A Glycemia-Based Nomogram for Predicting Outcome in Stroke Patients after Endovascular Treatment
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rinkel, L.A.; Nguyen, T.T.M.; Guglielmi, V.; Groot, A.E.; Posthuma, L.; Roos, Y.B.; Majoie, C.B.; Nijeholt, G.J.L.; Emmer, B.J.; van der Worp, H.B.; et al. High Admission Glucose Is Associated with Poor Outcome After Endovascular Treatment for Ischemic Stroke. Stroke 2020, 51, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vega, C.; A Domingo, R.; Tripathi, S.; Ramos-Fresnedo, A.; Kashyap, S.; Quinones-Hinojosa, A.; Lin, M.P.; Fox, W.C.; Tawk, R.G. Influence of glucose levels on clinical outcome after mechanical thrombectomy for large-vessel occlusion: A systematic review and meta-analysis. J. NeuroInterventional Surg. 2022, 14, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Tsivgoulis, G.; Pandhi, A.; Dillard, K.; Katsanos, A.H.; Magoufis, G.; Chang, J.J.; Zand, R.; Hoit, D.; Safouris, A.; et al. Admission hyperglycemia and outcomes in large vessel occlusion strokes treated with mechanical thrombectomy. J. NeuroInterventional Surg. 2018, 10, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Liu, R.; Gao, F.; Ma, N.; Mo, D.; Liao, X.; Wang, C.; Sun, X.; Song, L.; Jia, B.; et al. Effect of Hyperglycemia at Presentation on Outcomes in Acute Large Artery Occlusion Patients Treated with Solitaire Stent Thrombectomy. Front. Neurol. 2019, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Osei, E.; Hertog, H.M.D.; Berkhemer, O.A.; Fransen, P.S.; Roos, Y.B.; Beumer, D.; van Oostenbrugge, R.J.; Schonewille, W.J.; Boiten, J.; Zandbergen, A.A.; et al. Admission Glucose and Effect of Intra-Arterial Treatment in Patients with Acute Ischemic Stroke. Stroke 2017, 48, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Yong, M.; Kaste, M. Dynamic of Hyperglycemia as a Predictor of Stroke Outcome in the ECASS-II Trial. Stroke 2008, 39, 2749–2755. [Google Scholar] [CrossRef]
