Association between Genetically Proxied Inhibition of HMG-CoA Reductase and Age at Onset of Huntington’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Outcomes
2.3. Statistical Analysis
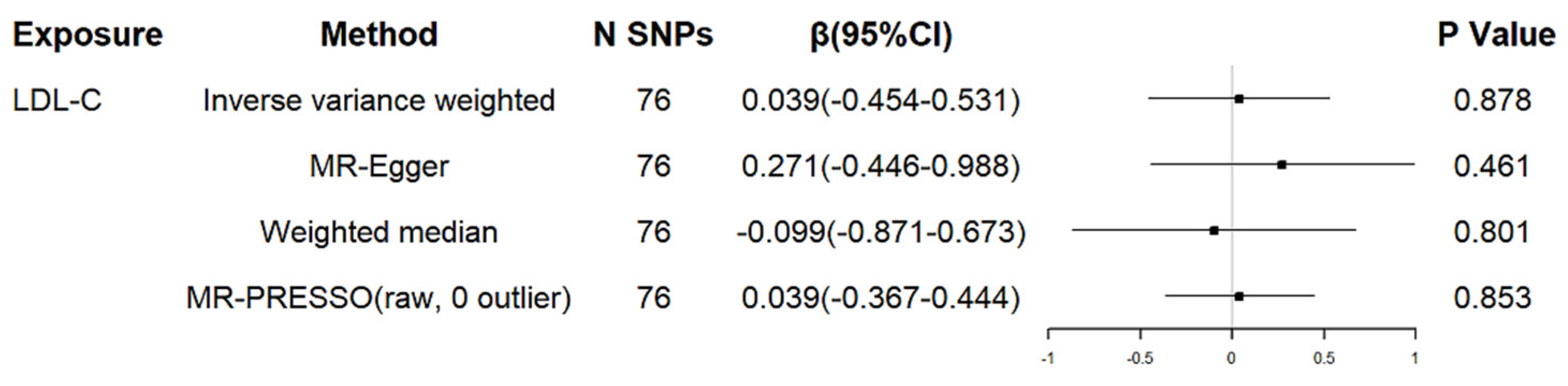
3. Results
3.1. Genetically Determined LDL Cholesterol and Age at Onset of HD
3.2. Genetic Proxies for Lipid-Lowering Drugs and AAO of HD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Gusella, J.F.; MacDonald, M.E.; Ambrose, C.M.; Duyao, M.P. Molecular genetics of Huntington’s disease. Arch. Neurol. 1993, 50, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.E. Huntington’s disease and the striatal medium spiny neuron: Cell-autonomous and non-cell-autonomous mechanisms of disease. Neurother. J. Am. Soc. Exp. Neurother. 2012, 9, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Knowlton, B.; Hayden, M.; Almqvist, E.W.; Brinkman, R.; Ross, C.; Margolis, R.; Rosenblatt, A.; Durr, A.; Dode, C.; et al. Interaction of normal and expanded CAG repeat sizes influences age at onset of Huntington disease. Am. J. Med. Genet. Part A 2003, 119a, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Wexler, N.S.; Lorimer, J.; Porter, J.; Gomez, F.; Moskowitz, C.; Shackell, E.; Marder, K.; Penchaszadeh, G.; Roberts, S.A.; Gayán, J.; et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington’s disease age of onset. Proc. Natl. Acad. Sci. USA 2004, 101, 3498–3503. [Google Scholar] [CrossRef]
- Schultz, J.L.; Kamholz, J.A.; Moser, D.J.; Feely, S.M.; Paulsen, J.S.; Nopoulos, P.C. Substance abuse may hasten motor onset of Huntington disease: Evaluating the Enroll-HD database. Neurology 2017, 88, 909–915. [Google Scholar] [CrossRef]
- Li, G.; Larson, E.B.; Sonnen, J.A.; Shofer, J.B.; Petrie, E.C.; Schantz, A.; Peskind, E.R.; Raskind, M.A.; Breitner, J.C.; Montine, T.J. Statin therapy is associated with reduced neuropathologic changes of Alzheimer disease. Neurology 2007, 69, 878–885. [Google Scholar] [CrossRef]
- Bai, S.; Song, Y.; Huang, X.; Peng, L.; Jia, J.; Liu, Y.; Lu, H. Statin Use and the Risk of Parkinson’s Disease: An Updated Meta-Analysis. PLoS ONE 2016, 11, e0152564. [Google Scholar] [CrossRef]
- Patassini, S.; Giampà, C.; Martorana, A.; Bernardi, G.; Fusco, F.R. Effects of simvastatin on neuroprotection and modulation of Bcl-2 and BAX in the rat quinolinic acid model of Huntington’s disease. Neurosci. Lett. 2008, 448, 166–169. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, N.; Mishra, J.; Kalonia, H. Synergistical neuroprotection of rofecoxib and statins against malonic acid induced Huntington’s disease like symptoms and related cognitive dysfunction in rats. Eur. J. Pharmacol. 2013, 709, 1–12. [Google Scholar] [CrossRef]
- Schultz, J.L.; Nopoulos, P.C.; Killoran, A.; Kamholz, J.A. Statin use and delayed onset of Huntington’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 281–285. [Google Scholar] [CrossRef]
