Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone
Abstract
1. Introduction
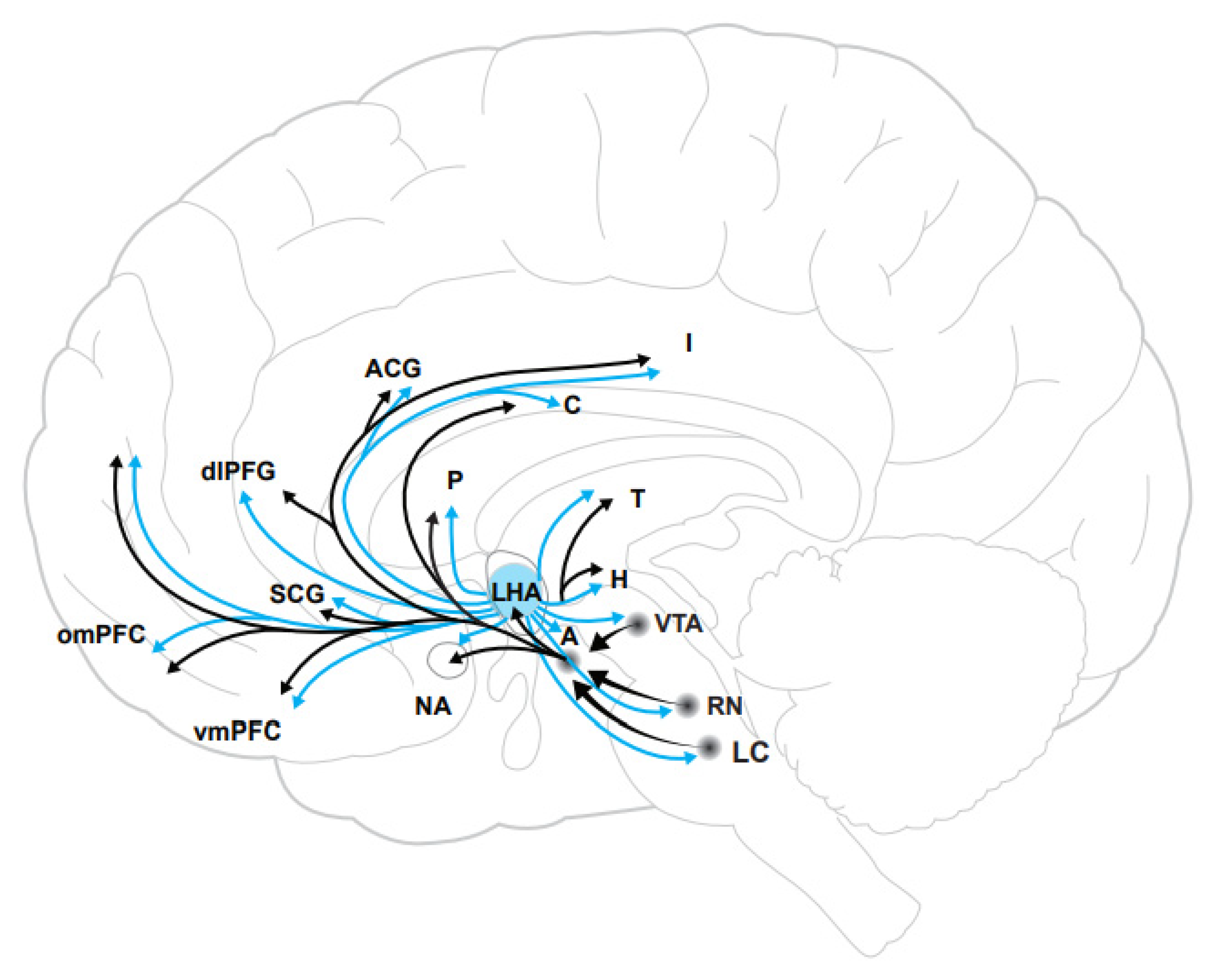
2. Neurobiology of the Orexin System
3. Overview of Physiological Roles of the Orexin System
3.1. Role of Orexin in the Regulation of Sleep and Arousal
3.2. Role of Orexin in the Regulation of Affect, Motivation, and Reward
3.3. Influence of Orexin on Attention/Cognition and Learning
3.4. Influence of Orexin on Mood and Mood and Psychiatric Disorders
3.4.1. Depression
3.4.2. Anxiety
3.4.3. Addiction
3.4.4. ADHD
3.4.5. Schizophrenia
3.4.6. Overview of Hedonic Tone and Anhedonia
4. Potential Role of Orexin in Modulation of Hedonic Tone and Anhedonia
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Lecea, L.; Sutcliffe, J.G. The hypocretins/orexins: Novel hypothalamic neuropeptides involved in different physiological systems. Cell Mol. Life Sci. 1999, 56, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richarson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef]
- Matzeu, A.; Martin-Fardon, R. Targeting the Orexin System for Prescription Opioid Use Disorder. Brain Sci. 2020, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Baimel, C.; Bartlett, S.E.; Chiou, L.C.; Lawrence, A.J.; Muschamp, J.W.; Patkar, O.; Tung, L.W.; Borgland, S.L. Orexin/hypocretin role in reward: Implications for opioid and other addictions. Br. J. Pharmacol. 2015, 172, 334–348. [Google Scholar] [CrossRef]
- Girault, E.M.; Yi, C.X.; Fliers, E.; Kalsbeek, A. Orexins, feeding, and energy balance. Prog. Brain Res. 2012, 198, 47–64. [Google Scholar] [CrossRef]
- Hoyer, D.; Jacobson, L.H. Orexin in sleep, addiction and more: Is the perfect insomnia drug at hand? Neuropeptides 2013, 47, 477–488. [Google Scholar] [CrossRef]
- James, M.H.; Mahler, S.V.; Moorman, D.E.; Aston-Jones, G. A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr. Top Behav. Neurosci. 2017, 33, 247–281. [Google Scholar] [CrossRef]
- Ahmadi-Soleimani, S.M.; Mianbandi, V.; Azizi, H.; Azhdari-Zarmehri, H.; Ghaemi-Jandabi, M.; Abbasi-Mazar, A.; Mohajer, Y.; Darana, S.P. Coregulation of sleep-pain physiological interplay by orexin system: An unprecedented review. Behav. Brain Res. 2020, 391, 112650. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M. The roles of orexins in sleep/wake regulation. Neurosci. Res. 2017, 118, 56–65. [Google Scholar] [CrossRef]
- Pan, S.; Cabral, C.S.; Ashley, E.A.; Perez, M.V. Orexin: A Missing Link Between Sleep Disorders and Heart Failure? Curr. Heart Fail. Rep. 2017, 14, 100–105. [Google Scholar] [CrossRef]
- Soya, S.; Sakurai, T. Evolution of Orexin Neuropeptide System: Structure and Function. Front. Neurosci. 2020, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Ammoun, S.; Holmqvist, T.; Shariatmadari, R.; Oonk, H.B.; Detheux, M.; Parmentier, M.; Akerman, K.E.; Kukkonen, J.P. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Ther. 2003, 305, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.N.; Aschkenasi, C.J.; Lee, C.E.; Chemelli, R.M.; Saper, C.B.; Yanagisawa, M.; Elmquist, J.K. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 2001, 435, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Cluderay, J.E.; Harrison, D.C.; Hervieu, G.J. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul. Pept. 2002, 104, 131–144. [Google Scholar] [CrossRef]
- Hervieu, G.J.; Cluderay, J.E.; Harrison, D.C.; Roberts, J.C.; Leslie, R.A. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 2001, 103, 777–797. [Google Scholar] [CrossRef]
- Trivedi, P.; Yu, H.; MacNeil, D.J.; Van der Ploeg, L.H.; Guan, X.M. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998, 438, 71–75. [Google Scholar] [CrossRef]
- Nevárez, N.; de Lecea, L. Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation. F1000Res 2018, 7. [Google Scholar] [CrossRef]
- Mahler, S.V.; Moorman, D.E.; Smith, R.J.; James, M.H.; Aston-Jones, G. Motivational activation: A unifying hypothesis of orexin/hypocretin function. Nat. Neurosci. 2014, 17, 1298–1303. [Google Scholar] [CrossRef]
