Corticomotor Plasticity Underlying Priming Effects of Motor Imagery on Force Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
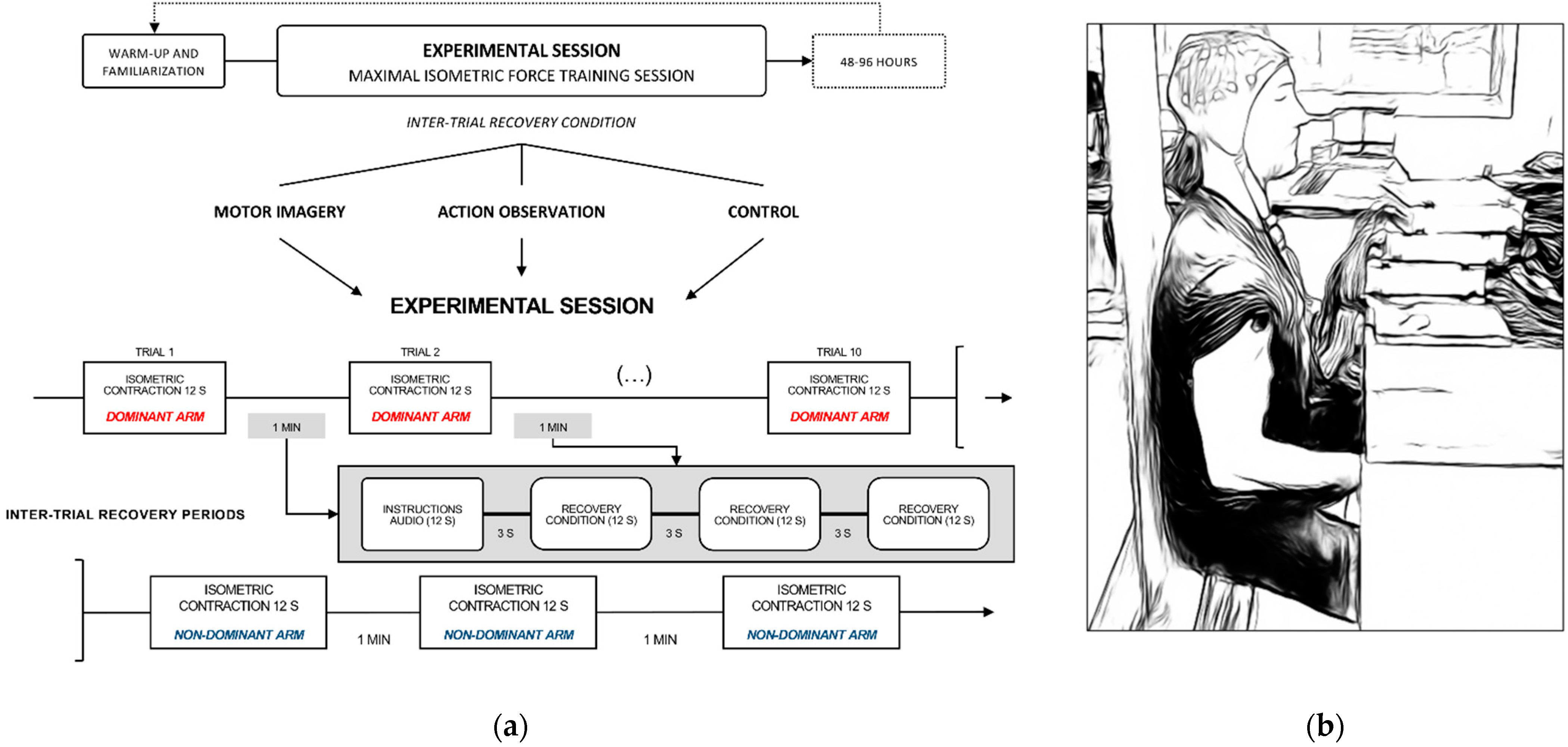
2.2.1. Experimental Design
2.2.2. Experimental Settings
2.3. Dependent Variables
2.3.1. Force Performance
2.3.2. Electromyography
2.3.3. Electroencephalography
- 1.
- Data acquisition
- 2.
- Pre-processing
- 3.