- Aziz, N.A. Statin use and delayed onset of Huntington’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 762–763. [Google Scholar] [CrossRef]
- Walker, V.M.; Davey Smith, G.; Davies, N.M.; Martin, R.M. Mendelian randomization: A novel approach for the prediction of adverse drug events and drug repurposing opportunities. Int. J. Epidemiol. 2017, 46, 2078–2089. [Google Scholar] [CrossRef]
- Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; Buchkovich, M.L.; Mora, S.; et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet. 2013, 45, 1274–1283. [Google Scholar] [CrossRef]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]
- Chen, L.; Yang, H.; Li, H.; He, C.; Yang, L.; Lv, G. Insights into modifiable risk factors of cholelithiasis: A Mendelian randomization study. Hepatology 2022, 75, 785–796. [Google Scholar] [CrossRef]
- Shim, H.; Chasman, D.I.; Smith, J.D.; Mora, S.; Ridker, P.M.; Nickerson, D.A.; Krauss, R.M.; Stephens, M. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PLoS ONE 2015, 10, e0120758. [Google Scholar] [CrossRef]
- CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell 2019, 178, 887–900.e814. [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ (Clin. Res. Ed.) 2018, 362, k601. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.F.; Minelli, C.; Sheehan, N.A.; Thompson, J.R. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 2015, 34, 2926–2940. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Dudbridge, F.; Thompson, S.G. Combining information on multiple instrumental variables in Mendelian randomization: Comparison of allele score and summarized data methods. Stat. Med. 2016, 35, 1880–1906. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cornelis, M.C.; Zhang, Z.; Liu, D.; Lian, X. Mendelian randomization study of coffee consumption and age at onset of Huntington’s disease. Clin. Nutr. 2021, 40, 5615–5618. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, D.; Yang, S.; Li, Y.; Lian, X. Smoking, alcohol consumption, and age at onset of Huntington’s disease: A Mendelian randomization study. Park. Relat. Disord. 2022, 97, 34–38. [Google Scholar] [CrossRef]
- Aziz, N.A.; Weydt, P. Telomere length as a modifier of age-at-onset in Huntington disease: A two-sample Mendelian randomization study. J. Neurol. 2018, 265, 2149–2151. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Thematic review series: Brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J. Lipid Res. 2004, 45, 1375–1397. [Google Scholar] [CrossRef]
- Wang, R.; Ross, C.A.; Cai, H.; Cong, W.N.; Daimon, C.M.; Carlson, O.D.; Egan, J.M.; Siddiqui, S.; Maudsley, S.; Martin, B. Metabolic and hormonal signatures in pre-manifest and manifest Huntington’s disease patients. Front. Physiol. 2014, 5, 231. [Google Scholar] [CrossRef]