- Villano, I.; Messina, A.; Valenzano, A.; Moscatelli, F.; Esposito, T.; Monda, V.; Esposito, M.; Precenzano, F.; Carotenuto, M.; Viggiano, A.; et al. Basal Forebrain Cholinergic System and Orexin Neurons: Effects on Attention. Front. Behav. Neurosci. 2017, 11, 10. [Google Scholar] [CrossRef]
- Eggermann, E.; Serafin, M.; Bayer, L.; Machard, D.; Saint-Mleux, B.; Jones, B.E.; Muhlethaler, M. Orexins/hypocretins excite basal forebrain cholinergic neurones. Neuroscience 2001, 108, 177–181. [Google Scholar] [CrossRef]
- Hagan, J.J.; Leslie, R.A.; Patel, S.; Evans, M.L.; Wattam, T.A.; Holmes, S.; Benham, C.D.; Taylor, S.G.; Routledge, C.; Hemmati, P.; et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. USA 1999, 96, 10911–10916. [Google Scholar] [CrossRef] [PubMed]
- Horvath, T.L.; Peyron, C.; Diano, S.; Ivanov, A.; Aston-Jones, G.; Kilduff, T.S.; van Den Pol, A.N. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol. 1999, 415, 145–159. [Google Scholar] [CrossRef]
- van den Pol, A.N.; Ghosh, P.K.; Liu, R.J.; Li, Y.; Aghajanian, G.K.; Gao, X.B. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J. Physiol. 2002, 541, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.E.; Brill, J.; Bonnavion, P.; Huguenard, J.R.; Huerta, R.; de Lecea, L. Mechanism for Hypocretin-mediated sleep-to-wake transitions. Proc. Natl. Acad. Sci. USA 2012, 109, E2635–E2644. [Google Scholar] [CrossRef]
- Boutrel, B.; Steiner, N.; Halfon, O. The hypocretins and the reward function: What have we learned so far? Front. Behav. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef]
- Giardino, W.J.; de Lecea, L. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr. Opin. Neurobiol. 2014, 29, 103–108. [Google Scholar] [CrossRef]
- Balcita-Pedicino, J.J.; Sesack, S.R. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J. Comp. Neurol. 2007, 503, 668–684. [Google Scholar] [CrossRef]
- Fadel, J.; Deutch, A.Y. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience 2002, 111, 379–387. [Google Scholar] [CrossRef]
- Lambe, E.K.; Olausson, P.; Horst, N.K.; Taylor, J.R.; Aghajanian, G.K. Hypocretin and nicotine excite the same thalamocortical synapses in prefrontal cortex: Correlation with improved attention in rat. J. Neurosci. 2005, 25, 5225–5229. [Google Scholar] [CrossRef]
- Gentile, T.A.; Simmons, S.J.; Watson, M.N.; Connelly, K.L.; Brailoiu, E.; Zhang, Y.; Muschamp, J.W. Effects of Suvorexant, a Dual Orexin/Hypocretin Receptor Antagonist, on Impulsive Behavior Associated with Cocaine. Neuropsychopharmacology 2018, 43, 1001–1009. [Google Scholar] [CrossRef]
- Blouin, A.M.; Fried, I.; Wilson, C.L.; Staba, R.J.; Behnke, E.J.; Lam, H.A.; Maidment, N.T.; Karlsson, K.; Lapierre, J.L.; Siegel, J.M. Human hypocretin and melanin-concentrating hormone levels are linked to emotion and social interaction. Nat. Commun. 2013, 4, 1547. [Google Scholar] [CrossRef] [PubMed]
- Deats, S.P.; Adidharma, W.; Lonstein, J.S.; Yan, L. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience 2014, 272, 252–260. [Google Scholar] [CrossRef]
- Nocjar, C.; Zhang, J.; Feng, P.; Panksepp, J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience 2012, 218, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Nollet, M.; Gaillard, P.; Minier, F.; Tanti, A.; Belzung, C.; Leman, S. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 2011, 61, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Brundin, L.; Petersen, A.; Bjorkqvist, M.; Traskman-Bendz, L. Orexin and psychiatric symptoms in suicide attempters. J. Affect. Disord. 2007, 100, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Brundin, L.; Bjorkqvist, M.; Petersen, A.; Traskman-Bendz, L. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur. Neuropsychopharmacol. 2007, 17, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Brundin, L.; Bjorkqvist, M.; Traskman-Bendz, L.; Petersen, A. Increased orexin levels in the cerebrospinal fluid the first year after a suicide attempt. J. Affect. Disord. 