- Coherence analysis
2.3.4. Self-Report Ratings
2.4. Statistical Analyzes
2.4.1. Linear Mixed-Effects Analysis
2.4.2. Power Considerations
3. Results
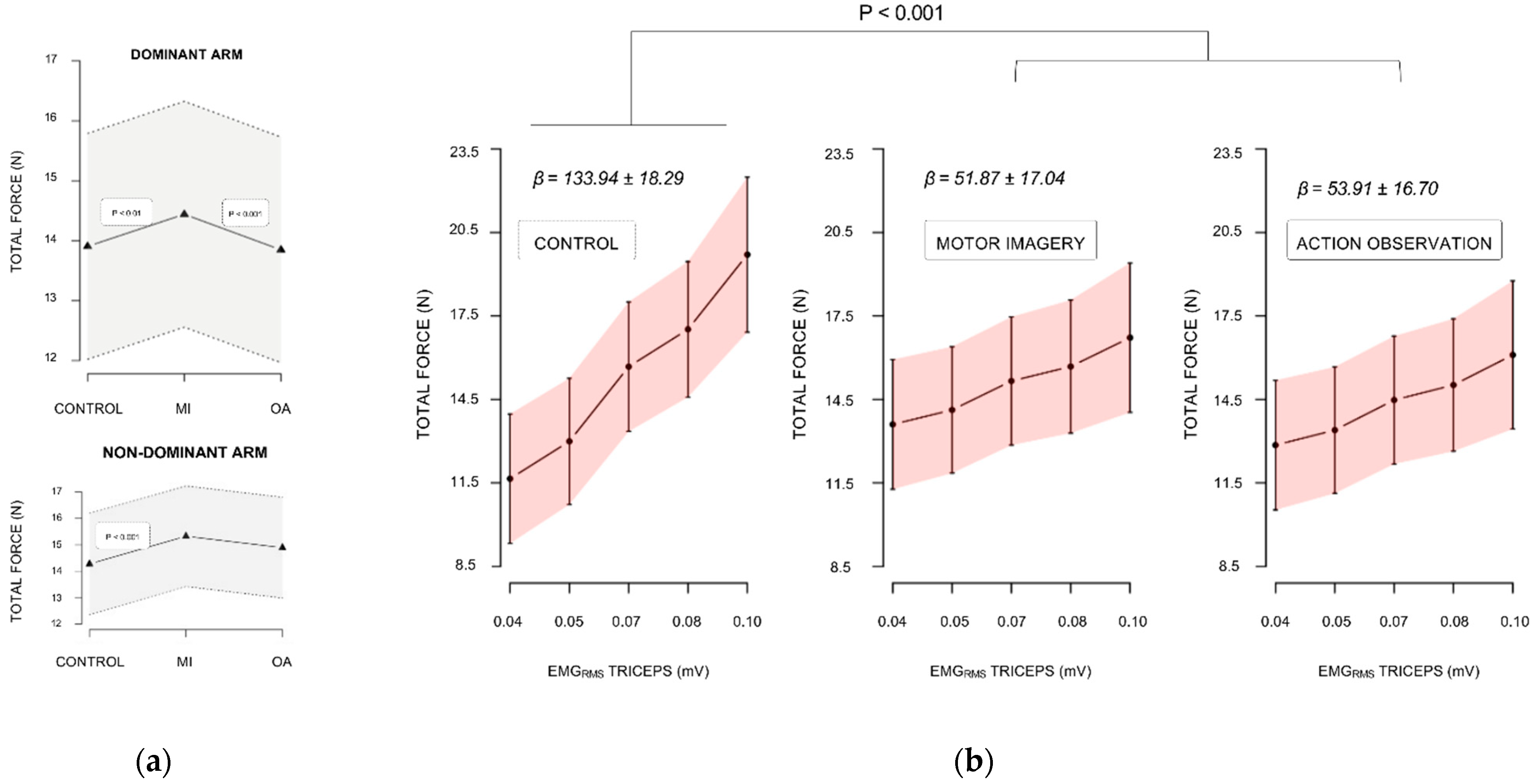
3.1. Force Performance Analysis
3.1.1. Baseline Levels
3.1.2. Maximal Isometric Force during Experimental Sessions
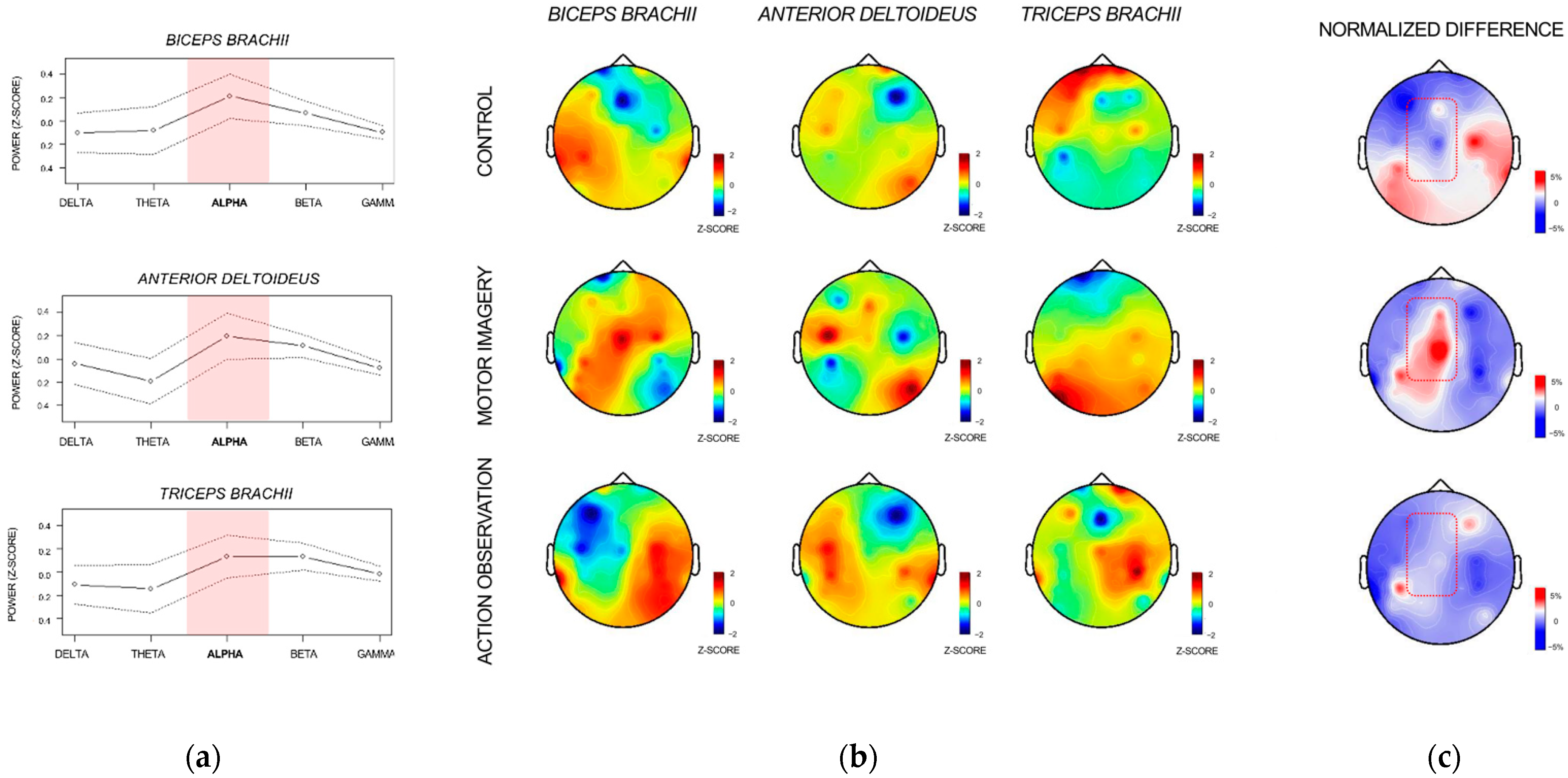
3.2. Corticomotor Coherence Analysis
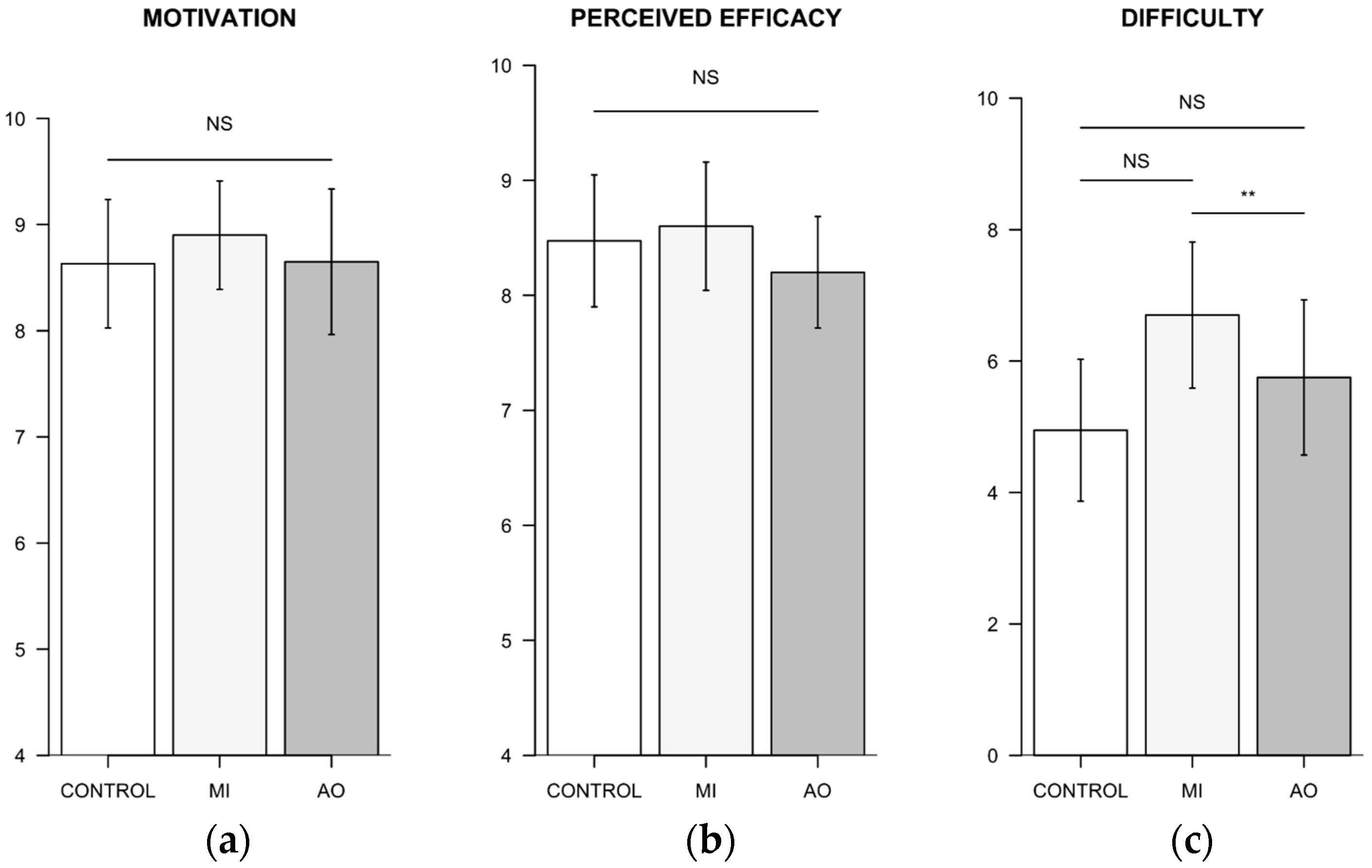
3.3. Analysis of Participant’s Subjective Ratings
3.3.1. Standardized Questionnaires
3.3.2. Standardized Questionnaires
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeannerod, M. The Representing Brain: Neural Correlates of Motor Intention and Imagery. Behav. Brain Sci. 1994, 17, 187–202. [Google Scholar] [CrossRef]
- Jeannerod, M. Neural Simulation of Action: A Unifying Mechanism for Motor Cognition. NeuroImage 2001, 14, S103–S109. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Caspers, S.; Eickhoff, S.B.; Swinnen, S.P. Neural Correlates of Action: Comparing Meta-Analyses of Imagery, Observation, and Execution. Neurosci. Biobehav. Rev. 2018, 94, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wieland, B.; Behringer, M.; Zentgraf, K. Motor Imagery and the Muscle System. Int. J. Psychophysiol. 2022, 174, 57–65. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, T.E.; Madan, C.R.; Moran, A.P.; Collet, C.; Guillot, A. Motor Imagery, Performance and Motor Rehabilitation. Prog. Brain Res. 2018, 240, 141–159. [Google Scholar]
- Vogt, S.; Rienzo, F.D.; Collet, C.; Collins, A.; Guillot, A. Multiple Roles of Motor Imagery during Action Observation. Front. Hum. Neurosci. 2013, 7, 807. [Google Scholar] [CrossRef]
- Holmes, P.; Calmels, C. A Neuroscientific Review of Imagery and Observation Use in Sport. J. Mot. Behav. 2008, 40, 433–445. [Google Scholar] [CrossRef]
- Munzert, J.; Zentgraf, K.; Stark, R.; Vaitl, D. Neural Activation in Cognitive Motor Processes: Comparing Motor Imagery and Observation of Gymnastic Movements. Exp. Brain Res. 2008, 188, 437–444. [Google Scholar] [CrossRef]
- Buccino, G.; Riggio, L. The Role of the Mirror Neuron System in Motor Learning. Kinesiology 2006, 38, 5–15. [Google Scholar]