- Osei, E.; Hertog, H.D.; Berkhemer, O.; Fransen, P.; Roos, Y.; Beumer, D.; van Oostenbrugge, R.; Schonewille, W.; Boiten, J.; Zandbergen, A.; et al. Increased admission and fasting glucose are associated with unfavorable short-term outcome after intra-arterial treatment of ischemic stroke in the MR CLEAN pretrial cohort. J. Neurol. Sci. 2016, 371, 1–5. [Google Scholar] [CrossRef]
- Wnuk, M.; Popiela, T.; Drabik, L.; Brzegowy, P.; Lasocha, B.; Wloch-Kopec, D.; Pulyk, R.; Jagiella, J.; Wiacek, M.; Kaczorowski, R.; et al. Fasting Hyperglycemia and Long-term Outcome in Patients with Acute Ischemic Stroke Treated with Mechanical Thrombectomy. J. Stroke Cerebrovasc. Dis. 2020, 29, 104774. [Google Scholar] [CrossRef]
- Yuan, L.; Sun, Y.; Huang, X.; Xu, X.; Xu, J.; Xu, Y.; Yang, Q.; Zhu, Y.; Zhou, Z. Fasting Blood-Glucose Level and Clinical Outcome in Anterior Circulation Ischemic Stroke of Different Age Groups After Endovascular Treatment. Neuropsychiatr. Dis. Treat. 2022, 18, 575–583. [Google Scholar] [CrossRef]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J.; for the A1c-Derived Average Glucose (ADAG) Study Group. Translating the A1C Assay into Estimated Average Glucose Values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef]
- Chang, J.Y.; Kim, W.-J.; Kwon, J.H.; Kim, B.J.; Kim, J.-T.; Lee, J.; Cha, J.K.; Kim, D.-H.; Cho, Y.-J.; Hong, K.-S.; et al. Prestroke Glucose Control and Functional Outcome in Patients with Acute Large Vessel Occlusive Stroke and Diabetes After Thrombectomy. Diabetes Care 2021, 44, 2140–2148. [Google Scholar] [CrossRef]
- Merlino, G.; Pez, S.; Gigli, G.L.; Sponza, M.; Lorenzut, S.; Surcinelli, A.; Smeralda, C.; Valente, M. Stress Hyperglycemia in Patients with Acute Ischemic Stroke Due to Large Vessel Occlusion Undergoing Mechanical Thrombectomy. Front. Neurol. 2021, 12, 725002. [Google Scholar] [CrossRef]
- Chen, G.; Ren, J.; Huang, H.; Shen, J.; Yang, C.; Hu, J.; Pan, W.; Sun, F.; Zhou, X.; Zeng, T.; et al. Admission Random Blood Glucose, Fasting Blood Glucose, Stress Hyperglycemia Ratio, and Functional Outcomes in Patients with Acute Ischemic Stroke Treated with Intravenous Thrombolysis. Front. Aging Neurosci. 2022, 14, 782282. [Google Scholar] [CrossRef]