2009, 113, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, J.; Balesar, R.; Fronczek, R.; Zhu, Q.B.; Wu, X.Y.; Hu, S.H.; Bao, A.M.; Swaab, D.F. Sexually Dimorphic Changes of Hypocretin (Orexin) in Depression. EBioMedicine 2017, 18, 311–319. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Brügel, M.; Kratzsch, J.; Strauss, M.; Sander, C.; Baum, P.; Thiery, J.; Hegerl, U.; Schönknecht, P. Cerebrospinal fluid hypocretin-1 (orexin A) levels in mania compared to unipolar depression and healthy controls. Neurosci. Lett. 2010, 483, 20–22. [Google Scholar] [CrossRef]
- Palotai, M.; Telegdy, G.; Jászberényi, M. Orexin A-induced anxiety-like behavior is mediated through GABA-ergic, α- and β-adrenergic neurotransmissions in mice. Peptides 2014, 57, 129–134. [Google Scholar] [CrossRef]
- Heydendael, W.; Sengupta, A.; Beck, S.; Bhatnagar, S. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol. Behav. 2014, 130, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.L.; Truitt, W.; Fitz, S.D.; Minick, P.E.; Dietrich, A.; Sanghani, S.; Traskman-Bendz, L.; Goddard, A.W.; Brundin, L.; Shekhar, A. A key role for orexin in panic anxiety. Nat. Med. 2010, 16, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, P.; Dugovic, C.; Shireman, B.; Preville, C.; Yun, S.; Lord, B.; Nepomuceno, D.; Wennerholm, M.; Lovenberg, T.; Carruthers, N.; et al. Evaluation of JNJ-54717793 a Novel Brain Penetrant Selective Orexin 1 Receptor Antagonist in Two Rat Models of Panic Attack Provocation. Front. Pharmacol. 2017, 8, 357. [Google Scholar] [CrossRef]
- Bonaventure, P.; Shelton, J.; Yun, S.; Nepomuceno, D.; Sutton, S.; Aluisio, L.; Fraser, I.; Lord, B.; Shoblock, J.; Welty, N.; et al. Characterization of JNJ-42847922, a Selective Orexin-2 Receptor Antagonist, as a Clinical Candidate for the Treatment of Insomnia. J. Pharmacol. Exp. Ther. 2015, 354, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.L.; Federici, L.M.; Fitz, S.D.; Renger, J.J.; Shireman, B.; Winrow, C.J.; Bonaventure, P.; Shekhar, A. Orexin 1 and 2 Receptor Involvement in CO2-Induced Panic-Associated Behavior and Autonomic Responses. Depress. Anxiety 2015, 32, 671–683. [Google Scholar] [CrossRef]
- Kordi Jaz, E.; Moghimi, A.; Fereidoni, M.; Asadi, S.; Shamsizadeh, A.; Roohbakhsh, A. SB-334867, an orexin receptor 1 antagonist, decreased seizure and anxiety in pentylenetetrazol-kindled rats. Fundam. Clin. Pharmacol. 2017, 31, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Staton, C.D.; Yaeger, J.D.W.; Khalid, D.; Haroun, F.; Fernandez, B.S.; Fernandez, J.S.; Summers, B.K.; Summers, T.R.; Sathyanesan, M.; Newton, S.S.; et al. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology 2018, 143, 79–94. [Google Scholar] [CrossRef]
- Avolio, E.; Alò, R.; Carelli, A.; Canonaco, M. Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav. Brain Res. 2011, 218, 288–295. [Google Scholar] [CrossRef]
- Lungwitz, E.A.; Molosh, A.; Johnson, P.L.; Harvey, B.P.; Dirks, R.C.; Dietrich, A.; Minick, P.; Shekhar, A.; Truitt, W.A. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol. Behav. 2012, 107, 726–732. [Google Scholar] [CrossRef]
- Akça, Ö.F.; Uzun, N.; Kılınç, İ. Orexin A in adolescents with anxiety disorders. Int. J. Psychiatry Clin. Pract. 2020, 24, 127–134. [Google Scholar] [CrossRef]
- Gottschalk, M.G.; Richter, J.; Ziegler, C.; Schiele, M.A.; Mann, J.; Geiger, M.J.; Schartner, C.; Homola, G.A.; Alpers, G.W.; Büchel, C.; et al. Orexin in the anxiety spectrum: Association of a HCRTR1 polymorphism with panic disorder/agoraphobia, CBT treatment response and fear-related intermediate phenotypes. Transl. Psychiatry 2019, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.C.; Wimmer, M.; Aston-Jones, G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 2005, 437, 556–559. [Google Scholar] [CrossRef]