- Zentgraf, K.; Munzert, J.; Bischoff, M.; Newman-Norlund, R.D. Simulation during Observation of Human Actions—Theories, Empirical Studies, Applications. Vis. Res. 2011, 51, 827–835. [Google Scholar] [CrossRef]
- Malouin, F.; Jackson, P.L.; Richards, C.L. Towards the Integration of Mental Practice in Rehabilitation Programs. A Critical Review. Front. Hum. Neurosci. 2013, 7, 576. [Google Scholar] [CrossRef]
- Feltz, D.L.; Landers, D.M. The Effects of Mental Practice on Motor Skill Learning and Performance: A Meta-Analysis. J. Sport Psychol. 1983, 5, 25–57. [Google Scholar] [CrossRef]
- Eaves, D.L.; Riach, M.; Holmes, P.S.; Wright, D.J. Motor Imagery during Action Observation: A Brief Review of Evidence, Theory and Future Research Opportunities. Front. Neurosci. 2016, 10, 514. [Google Scholar] [CrossRef]
- Jackson, P.L.; Lafleur, M.F.; Malouin, F.; Richards, C.L.; Doyon, J. Functional Cerebral Reorganization following Motor Sequence Learning through Mental Practice with Motor Imagery. NeuroImage 2003, 20, 1171–1180. [Google Scholar] [CrossRef]
- Lafleur, M.F.; Jackson, P.L.; Malouin, F.; Richards, C.L.; Evans, A.C.; Doyon, J. Motor Learning Produces Parallel Dynamic Functional Changes during the Execution and Imagination of Sequential Foot Movements. NeuroImage 2002, 16, 142–157. [Google Scholar] [CrossRef]
- Tkach, D.; Reimer, J.; Hatsopoulos, N.G. Congruent Activity during Action and Action Observation in Motor Cortex. J. Neurosci. 2007, 27, 13241–13250. [Google Scholar] [CrossRef]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.-P.; Eugène, F.; Michon, P.-E.; Jackson, P.L. The Neural Network of Motor Imagery: An ALE Meta-Analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef]
- Lotze, M.; Halsband, U. Motor Imagery. J. Physiol.-Paris 2006, 99, 386–395. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Nguyet, D.; Cohen, L.G.; Brasil-Neto, J.P.; Cammarota, A.; Hallett, M. Modulation of Muscle Responses Evoked by Transcranial Magnetic Stimulation during the Acquisition of New Fine Motor Skills. J. Neurophysiol. 1995, 74, 1037–1045. [Google Scholar] [CrossRef]