- Yang, C.-J.; Liao, W.-I.; Wang, J.-C.; Tsai, C.-L.; Lee, J.-T.; Peng, G.-S.; Lee, C.-H.; Hsu, C.-W.; Tsai, S.-H. Usefulness of glycated hemoglobin A1c-based adjusted glycemic variables in diabetic patients presenting with acute ischemic stroke. Am. J. Emerg. Med. 2017, 35, 1240–1246. [Google Scholar] [CrossRef]
- Roberts, G.; Sires, J.; Chen, A.; Thynne, T.; Sullivan, C.; Quinn, S.; Chen, W.S.; Meyer, E. A comparison of the stress hyperglycemia ratio, glycemic gap, and glucose to assess the impact of stress-induced hyperglycemia on ischemic stroke outcome. J. Diabetes 2021, 13, 1034–1042. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Meng, L.; Wang, H.; Yang, H.; Zhang, X.; Zhang, Q.; Dong, Q.; Shu, Z.; Chen, L.; Gong, L.; Zhao, Y. Nomogram to Predict Poor Outcome after Mechanical Thrombectomy at Older Age and Histological Analysis of Thrombus Composition. Oxidative Med. Cell. Longev. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Cappellari, M.; Mangiafico, S.; Saia, V.; Pracucci, G.; Nappini, S.; Nencini, P.; Konda, D.; Sallustio, F.; Vallone, S.; Zini, A.; et al. IER-START nomogram for prediction of three-month unfavorable outcome after thrombectomy for stroke. Int. J. Stroke 2020, 15, 412–420. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Wang, J.-H.; Yang, W.-H.; Zhu, X.-Q.; Xue, J.; Li, Z.-Z.; Kong, Y.-M.; Hu, L.; Jiang, S.-S.; Xu, X.-S.; et al. Nomogram to predict 3-month unfavorable outcome after thrombectomy for stroke. BMC Neurol. 2022, 22, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Zou, Y.; Hu, J.; Li, X.M.; Huang, C.P.; Shan, Y.J.; Nyame, L.; Zhao, Z.; Sun, C.; Ibrahim, M.; et al. A NAC nomogram to predict the probability of three-month unfavorable outcome in Chinese acute ischemic stroke patients treated with mechanical thrombectomy. Int. J. Neurosci. 2021, 131, 163–169. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, D.; Wang, H.; Zi, W.; Zhang, M.; Geng, Y.; Zhou, Z.; Wang, W.; Xu, H.; Tian, X.; et al. Predictors for Symptomatic Intracranial Hemorrhage After Endovascular Treatment of Acute Ischemic Stroke. Stroke 2017, 48, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418, Correction in Stroke 2019, 50, e440–e441. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, Y.; Ohira, T.; Itabashi, R.; Uchida, K.; Sakai, N.; Yamagami, H.; Morimoto, T.; Yoshimura, S. Association of Admission Hyperglycemia with Clinical Outcomes in Japanese Patients with Acute Large Vessel Occlusion Stroke: A post hoc Analysis of the Recovery by Endovascular Salvage for Cerebral Ultra-Acute Embolism Japan Registry 2. Cerebrovasc. Dis. 2021, 50, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ren, Y.; Cui, X.; Liu, P.; Chen, F.; Zhao, H.; Han, Z.; Huang, Y.; Ma, Q.; Luo, Y. Postoperative hyperglycemia predicts symptomatic intracranial hemorrhage after endovascular treatment in patients with acute anterior circulation large artery occlusion. J. Neurol. Sci. 2020, 409, 116588. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Guo, S.; Pan, J.; Xu, C.; Geng, Y.; Zheng, S. Increased Postoperative Fasting Glucose Is Associated with Unfavorable Outcomes in Patients Treated With Mechanical Thrombectomy Treatment. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Miao, J.; Zheng, W.; Yang, Q.; Ye, X.; Zhuang, X.; Peng, F. High Stress Hyperglycemia Ratio Predicts Poor Outcome after Mechanical Thrombectomy for Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1668–1673. [Google Scholar] [CrossRef]
- Choi, K.H.; Kim, J.H.; Kang, K.W.; Kim, J.T.; Choi, S.-M.; Lee, S.-H.; Park, M.-S.; Kim, B.-C.; Kim, M.-K.; Cho, K.-H. HbA1c (Glycated Hemoglobin) Levels and Clinical Outcome Post-Mechanical Thrombectomy in Patients with Large Vessel Occlusion. Stroke 2018, 50, 119–126. [Google Scholar] [CrossRef]
- Diprose, W.K.; Wang, M.T.M.; McFetridge, A.; Sutcliffe, J.; Barber, P.A. Glycated hemoglobin (HbA1c) and outcome following endovascular thrombectomy for ischemic stroke. J. NeuroInterventional Surg. 2020, 12, 30–32. [Google Scholar] [CrossRef]