- Fragale, J.E.; James, M.H.; Aston-Jones, G. Intermittent self-administration of fentanyl induces a multifaceted addiction state associated with persistent changes in the orexin system. Addict. Biol. 2021, 26, e12946. [Google Scholar] [CrossRef] [PubMed]
- James, M.H.; Stopper, C.M.; Zimmer, B.A.; Koll, N.E.; Bowrey, H.E.; Aston-Jones, G. Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol. Psychiatry 2019, 85, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, T.C.; John, J.; Shan, L.; Swaab, D.F.; Wu, M.F.; Ramanathan, L.; McGregor, R.; Chew, K.T.; Cornford, M.; Yamanaka, A.; et al. Opiates increase the number of hypocretin-producing cells in human and mouse brain and reverse cataplexy in a mouse model of narcolepsy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Baykal, S.; Albayrak, Y.; Durankuş, F.; Güzel, S.; Abbak, Ö.; Potas, N.; Beyazyüz, M.; Karabekiroğlu, K.; Donma, M.M. Decreased serum orexin A levels in drug-naive children with attention deficit and hyperactivity disorder. Neurol. Sci. 2019, 40, 593–602. [Google Scholar] [CrossRef]
- Filardi, M.; Pizza, F.; Tonetti, L.; Antelmi, E.; Natale, V.; Plazzi, G. Attention impairments and ADHD symptoms in adult narcoleptic patients with and without hypocretin deficiency. PLoS ONE 2017, 12, e0182085. [Google Scholar] [CrossRef]
- Perez, S.M.; Lodge, D.J. Orexin Modulation of VTA Dopamine Neuron Activity: Relevance to Schizophrenia. Int. J. Neuropsychopharmacol. 2021, 24, 344–353. [Google Scholar] [CrossRef]
- Dalal, M.A.; Schuld, A.; Pollmacher, T. Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol. Psychiatry 2003, 8, 836–837. [Google Scholar] [CrossRef]
- Chien, Y.L.; Liu, C.M.; Shan, J.C.; Lee, H.J.; Hsieh, M.H.; Hwu, H.G.; Chiou, L.C. Elevated plasma orexin A levels in a subgroup of patients with schizophrenia associated with fewer negative and disorganized symptoms. Psychoneuroendocrinology 2015, 53, 1–9. [Google Scholar] [CrossRef]
- Sternat, T.; Katzman, M.A. Neurobiology of hedonic tone: The relationship between treatment-resistant depression, attention-deficit hyperactivity disorder, and substance abuse. Neuropsychiatr. Dis. Treat. 2016, 12, 2149–2164. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.C.; Berridge, K.C. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc. Natl. Acad. Sci. USA 2017, 114, E9125–E9134. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Pan, H.; Kocsis, J.H.; Yang, Y.; Butler, T.; Chusid, J.; Hochberg, H.; Murrough, J.; Strohmayer, E.; Stern, E.; et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am. J. Psychiatry 2006, 163, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Young, C.B.; Kelley, E.; Prater, K.; Levitin, D.J.; Menon, V. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. J. Psychiatr. Res. 2013, 47, 1319–1328. [Google Scholar] [CrossRef]
- Mitterschiffthaler, M.T.; Kumari, V.; Malhi, G.S.; Brown, R.G.; Giampietro, V.P.; Brammer, M.J.; Suckling, J.; Poon, L.; Simmons, A.; Andrew, C.; et al. Neural response to pleasant stimuli in anhedonia: An fMRI study. NeuroReport 2003, 14, 177–182. [Google Scholar] [CrossRef]
- Light, S.N.; Heller, A.S.; Johnstone, T.; Kolden, G.G.; Peterson, M.J.; Kalin, N.H.; Davidson, R.J. Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorder. Biol. Psychiatry 2011, 70, 962–968. [Google Scholar] [CrossRef]
- Harvey, P.O.; Pruessner, J.; Czechowska, Y.; Lepage, M. Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non-clinical subjects. Mol. Psychiatry 2007, 12, 703, 767–775. [Google Scholar] [CrossRef]
- Wacker, J.; Dillon, D.G.; Pizzagalli, D.A. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 2009, 46, 327–337. [Google Scholar] [CrossRef]
- Lei, K.; Wegner, S.A.; Yu, J.H.; Mototake, A.; Hu, B.; Hopf, F.W. Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking. Front. Neurosci. 2016, 10, 400. [Google Scholar] [CrossRef]
- Castro, D.C.; Terry, R.A.; Berridge, K.C. Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose ‘Liking’ and Intake but Scopolamine in Caudal Shell Shifts ‘Liking’ Toward ‘Disgust’ and ‘Fear’. Neuropsychopharmacology 2016, 41, 2101–2111. [Google Scholar] [CrossRef]
