18 (6.8) | 3.5 (2.05; 5.47) |
| Lymphopenia | 44 (7.0) | 3.7 (2.66; 4.91) | 28 (7.8) | 4.1 (2.73; 5.94) | 16 (6.0) | 3.1 (1.76; 4.99) |
| Depression | 41 (6.5) | 3.4 (2.43; 4.59) | 26 (7.2) | 3.8 (2.47; 5.54) | 15 (5.6) | 2.9 (1.60; 4.71) |
| Diarrhea | 33 (5.3) | 2.7 (1.87; 3.81) | 21 (5.8) | 3.0 (1.88; 4.64) | 12 (4.5) | 2.3 (1.18; 3.99) |
| Muscular weakness | 32 (5.1) | 2.6 (1.79; 3.69) | 19 (5.3) | 2.7 (1.64; 4.25) | 13 (4.9) | 2.5 (1.32; 4.23) |
| Constipation | 31 (4.9) | 2.5 (1.71; 3.58) | 22 (6.1) | 3.2 (1.99; 4.80) | 9 (3.4) | 1.7 (0.77; 3.20) |
| Back pain | 30 (4.8) | 2.5 (1.66; 3.50) | 16 (4.4) | 2.3 (1.32; 3.74) | 14 (5.3) | 2.7 (1.45; 4.46) |
| Fall | 28 (4.5) | 2.3 (1.51; 3.29) | 15 (4.2) | 2.1 (1.20; 3.53) | 13 (4.9) | 2.5 (1.31; 4.19) |
| Insomnia | 27 (4.3) | 2.2 (1.45; 3.19) | 14 (3.9) | 2.0 (1.09; 3.35) | 13 (4.9) | 2.5 (1.31; 4.21) |
| Hypertension | 26 (4.1) | 2.1 (1.39; 3.11) | 13 (3.6) | 1.9 (0.99; 3.17) | 13 (4.9) | 2.5 (1.33; 4.26) |
| Nausea | 26 (4.1) | 2.1 (1.39; 3.11) | 20 (5.5) | 2.9 (1.77; 4.48) | 6 (2.3) | 1.1 (0.41; 2.44) |
| Total SAEs | 80 (12.7) | 6.9 (5.50; 8.63) | 47 (13.0) | 7.2 (5.30; 9.59) | 33 (12.4) | 6.6 (4.53; 9.24) |
| Multiple sclerosis relapse | 13 (2.1) | 1.0 (0.56; 1.78) | 7 (1.94) | 1.0 (0.40; 2.04) | 6 (2.3) | 1.1 (0.41; 2.42) |
| Pre-Baseline | Month 12 | Month 24 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤8 Weeks | >8 Weeks | Overall Population | ≤8 Weeks | >8 Weeks | Overall Population | ≤8 Weeks | >8 Weeks | Overall Population | |
| MRI performed, n | 154 | 449 | 605 | 46 | 159 | 205 | 15 | 39 | 54 |
| Worsening in MRI since the last scan was performed, n (%) | 24 (15.6) | 86 (19.2) | 110 (18.2) | 11 (23.9) | 31 (19.5) | 42 (20.5) | 4 (26.7) | 10 (25.6) | 14 (25.9) |
| Presence of Gd+ T1 lesions | 8 | 39 | 47 | 5 | 7 | 12 | 1 | 4 | 5 |
| Increase in number of T2 lesions | 15 | 42 | 57 | 5 | 16 | 21 | 4 | 5 | 9 |
| Other | 6 | 17 | 23 | 3 | 12 | 15 | 0 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butzkueven, H.; Giacomini, P.S.; Cohan, S.; Ziemssen, T.; Sienkiewicz, D.; Zhang, Y.; Geissbühler, Y.; Silva, D.; Tomic, D.; Kropshofer, H.; et al. Safety of Fingolimod in Patients with Multiple Sclerosis Switched from Natalizumab: Results from TRANSITION―A 2-Year, Multicenter, Observational, Cohort Study. Brain Sci. 2022, 12, 215. https://doi.org/10.3390/brainsci12020215
Butzkueven H, Giacomini PS, Cohan S, Ziemssen T, Sienkiewicz D, Zhang Y, Geissbühler Y, Silva D, Tomic D, Kropshofer H, et al. Safety of Fingolimod in Patients with Multiple Sclerosis Switched from Natalizumab: Results from TRANSITION―A 2-Year, Multicenter, Observational, Cohort Study. Brain Sciences. 2022; 12(2):215. https://doi.org/10.3390/brainsci12020215
Chicago/Turabian StyleButzkueven, Helmut, Paul S. Giacomini, Stanley Cohan, Tjalf Ziemssen, Daniel Sienkiewicz, Ying Zhang, Yvonne Geissbühler, Diego Silva, Davorka Tomic, Harald Kropshofer, and et al. 2022. "Safety of Fingolimod in Patients with Multiple Sclerosis Switched from Natalizumab: Results from TRANSITION―A 2-Year, Multicenter, Observational, Cohort Study" Brain Sciences 12, no. 2: 215. https://doi.org/10.3390/brainsci12020215
APA StyleButzkueven, H., Giacomini, P. S., Cohan, S., Ziemssen, T., Sienkiewicz, D., Zhang, Y., Geissbühler, Y., Silva, D., Tomic, D., Kropshofer, H., & Trojano, M. (2022). Safety of Fingolimod in Patients with Multiple Sclerosis Switched from Natalizumab: Results from TRANSITION―A 2-Year, Multicenter, Observational, Cohort Study. Brain Sciences, 12(2), 215. https://doi.org/10.3390/brainsci12020215