- Zhang, H.; Long, Z.; Ge, R.; Xu, L.; Jin, Z.; Yao, L.; Liu, Y. Motor Imagery Learning Modulates Functional Connectivity of Multiple Brain Systems in Resting State. PLoS ONE 2014, 9, e85489. [Google Scholar] [CrossRef]
- Ge, R.; Zhang, H.; Yao, L.; Long, Z. Motor Imagery Learning Induced Changes in Functional Connectivity of the Default Mode Network. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Hodges, N.J.; Williams, A.M. Current Status of Observational Learning Research and the Role of Demonstrations in Sport; Taylor & Francis: New York, NY, USA, 2007; ISBN 0264-0414. [Google Scholar]
- Wesch, N.N.; Law, B.; Hall, C.R. The Use of Observational Learning by Athletes. J. Sport Behav. 2007, 30, 219. [Google Scholar]
- Gatti, R.; Rocca, M.A.; Preziosa, P.; Fumagalli, S.; Pagani, E.; Messina, R.; Filippi, M. Structural Brain Plasticity Modified by Action Observation Training in Healthy Subjects. Physiotherapy 2015, 101, e446–e447. [Google Scholar] [CrossRef][Green Version]
- Rocca, M.A.; Fumagalli, S.; Pagani, E.; Gatti, R.; Riccitelli, G.C.; Preziosa, P.; Comi, G.; Falini, A.; Filippi, M. Action Observation Training Modifies Brain Gray Matter Structure in Healthy Adult Individuals. Brain Imaging Behav. 2017, 11, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Porro, C.A.; Facchin, P.; Fusi, S.; Dri, G.; Fadiga, L. Enhancement of Force after Action Observation. Neuropsychologia 2007, 45, 3114–3121. [Google Scholar] [CrossRef]
- Byrne, R.W.; Russon, A.E. Learning by Imitation: A Hierarchical Approach. Behav. Brain Sci. 1998, 21, 667–684. [Google Scholar] [CrossRef]
- Paravlic, A.H.; Slimani, M.; Tod, D.; Marusic, U.; Milanovic, Z.; Pisot, R. Effects and Dose-Response Relationships of Motor Imagery Practice on Strength Development in Healthy Adult Populations: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 1165–1187. [Google Scholar] [CrossRef]
- Tod, D.; Edwards, C.; McGuigan, M.; Lovell, G. A Systematic Review of the Effect of Cognitive Strategies on Strength Performance. Sports Med. 2015, 45, 1589–1602. [Google Scholar] [CrossRef]