- Yue, F.; Wang, Z.; Pu, J.; Zhang, M.; Liu, Y.; Han, H.; Liu, W.; Wang, X.; Li, R.; Xue, D.; et al. HbA1c and clinical outcomes after endovascular treatment in patients with posterior circulation large vessel occlusion: A subgroup analysis of a nationwide registry (BASILAR). Ther. Adv. Neurol. Disord. 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Shi, M.; Qu, Y.; Huo, L.; Hao, Z.; Yue, F.; Gan, L.; Wang, S. Which glucose parameter best predicts poor outcome after mechanical thrombectomy for acute large vessel occlusion stroke? Intern. Med. J. 2021, 52, 1374–1380. [Google Scholar] [CrossRef]
- Lu, G.-D.; Ren, Z.-Q.; Zhang, J.-X.; Zu, Q.-Q.; Shi, H.-B. Effects of Diabetes Mellitus and Admission Glucose in Patients Receiving Mechanical Thrombectomy: A Systematic Review and Meta-analysis. Neurocritical Care 2018, 29, 426–434. [Google Scholar] [CrossRef]
- Merlino, G.; Smeralda, C.; Sponza, M.; Gigli, G.L.; Lorenzut, S.; Marini, A.; Surcinelli, A.; Pez, S.; Vit, A.; Gavrilovic, V.; et al. Dynamic Hyperglycemic Patterns Predict Adverse Outcomes in Patients with Acute Ischemic Stroke Undergoing Mechanical Thrombectomy. J. Clin. Med. 2020, 9, 1932. [Google Scholar] [CrossRef]
- Robbins, N.M.; Swanson, R.A. Opposing effects of glucose on stroke and reperfusion injury: Acidosis, oxidative stress, and energy metabolism. Stroke 2014, 45, 1881–1886. [Google Scholar] [CrossRef]
- Linfante, I.; Starosciak, A.K.; Walker, G.R.; Dabus, G.; Castonguay, A.C.; Gupta, R.; Sun, C.-H.J.; Martin, C.; E Holloway, W.; Mueller-Kronast, N.; et al. Predictors of poor outcome despite recanalization: A multiple regression analysis of the NASA registry. J. NeuroInterventional Surg. 2016, 8, 224–229. [Google Scholar] [CrossRef]
- Seker, F.; Pfaff, J.; Wolf, M.; Ringleb, P.; Nagel, S.; Schönenberger, S.; Herweh, C.; Möhlenbruch, M.; Bendszus, M.; Pham, M. Correlation of Thrombectomy Maneuver Count with Recanalization Success and Clinical Outcome in Patients with Ischemic Stroke. Am. J. Neuroradiol. 2017, 38, 1368–1371. [Google Scholar] [CrossRef]