- Liu, X.; Gao, S.; Zhang, N.; Jin, T.; Sun, X.; Luan, X.; Xu, L.; Guo, F. The orexinergic neural pathway from the lateral hypothalamus to the nucleus accumbens and its regulation of palatable food intake. Neuropeptides 2020, 80, 102028. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Berridge, K.C. An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology 2013, 38, 1655–1664. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patyal, R.; Woo, E.Y.; Borgland, S.L. Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front. Behav. Neurosci. 2012, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Cucca, F.; Frau, R.; Corrias, F.; Schlich, M.; Caboni, P.; Fadda, A.M.; Bassareo, V. Systemic Administration of Orexin a Loaded Liposomes Potentiates Nucleus Accumbens Shell Dopamine Release by Sucrose Feeding. Front. Psychiatry 2018, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- España, R.A.; Melchior, J.R.; Roberts, D.C.; Jones, S.R. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology 2011, 214, 415–426. [Google Scholar] [CrossRef]
- Moorman, D.E.; Aston-Jones, G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: Diurnal influences. J. Neurosci. 2010, 30, 15585–15599. [Google Scholar] [CrossRef]
- Naghavi, F.S.; Namvar, P.; Sadeghzadeh, F.; Haghparast, A. The Involvement of Intra-Hippocampal Dopamine Receptors in the Conditioned Place Preference Induced by Orexin Administration into the Rat Ventral Tegmental Area. Iran J. Pharm. Res. 2019, 18, 328–338. [Google Scholar]
- Mohammadkhani, A.; Fragale, J.E.; Pantazis, C.B.; Bowrey, H.E.; James, M.H.; Aston-Jones, G. Orexin-1 Receptor Signaling in Ventral Pallidum Regulates Motivation for the Opioid Remifentanil. J. Neurosci. 2019, 39, 9831–9840. [Google Scholar] [CrossRef]
- Assar, N.; Mahmoudi, D.; Mousavi, Z.; Zarrabian, S.; Haghparast, A. Role of orexin-1 and -2 receptors within the nucleus accumbens in the acquisition of sensitization to morphine in rats. Behav. Brain Res. 2019, 373, 112090. [Google Scholar] [CrossRef]
- Fartootzadeh, R.; Azizi, F.; Alaei, H.; Reisi, P. Orexin type-2 receptor blockade prevents the nicotine-induced excitation of nucleus accumbens core neurons in rats: An electrophysiological perspective. Pharmacol. Rep. 2019, 71, 361–366. [Google Scholar] [CrossRef]
- Mayannavar, S.; Rashmi, K.S.; Rao, Y.D.; Yadav, S.; Ganaraja, B. Effect of Orexin A antagonist (SB-334867) infusion into the nucleus accumbens on consummatory behavior and alcohol preference in Wistar rats. Indian J. Pharmacol. 2016, 48, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Sahafzadeh, M.; Karimi-Haghighi, S.; Mousavi, Z.; Haghparast, A. Role of the orexin receptors within the nucleus accumbens in the drug priming-induced reinstatement of morphine seeking in the food deprived rats. Brain Res. Bull. 2018, 137, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.; Lei, K.; Pedrozo, V.; Anderson, L.; Ghotra, S.; Walsh, M.; Li, L.; Yu, J.; Hopf, F.W. Differential importance of nucleus accumbens Ox1Rs and AMPARs for female and male mouse binge alcohol drinking. Sci. Rep. 2021, 11, 231. [Google Scholar] [CrossRef]
- Lei, K.; Kwok, C.; Darevsky, D.; Wegner, S.A.; Yu, J.; Nakayama, L.; Pedrozo, V.; Anderson, L.; Ghotra, S.; Fouad, M.; et al. Nucleus Accumbens Shell Orexin-1 Receptors Are Critical Mediators of Binge Intake in Excessive-Drinking Individuals. Front. Neurosci. 2019, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Azizbeigi, R.; Farzinpour, Z.; Haghparast, A. Role of Orexin-1 Receptor Within the Ventral Tegmental Area in Mediating Stress- and Morphine Priming-induced Reinstatement of Conditioned Place Preference in Rats. Basic Clin. Neurosci. 2019, 10, 373–382. [Google Scholar] [CrossRef]
- Azizbeigi, R.; Haghparast, A. Involvement of orexin-2 receptor in the ventral tegmental area in stress- and drug priming-induced reinstatement of conditioned place preference in rats. Neurosci. Lett. 2019, 696, 121–126. [Google Scholar] [CrossRef]