- Ranganathan, V.K.; Siemionow, V.; Liu, J.Z.; Sahgal, V.; Yue, G.H. From Mental Power to Muscle Power—Gaining Strength by Using the Mind. Neuropsychologia 2004, 42, 944–956. [Google Scholar] [CrossRef]
- Yao, W.X.; Ranganathan, V.K.; Allexandre, D.; Siemionow, V.; Yue, G.H. Kinesthetic Imagery Training of Forceful Muscle Contractions Increases Brain Signal and Muscle Strength. Front. Hum. Neurosci. 2013, 7, 561. [Google Scholar] [CrossRef]
- Di Rienzo, F.; Blache, Y.; Kanthack, T.F.D.; Monteil, K.; Collet, C.; Guillot, A. Short-Term Effects of Integrated Motor Imagery Practice on Muscle Activation and Force Performance. Neuroscience 2015, 305, 146–156. [Google Scholar] [CrossRef]
- Stoykov, M.E.; Corcos, D.M.; Madhavan, S. Movement-Based Priming: Clinical Applications and Neural Mechanisms. J. Mot. Behav. 2017, 49, 88–97. [Google Scholar] [CrossRef]
- Di Rienzo, F.; Joassy, P.; Kanthack, T.; MacIntyre, T.E.; Debarnot, U.; Blache, Y.; Hautier, C.; Collet, C.; Guillot, A. Effects of Action Observation and Action Observation Combined with Motor Imagery on Maximal Isometric Strength. Neuroscience 2019, 418, 82–95. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Lebon, F.; Papaxanthis, C.; Martin, A. New Evidence of Corticospinal Network Modulation Induced by Motor Imagery. J. Neurophysiol. 2016, 115, 1279–1288. [Google Scholar] [CrossRef]
- Yue, G.; Cole, K.J. Strength Increases from the Motor Program: Comparison of Training with Maximal Voluntary and Imagined Muscle Contractions. J. Neurophysiol. 1992, 67, 1114–1123. [Google Scholar] [CrossRef]
- Veale, J.F. Edinburgh Handedness Inventory—Short Form: A Revised Version Based on Confirmatory Factor Analysis. Laterality 2014, 19, 164–177. [Google Scholar] [CrossRef]
- Williams, S.E.; Cumming, J.; Ntoumanis, N.; Nordin-Bates, S.M.; Ramsey, R.; Hall, C. Further Validation and Development of the Movement Imagery Questionnaire. J. Sport Exerc. Psychol. 2012, 34, 621–646. [Google Scholar] [CrossRef]
- Frazer, A.K.; Pearce, A.J.; Howatson, G.; Thomas, K.; Goodall, S.; Kidgell, D.J. Determining the Potential Sites of Neural Adaptation to Cross-Education: Implications for the Cross-Education of Muscle Strength. Eur. J. Appl. Physiol. 2018, 118, 1751–1772. [Google Scholar] [CrossRef]