- Leoni, V.; Mariotti, C.; Nanetti, L.; Salvatore, E.; Squitieri, F.; Bentivoglio, A.R.; Bandettini di Poggio, M.; Piacentini, S.; Monza, D.; Valenza, M.; et al. Whole body cholesterol metabolism is impaired in Huntington’s disease. Neurosci. Lett. 2011, 494, 245–249. [Google Scholar] [CrossRef]
- Aziz, N.A.; van der Burg, J.M.; Landwehrmeyer, G.B.; Brundin, P.; Stijnen, T.; Roos, R.A. Weight loss in Huntington disease increases with higher CAG repeat number. Neurology 2008, 71, 1506–1513. [Google Scholar] [CrossRef]
- Rosenblatt, A.; Abbott, M.H.; Gourley, L.M.; Troncoso, J.C.; Margolis, R.L.; Brandt, J.; Ross, C.A. Predictors of neuropathological severity in 100 patients with Huntington’s disease. Ann. Neurol. 2003, 54, 488–493. [Google Scholar] [CrossRef]
- Petersén, A.; Björkqvist, M. Hypothalamic-endocrine aspects in Huntington’s disease. Eur. J. Neurosci. 2006, 24, 961–967. [Google Scholar] [CrossRef]
- Ando, H.; Takamura, T.; Ota, T.; Nagai, Y.; Kobayashi, K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J. Pharmacol. Exp. Ther. 2000, 294, 1043–1046. [Google Scholar]
- Chaudhry, M.Z.; Wang, J.H.; Blankson, S.; Redmond, H.P. Statin (cerivastatin) protects mice against sepsis-related death via reduced proinflammatory cytokines and enhanced bacterial clearance. Surg. Infect. 2008, 9, 183–194. [Google Scholar] [CrossRef]
- Del Toro, D.; Xifró, X.; Pol, A.; Humbert, S.; Saudou, F.; Canals, J.M.; Alberch, J. Altered cholesterol homeostasis contributes to enhanced excitotoxicity in Huntington’s disease. J. Neurochem. 2010, 115, 153–167. [Google Scholar] [CrossRef]

| Exposure | N SNPs | Heterogeneity Analysis | Pleiotropy Analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Method | Q | Degree of Freedom | p-Value | Method | Egger Intercept | SE | p-Value | ||
| LDL-C | 76 | MR Egger | 50.0 | 74 | 0.985 | MR Egger intercept | −0.021 | 0.024 | 0.385 |
| IVW | 50.8 | 75 | 0.986 | MR-PRESSO Global test | 0.980 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Li, M.; Wang, H.; Yang, F.; Wang, J.; Huang, X. Association between Genetically Proxied Inhibition of HMG-CoA Reductase and Age at Onset of Huntington’s Disease. Brain Sci. 2022, 12, 1551. https://doi.org/10.3390/brainsci12111551
Zhu Y, Li M, Wang H, Yang F, Wang J, Huang X. Association between Genetically Proxied Inhibition of HMG-CoA Reductase and Age at Onset of Huntington’s Disease. Brain Sciences. 2022; 12(11):1551. https://doi.org/10.3390/brainsci12111551
Chicago/Turabian StyleZhu, Yahui, Mao Li, Hongfen Wang, Fei Yang, Jiao Wang, and Xusheng Huang. 2022. "Association between Genetically Proxied Inhibition of HMG-CoA Reductase and Age at Onset of Huntington’s Disease" Brain Sciences 12, no. 11: 1551. https://doi.org/10.3390/brainsci12111551
APA StyleZhu, Y., Li, M., Wang, H., Yang, F., Wang, J., & Huang, X. (2022). Association between Genetically Proxied Inhibition of HMG-CoA Reductase and Age at Onset of Huntington’s Disease. Brain Sciences, 12(11), 1551. https://doi.org/10.3390/brainsci12111551