- Taslimi, Z.; Arezoomandan, R.; Omranifard, A.; Ghalandari-Shamami, M.; Riahi, E.; Vafaei, A.A.; Rashidy-Pour, A.; Haghparast, A. Orexin A in the ventral tegmental area induces conditioned place preference in a dose-dependent manner: Involvement of D1/D2 receptors in the nucleus accumbens. Peptides 2012, 37, 225–232. [Google Scholar] [CrossRef]
- Yazdi, F.; Jahangirvand, M.; Pirasteh, A.H.; Moradi, M.; Haghparast, A. Functional interaction between OX2 and CB1 receptors in the ventral tegmental area and the nucleus accumbens in response to place preference induced by chemical stimulation of the lateral hypothalamus. Pharmacol. Biochem. Behav. 2015, 139, 39–46. [Google Scholar] [CrossRef]
- Azizi, F.; Fartootzadeh, R.; Alaei, H.; Reisi, P. Effects of concurrent blockade of OX2 and CB1 receptors in the ventral tegmental area on nicotine-induced place preference in rats. Neurosci. Lett. 2018, 684, 121–126. [Google Scholar] [CrossRef]
- Bernstein, D.L.; Badve, P.S.; Barson, J.R.; Bass, C.E.; España, R.A. Hypocretin receptor 1 knockdown in the ventral tegmental area attenuates mesolimbic dopamine signaling and reduces motivation for cocaine. Addict. Biol. 2018, 23, 1032–1045. [Google Scholar] [CrossRef]
- Brown, R.M.; Kim, A.K.; Khoo, S.Y.; Kim, J.H.; Jupp, B.; Lawrence, A.J. Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in iP rats. Addict. Biol. 2016, 21, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Farahimanesh, S.; Zarrabian, S.; Haghparast, A. Role of orexin receptors in the ventral tegmental area on acquisition and expression of morphine-induced conditioned place preference in the rats. Neuropeptides 2017, 66, 45–51. [Google Scholar] [CrossRef] [PubMed]
- James, M.H.; Charnley, J.L.; Levi, E.M.; Jones, E.; Yeoh, J.W.; Smith, D.W.; Dayas, C.V. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int. J. Neuropsychopharmacol. 2011, 14, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Muschamp, J.W.; Hollander, J.A.; Thompson, J.L.; Voren, G.; Hassinger, L.C.; Onvani, S.; Kamenecka, T.M.; Borgland, S.L.; Kenny, P.J.; Carlezon, W.A., Jr. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc. Natl. Acad. Sci. USA 2014, 111, E1648–E1655. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.J.; Navarro, M.; Thiele, T.E. The Role of Orexin Signaling in the Ventral Tegmental Area and Central Amygdala in Modulating Binge-Like Ethanol Drinking Behavior. Alcohol. Clin. Exp. Res. 2017, 41, 551–561. [Google Scholar] [CrossRef]
- Richardson, K.A.; Aston-Jones, G. Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. J. Neurosci. 2012, 32, 3809–3817. [Google Scholar] [CrossRef]
- Safari-Sandiani, E.; Rahimitabar, N.; Rezaee, L.; Behnaz, M.; Haghparast, A. The contribution of orexin receptors within the ventral tegmental area to modulation of antinociception induced by chemical stimulation of the lateral hypothalamus in the animal model of orofacial pain in the rats. Behav. Pharmacol. 2019, 31, 500–509. [Google Scholar] [CrossRef]
- Srinivasan, S.; Simms, J.A.; Nielsen, C.K.; Lieske, S.P.; Bito-Onon, J.J.; Yi, H.; Hopf, F.W.; Bonci, A.; Bartlett, S.E. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS ONE 2012, 7, e44726. [Google Scholar] [CrossRef]
- Terrill, S.J.; Hyde, K.M.; Kay, K.E.; Greene, H.E.; Maske, C.B.; Knierim, A.E.; Davis, J.F.; Williams, D.L. Ventral tegmental area orexin 1 receptors promote palatable food intake and oppose postingestive negative feedback. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R592–R599. [Google Scholar] [CrossRef]
- Wang, B.; You, Z.B.; Wise, R.A. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: Independence from the local corticotropin-releasing factor network. Biol. Psychiatry 2009, 65, 857–862. [Google Scholar] [CrossRef]