- Asaithambi, G.; Tipps, M.E. Effect of Intensive Glucose Control on Outcomes of Hyperglycemic Stroke Patients Receiving Mechanical Thrombectomy: Secondary Analysis of the SHINE Trial. J. Neurosurg. Anesthesiol. 2022, 34, 415–418. [Google Scholar] [CrossRef]
- Bains, N.K.; Huang, W.; French, B.R.; Siddiq, F.; Gomez, C.R.; Qureshi, A.I. Hyperglycemic control in acute ischemic stroke patients undergoing endovascular treatment: Post hoc analysis of the Stroke Hyperglycemia Insulin Network Effort trial. J. Neurointerv. Surg. 2022, 1–6. [Google Scholar]

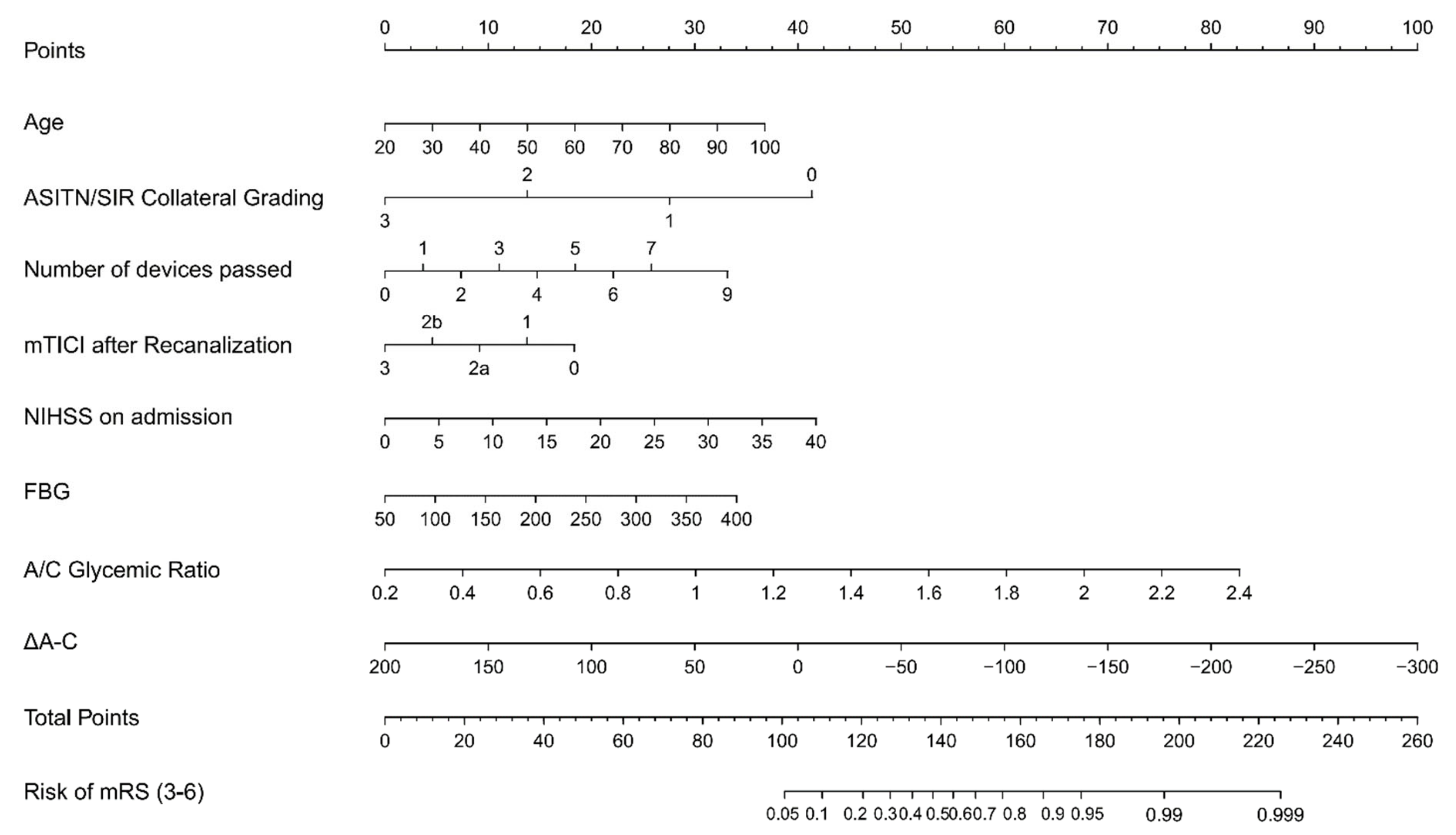
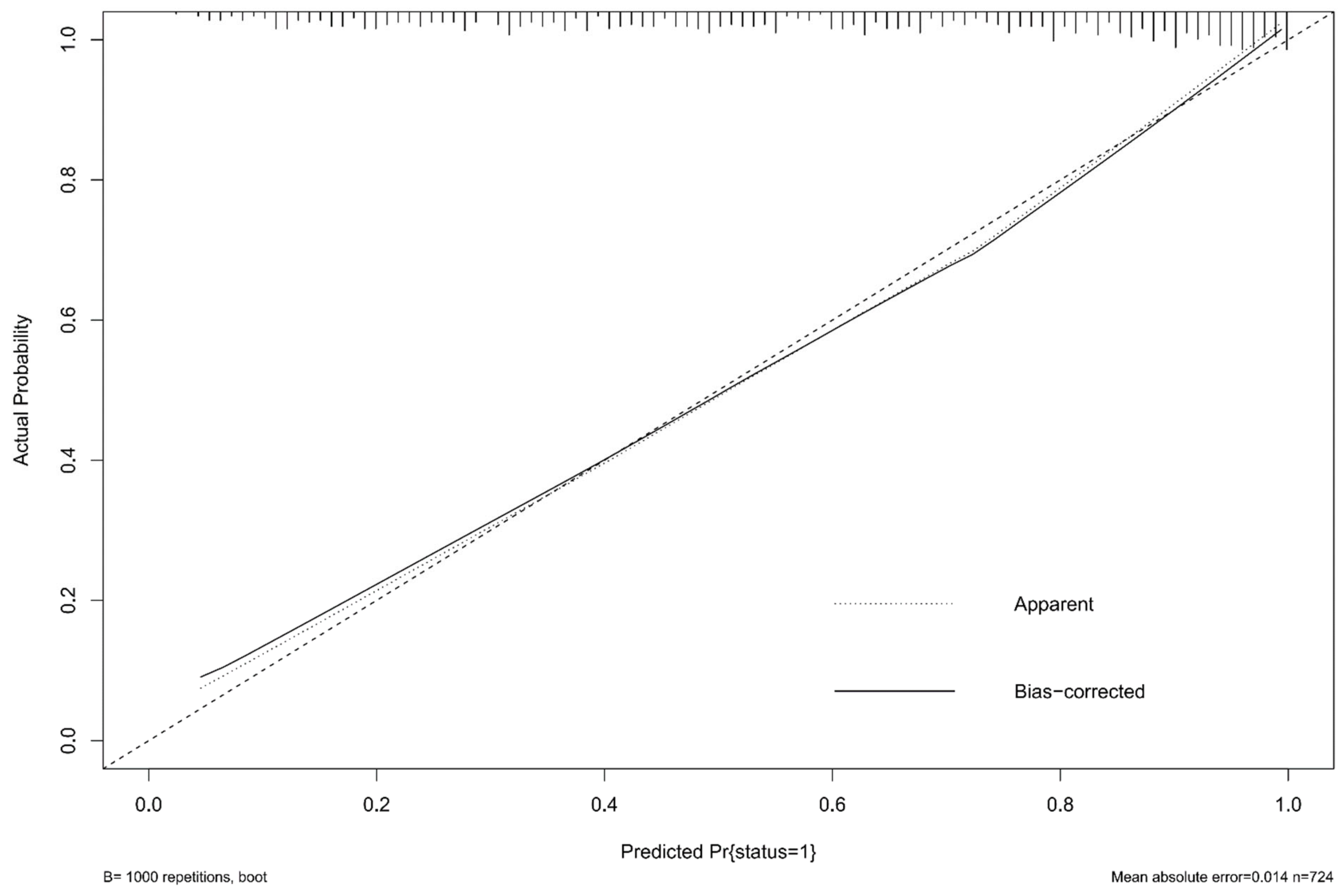
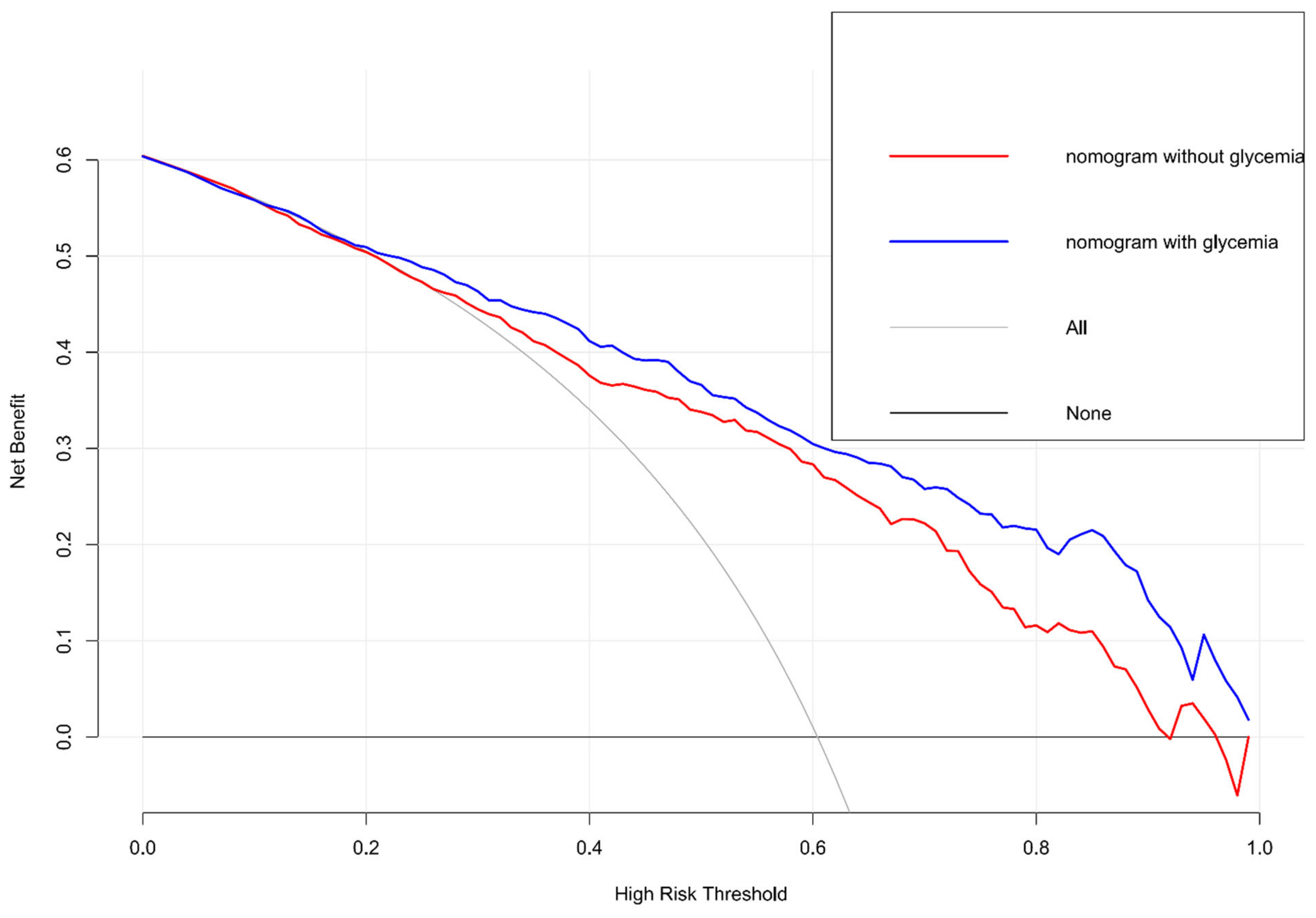
| Total (739) | mRS 0–2 (n = 291) | mRS 3–6 (n = 448) | p | |
|---|---|---|---|---|
| Age (years), mean ± SD | 70.0 ± 12.2 | 65.5 ± 12.7 | 72.9 ± 10.9 | <0.001 |
| Sex, male, n (%) | 471 (63.7%) | 208 (71.5%) | 263 (58.7%) | <0.001 |
| Medical history, n (%) | ||||
| Hypertension | 547 (74.0%) | 205 (70.4%) | 342 (76.3%) | 0.074 |
| Diabetes | 234 (31.7%) | 80 (27.5%) | 154 (34.4%) | 0.049 |
| Atrial fibrillation | 334 (45.2%) | 99 (34.0%) | 235 (52.5%) | <0.001 |
| Prior stroke | 152 (20.6%) | 49 (17.0%) | 103 (23.0%) | 0.048 |
| Laboratory examination, mean ± SD | ||||
| FBG, mg/dL | 128 ± 45 | 112 ± 36 | 138 ± 48 | <0.001 |
| HbA1c, % | 6.3 ± 1.4 | 6.2 ± 1.2 | 6.4 ± 1.5 | 0.013 |
| Average chronic glycemia, mg/dL | 135 ± 40 | 130 ± 35 | 138 ± 43 | 0.013 |
| A/C glycemic ratio | 0.97 ± 0.28 | 0.88 ± 0.22 | 1.03 ± 0.30 | <0.001 |
| ΔA-C, mg/dL | −6 ± 43 | −18 ± 36 | 1 ± 46 | <0.001 |
| Serum creatinine, μmol/L | 77.2 ± 32.6 | 75 ± 33 | 78 ± 32 | 0.245 |
| Total cholesterol, mg/dL | 76 ± 21 | 77 ± 20 | 76 ± 22 | 0.489 |
| Triglycerides, mg/dL | 23 ± 16 | 23 ± 14 | 23 ± 17 | 0.932 |
| HDL, mg/dL | 20 ± 6 | 20 ± 5 | 20 ± 7 | 0.077 |
| LDL, mg/dL | 46 ± 17 | 47 ± 17 | 46 ± 17 | 0.275 |
| Baseline NIHSS score, median (IQR) | 14 (10–18) | 12 (7–16) | 16 (12–20) | <0.001 |
| Infarct circulation, n (%) | 0.464 | |||
| Anterior | 626 (84.7%) | 243 (83.5%) | 383 (85.5%) | |