- Shima, N.; Ishida, K.; Katayama, K.; Morotome, Y.; Sato, Y.; Miyamura, M. Cross Education of Muscular Strength during Unilateral Resistance Training and Detraining. Eur. J. Appl. Physiol. 2002, 86, 287–294. [Google Scholar] [CrossRef]
- Manca, A.; Hortobágyi, T.; Carroll, T.J.; Enoka, R.M.; Farthing, J.P.; Gandevia, S.C.; Kidgell, D.J.; Taylor, J.L.; Deriu, F. Contralateral Effects of Unilateral Strength and Skill Training: Modified Delphi Consensus to Establish Key Aspects of Cross-Education. Sport. Med. 2021, 51, 11–20. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Uusitalo, M.A.; Ilmoniemi, R.J. Signal-Space Projection Method for Separating MEG or EEG into Components. Med. Biol. Eng. Comput. 1997, 35, 135–140. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, N.E. Ensemble empirical mode decomposition: A noise-assisted data analysis method. Adv. Adapt. Data Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Ushiyama, J.; Takahashi, Y.; Ushiba, J. Muscle Dependency of Corticomuscular Coherence in Upper and Lower Limb Muscles and Training-Related Alterations in Ballet Dancers and Weightlifters. J. Appl. Physiol. 2010, 109, 1086–1095. [Google Scholar] [CrossRef]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Effect Size. Restor. Dent. Endod. 2015, 40, 328–331. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Linear and Nonlinear Mixed Effects Models. R Package Version 2014, 3, 1–89. [Google Scholar]
- Ben-Shachar, M.; Lüdecke, D.; Makowski, D. Effectsize: Estimation of Effect Size Indices and Standardized Parameters. JOSS 2020, 5, 2815. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013; ISBN 1-134-74270-3. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2016; ISBN 978-0-429-13954-3. [Google Scholar]
- Champely, S.; Ekstrom, C.; Dalgaard, P.; Gill, J.; Weibelzahl, S.; Anandkumar, A.; Ford, C.; Volcic, R.; De Rosario, H.; De Rosario, M.H. Package ‘Pwr’. 2018. Available online: http://archive.linux.duke.edu/cran/web/packages/pwr/pwr.pdf (accessed on 7 October 2022).