- Zarepour, L.; Fatahi, Z.; Sarihi, A.; Haghparast, A. Blockade of orexin-1 receptors in the ventral tegmental area could attenuate the lateral hypothalamic stimulation-induced potentiation of rewarding properties of morphine. Neuropeptides 2014, 48, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Mayer, H.S.; Petrovich, G.D. Orexin/Hypocretin-1 Receptor Antagonism Selectively Reduces Cue-Induced Feeding in Sated Rats and Recruits Medial Prefrontal Cortex and Thalamus. Sci. Rep. 2015, 5, 16143. [Google Scholar] [CrossRef]
- Dimatelis, J.J.; Mtintsilana, A.; Naidoo, V.; Stein, D.J.; Russell, V.A. Chronic light exposure alters serotonergic and orexinergic systems in the rat brain and reverses maternal separation-induced increase in orexin receptors in the prefrontal cortex. Metab. Brain Dis. 2018, 33, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Jhou, T.C. The rostromedial tegmental (RMTg) “brake” on dopamine and behavior: A decade of progress but also much unfinished work. Neuropharmacology 2021, 198, 108763. [Google Scholar] [CrossRef] [PubMed]
| Reference | Effects of Orexin |
|---|---|
| Nucleus Accumbens | |
| Assar et al., 2019 [79] | Administration of OX1R antagonists reduced acquisition of morphine sensitization OX2R antagonist produced a similar effect, but at a higher dose |
| Fartootzadeh, 2019 [80] | Administration of OX2R antagonists in NAcc inhibited nicotine-induced increase in neuronal excitation |
| Lai et al., 2018 [74] | Activation of OX1R by orexin A facilitated sucrose-stimulated DA transmission by increasing the basal activity of VTA DA neurons |
| Lei et al., 2016 [69] | Administration of OX1R antagonist in the medial NAcc shell and mPFC significantly reduced excessive alcohol intake |
| Castro et al., 2016 [70] | Orexin enhances sucrose “liking” and intake but scopolamine in the caudal shell shifts “liking” toward “disgust” and “fear” |
| Liu et al., 2020 [71] | Microinjection orexin-A significantly increased palatable food intake; the effect was inhibited by pretreatment with OX1R antagonist |
| Mayannavar et al., 2016 [81] | Microinjection of orexin A antagonist in the NAc reduced water and alcohol intake, but did not affect preference to alcohol |
| Patyal et al., 2012 [73] | Application of Orexin A increased dopamine release in the NAcc shell without altering reuptake at dopamine terminals, indicating that locally released orexin A can modulate dopamine release in NAcc shell |
| Sahafzadeh et al., 2018 [82] | Administration of OX1R and OX2R antagonists into the NAcc attenuated the effect of food deprivation on morphine reinstatement |
| Kwok, C et al., 2021 [83] | OX1R inhibition in NAcc shell altered alcohol intake in male, but not female mice. OX1R inhibition reduced compulsion-like alcohol intake in both sexes |
| Lei, K., et al., 2019 [84] | Activation of OX1Rs promoted alcohol intake during intermittent-access |
| Ventral Tegmental Area | |
| Azizbeigi et al., 2019 [85] | OX1R antagonist suppressed morphine reinstatement induced by stress or drug priming |
| Azizbeigi et al., 2019 [86] | OX2R antagonist suppressed morphine reinstatement induced by stress or drug priming |
| Taslimi et al., 2012 [87] | Orexin A elicited conditioned place preference; the response was inhibited by administration of D1 and D2 receptor antagonists into the NAcc |
| Yazdi et al., 2015 [88] | Administration of OX2R antagonist into the VTA or NAcc dose-dependently inhibited the development of LH stimulation-induced conditioned place preference Co-administration of low doses of OX2R antagonist and CB1 receptor antagonist into the NAcc reduced conditioning scores |
| Azizi et al., 2018 [89] | OX2R antagonist inhibited the development of nicotine-induced conditioned place preference |
| Bernstein et al., 2018 [90] | OX1R knock-down delayed cocaine self-administration, indicating that OX1R are involved in motivation for cocaine |
| Brown et al., 2016 [91] | OX1R antagonist attenuated cue-induced reinstatement of ethanol-seeking |
| España et al., 2011 [75] | Orexin 1 increased the effect of cocaine on tonic and phasic DA signaling and increased the motivation to self-administer cocaine |