| Posterior | 113 (15.3%) | 48 (16.5%) | 65 (14.5%) | |
| Stroke subtypes, n (%) | <0.001 | |||
| LAA | 332 (44.9%) | 152 (52.2%) | 180 (40.2%) | |
| CE | 349 (47.2%) | 106 (36.4%) | 243 (54.2%) | |
| SOE | 22 (3.0%) | 17 (5.8%) | 5 (1.1%) | |
| SUE | 36 (4.9%) | 16 (5.5%) | 20 (4.5%) | |
| ASITN/SIR, median (IQR) | 2 (1-2) | 2(2-2) | 1 (1-2) | <0.001 |
| Interval time, min, median (IQR) | ||||
| Onset to door | 175 (86–308) | 175 (85–305) | 175 (81–300) | 0.924 |
| Door to groin puncture | 107 (80–140) | 108 (80–138) | 104 (78–140) | 0.283 |
| Door to first recanalization | 184 (149–228) | 170 (144–214) | 190 (150–230) | 0.003 |
| Intravenous thrombolysis, n (%) | 309 (41.8%) | 132 (45.3%) | 177 (39.5%) | 0.119 |
| Number of devices passed, median (IQR) | 2 (1-3) | 1 (1-2) | 2 (1-3) | <0.001 |
| mTICI score, n (%) | <0.001 | |||
| 2b-3 | 644(87.1%) | 276(94.8%) | 368(82.1%) | |
| 0-2a | 95(12.9%) | 15(5.2%) | 80(17.9%) |
| Crude OR (95% CI) | p | Adjusted OR (95% CI) | p | |
|---|---|---|---|---|
| FBG | 1.017 (1.012–1.022) | <0.001 | 1.012 (1.006–1.018) | <0.001 |
| Chronic glycemia | 1.005 (1.001–1.009) | 0.019 | 1.005 (0.999–1.011) | 0.122 |
| A/C glycemic ratio | 10.720 (5.559–20.671) | <0.001 | 4.783 (2.183–10.478) | <0.001 |
| ΔA-C | 1.011 (1.007–1.015) | <0.001 | 1.007 (1.002–1.011) | 0.006 |
| Age | 1.055 (1.040–1.069) | <0.001 | 1.041 (1.022–1.060) | <0.001 |
| Sex | 0.567 (0.413–0.778) | <0.001 | 0.792 (0.533–1.177) | 0.249 |
| Hypertension | 1.354 (0.970–1.888) | 0.075 | ||
| Diabetes | 1.382 (1.000–1.908) | 0.050 | ||
| Atrial fibrillation | 2.140 (1.577–2.904) | <0.001 | 0.823 (0.538–1.259) | 0.369 |
| Prior stroke | 1.462 (1.002–2.134) | 0.049 | 0.970 (0.609–1.544) | 0.896 |
| HDL | 1.024 (0.997–1.051) | 0.079 | ||
| Baseline NIHSS score | 1.122 (1.093–1.151) | <0.001 | 1.098 (1.067–1.130) | <0.001 |
| Stroke subtypes | 0.995 (0.855–1.157) | 0.946 | ||
| ASITN/SIR | 0.253 (0.189–0.339) | <0.001 | 0.289 (0.208–0.402) | <0.001 |
| Door to first recanalization | 1.001 (0.999–1.003) | 0.213 | ||