- Bellemare, Charles and Bissonnette, Luc and Kroger, Sabine, Statistical Power of Within and Between-Subjects Designs in Economic Experiments (November 24, 2014). CESifo Working Paper Series No. 5055. Available online: https://ssrn.com/abstract=2529895 or http://dx.doi.org/10.2139/ssrn.2529895 (accessed on 7 October 2022).
- Thompson, V.A.; Campbell, J.I.D. A Power Struggle: Between- vs. within-Subjects Designs in Deductive Reasoning Research. Psychol. Int. J. Psychol. Orient 2004, 47, 277–296. [Google Scholar] [CrossRef][Green Version]
- Grosprêtre, S.; Ruffino, C.; Lebon, F. Motor Imagery and Cortico-Spinal Excitability: A Review. Eur. J. Sport Sci. 2016, 16, 317–324. [Google Scholar] [CrossRef]
- Zijdewind, I.; Toering, S.T.; Bessem, B.; van der Laan, O.; Diercks, R.L. Effects of Imagery Motor Training on Torque Production of Ankle Plantar Flexor Muscles. Muscle Nerve 2003, 28, 168–173. [Google Scholar] [CrossRef]
- Cornwall, M.W.; Bruscato, M.P.; Barry, S. Effect of Mental Practice on Isometric Muscular Strength. J. Orthop. Sport. Phys. Ther. 1991, 13, 231–234. [Google Scholar] [CrossRef]
- Sidaway, B.; Trzaska, A. (Robinson) Can Mental Practice Increase Ankle Dorsiflexor Torque? Phys. Ther. 2005, 85, 1053–1060. [Google Scholar] [CrossRef]
- Salama, I.M.; Turner, S.; Edwards, M.G. Rapid Communication: Automatic Priming of Grip Force Following Action Observation. Q. J. Exp. Psychol. 2011, 64, 833–838. [Google Scholar] [CrossRef]
- Macuga, K.L.; Frey, S.H. Neural Representations Involved in Observed, Imagined, and Imitated Actions Are Dissociable and Hierarchically Organized. NeuroImage 2012, 59, 2798–2807. [Google Scholar] [CrossRef]
- Wright, D.J.; Williams, J.; Holmes, P.S. Combined Action Observation and Imagery Facilitates Corticospinal Excitability. Front. Hum. Neurosci. 2014, 8, 951. [Google Scholar] [CrossRef]
- Meers, R.; Nuttall, H.E.; Vogt, S. Motor Imagery Alone Drives Corticospinal Excitability during Concurrent Action Observation and Motor Imagery. Cortex 2020, 126, 322–333. [Google Scholar] [CrossRef]
- Hodges, N.J.; Williams, A.M.; Hayes, S.J.; Breslin, G. What Is Modelled during Observational Learning? J. Sport. Sci. 2007, 25, 531–545. [Google Scholar] [CrossRef]
- Bouguetoch, A.; Martin, A.; Grosprêtre, S. Does Partial Activation of the Neuromuscular System Induce Cross-Education Training Effect? Case of a Pilot Study on Motor Imagery and Neuromuscular Electrical Stimulation. Eur. J. Appl. Physiol. 2021, 121, 2337–2348. [Google Scholar] [CrossRef]
- Leung, M.; Rantalainen, T.; Teo, W.-P.; Kidgell, D. Motor Cortex Excitability Is Not Differentially Modulated Following Skill and Strength Training. Neuroscience 2015, 305, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.-Y.; Morris, L.; Gou, W.; Alexander, E.; Gay, E. Motor Cortical Circuits Contribute to Crossed Facilitation of Trunk Muscles Induced by Rhythmic Arm Movement. Sci. Rep. 2020, 10, 17067. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, E. Electrophysiology of Mental Activities. Am. J. Psychol. 1932, 44, 677. [Google Scholar] [CrossRef]