| Farahimanesh et al., 2017 [92] | OXR1 and OXR2 antagonists attenuated morphine conditioned place preference acquisition during the conditioning phase, and expression during the post-conditioning phase |
| James et al., 2011 [93] | OXR1 antagonist dose-dependently attenuated cue-induced reinstatement of cocaine-seeking |
| Moorman et al., 2010 [76] | Orexin (not specified if A or B) increased the activity of DA neurons and augmented excitatory responses to mPFC stimulation OX1R antagonist decreased tonic DA cell activity during active but not rest period |
| Muschamp et al., 2014 [94] | OX1R antagonism increased the threshold for intracranial self-stimulation; the response was blocked by a dynorphin receptor antagonist OXR1 antagonism reduced cocaine-induced impulsive behaviour Orexin A excited DA neurons in the VTA OX1R antagonist in the VTA reduced cocaine intake |
| Naghavi et al., 2019 [77] | Administration of D1 and D2 receptor antagonists attenuated the acquisition of place preference by orexin A |
| Olney et al., 2017 [95] | OX1R antagonist reduced binge-like ethanol intake but did not affect sucrose intake |
| Richardson et al., 2012 [96] | OX1R antagonist administration attenuated the morphine conditioned place preference score induced by administration of carbachol into the LGA, indicating that OX1R plays a role in sensitization to morphine |
| Saferi et al., 2019 [97] | OX1R and OXR2 antagonists reduced antinociceptive effect induced by carbachol administration into the lateral hypothalamus |
| Srinivasan et al., 2012 [98] | Administration of a dual OX1R and OXR2 antagonist into the VTA decreased operant self-administration of ethanol but not and sucrose Orexin A increased firing of VTA neurons |
| Taslimi et al., 2012 [87] | Orexin A administration induced conditioned place preference; the effect was inhibited by administration of D1 and D2 receptor antagonists |
| Terrill et al., 2016 [99] | Orexin-A increased intake of palatable food (high-fat food and sucrose), whereas OX1R antagonist suppressed sucrose intake |
| Wang et al., 2009 [100] | Orexin-A administration reinstated cocaine seeking and caused release of glutamate and dopamine in the VTA |
| Zarepour et al., 2014 [101] | OX1R antagonist inhibited the acquisition but not expression of LH stimulation-induced morphine conditioned place preference |
| Ventral Pallidum | |
| Ho et al., 2013 [72] | Orexin amplified hedonic liking for sweetness |
| Mohammadkhani et al., [78] | OXR1 antagonist decreased motivation for remifentanil without affecting remifentanil consumption Thus, Orexin amplifies motivation to gain reward from drugs and sucrose. |
| Medial prefrontal cortex | |
| Lei et al., 2016 [69] | OX1R antagonist reduced alcohol drinking |
| Cole et al., 2015 [102] | Systemic OX1R antagonist significantly reduced cue-driven consumption in sated rats and increased Fos expression in mPFC |
| Dimatelis et al., 2018 [103] | Maternal separation increased OXR1 and OXR2 levels in the PFC |
| Lambe et al., 2005 [29] | Similar to nicotine, orexin B infusion into the PFC improved accuracy under high attentional demand |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katzman, M.A.; Katzman, M.P. Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone. Brain Sci. 2022, 12, 150. https://doi.org/10.3390/brainsci12020150
Katzman MA, Katzman MP. Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone. Brain Sciences. 2022; 12(2):150. https://doi.org/10.3390/brainsci12020150
Chicago/Turabian StyleKatzman, Martin A., and Matthew P. Katzman. 2022. "Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone" Brain Sciences 12, no. 2: 150. https://doi.org/10.3390/brainsci12020150
APA StyleKatzman, M. A., & Katzman, M. P. (2022). Neurobiology of the Orexin System and Its Potential Role in the Regulation of Hedonic Tone. Brain Sciences, 12(2), 150. https://doi.org/10.3390/brainsci12020150