| Number of devices passed | 1.507 (1.317–1.724) | <0.001 | 1.352 (1.147–1.594) | <0.001 |
| mTICI score | 0.250 (0.141–0.443) | <0.001 | 0.334 (0.169–0.660) | 0.002 |
| Patients with Diabetes (n = 234) | Patients without Diabetes (n = 505) | P-Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | P | Adjusted OR (95% CI) | P | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P | Diabetes and glycemia | |
| FBG | 1.008 (1.002–1.014) | 0.004 | 1.006 (0.999–1.012) | 0.091 | 1.035 (1.026–1.044) | <0.001 | 1.025 (1.014–1.035) | <0.001 | 0.004 |
| Chronic glycemia | 1.002 (0.996–1.008) | 0.495 | 1.011 (1.000–1.023) | 0.056 | 0.039 | ||||
| A/C glycemic ratio | 3.092 (1.299–7.359) | 0.011 | 1.656 (0.590–4.649) | 0.339 | 46.832 (16.923–129.602) | <0.001 | 15.735 (4.588–53.969) | <0.001 | 0.005 |
| ΔA-C | 1.004 (1.000–1.009) | 0.046 | 1.001 (0.996–1.006) | 0.753 | 1.033 (1.024–1.041) | <0.001 | 1.024 (1.014–1.035) | <0.001 | 0.123 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhang, Y.; Li, X.; Liu, Y.; Jiang, T.; Wang, M.; Deng, Q.; Zhou, J. A Glycemia-Based Nomogram for Predicting Outcome in Stroke Patients after Endovascular Treatment. Brain Sci. 2022, 12, 1576. https://doi.org/10.3390/brainsci12111576
Liu C, Zhang Y, Li X, Liu Y, Jiang T, Wang M, Deng Q, Zhou J. A Glycemia-Based Nomogram for Predicting Outcome in Stroke Patients after Endovascular Treatment. Brain Sciences. 2022; 12(11):1576. https://doi.org/10.3390/brainsci12111576
Chicago/Turabian StyleLiu, Chengfang, Yuqiao Zhang, Xiaohui Li, Yukai Liu, Teng Jiang, Meng Wang, Qiwen Deng, and Junshan Zhou. 2022. "A Glycemia-Based Nomogram for Predicting Outcome in Stroke Patients after Endovascular Treatment" Brain Sciences 12, no. 11: 1576. https://doi.org/10.3390/brainsci12111576
APA StyleLiu, C., Zhang, Y., Li, X., Liu, Y., Jiang, T., Wang, M., Deng, Q., & Zhou, J. (2022). A Glycemia-Based Nomogram for Predicting Outcome in Stroke Patients after Endovascular Treatment. Brain Sciences, 12(11), 1576. https://doi.org/10.3390/brainsci12111576