- Stoykov, M.E.; Madhavan, S. Motor Priming in Neurorehabilitation. J. Neurol. Phys. Ther. 2015, 39, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Grosprêtre, S.; Jacquet, T.; Lebon, F.; Papaxanthis, C.; Martin, A. Neural Mechanisms of Strength Increase after One-Week Motor Imagery Training. Eur. J. Sport. Sci. 2018, 18, 209–218. [Google Scholar] [CrossRef]
- Grosprêtre, S.; Lebon, F.; Papaxanthis, C.; Martin, A. Spinal Plasticity with Motor Imagery Practice. J. Physiol. 2019, 597, 921–934. [Google Scholar] [CrossRef]
- Joundi, R.A.; Jenkinson, N.; Brittain, J.-S.; Aziz, T.Z.; Brown, P. Driving Oscillatory Activity in the Human Cortex Enhances Motor Performance. Curr. Biol. 2012, 22, 403–407. [Google Scholar] [CrossRef]
- Yang, Y.; Solis-Escalante, T.; Yao, J.; van der Helm, F.C.T.; Dewald, J.P.A.; Schouten, A.C. Nonlinear Connectivity in the Human Stretch Reflex Assessed by Cross-Frequency Phase Coupling. Int. J. Neural Syst. 2016, 26, 1650043. [Google Scholar] [CrossRef]
- Cremoux, S.; Tallet, J.; Dal Maso, F.; Berton, E.; Amarantini, D. Impaired Corticomuscular Coherence during Isometric Elbow Flexion Contractions in Humans with Cervical Spinal Cord Injury. Eur. J. Neurosci. 2017, 46, 1991–2000. [Google Scholar] [CrossRef]
- Desmyttere, G.; Mathieu, E.; Begon, M.; Simoneau-Buessinger, E.; Cremoux, S. Effect of the Phase of Force Production on Corticomuscular Coherence with Agonist and Antagonist Muscles. Eur. J. Neurosci. 2018, 48, 3288–3298. [Google Scholar] [CrossRef]
- Cremoux, S.; Elie, D.; Rovsing, C.; Rovsing, H.; Jochumsen, M.; Haavik, H.; Niazi, I.K. Functional and Corticomuscular Changes Associated with Early Phase of Motor Training. In Converging Clinical and Engineering Research on Neurorehabilitation III; Masia, L., Micera, S., Akay, M., Pons, J.L., Eds.; Biosystems & Biorobotics; Springer International Publishing: Cham, Switzerland, 2019; Volume 21, pp. 759–763. ISBN 978-3-030-01844-3. [Google Scholar]
- Liu, J.; Sheng, Y.; Liu, H. Corticomuscular Coherence and Its Applications: A Review. Front. Hum. Neurosci. 2019, 13, 100. [Google Scholar] [CrossRef]
- Hansen, S.; Hansen, N.L.; Christensen, L.O.D.; Petersen, N.T.; Nielsen, J.B. Coupling of Antagonistic Ankle Muscles during Co-Contraction in Humans. Exp. Brain Res. 2002, 146, 282–292. [Google Scholar] [CrossRef]
- Vecchio, F.; Del Percio, C.; Marzano, N.; Fiore, A.; Toran, G.; Aschieri, P.; Gallamini, M.; Cabras, J.; Rossini, P.M.; Babiloni, C.; et al. Functional Cortico-Muscular Coupling during Upright Standing in Athletes and Nonathletes: A Coherence Electroencephalographic-Electromyographic Study. Behav. Neurosci. 2008, 122, 917–927. [Google Scholar] [CrossRef]
- Wright, D.J.; Wood, G.; Eaves, D.L.; Bruton, A.M.; Frank, C.; Franklin, Z.C. Corticospinal Excitability Is Facilitated by Combined Action Observation and Motor Imagery of a Basketball Free Throw. Psychol. Sport Exerc. 2018, 39, 114–121. [Google Scholar] [CrossRef]
- Sale, D.G. Neural Adaptation to Resistance Training. Med. Sci. Sport. Exerc. 1988, 20, S135–S145. [Google Scholar] [CrossRef]
- Carolan, B.; Cafarelli, E. Adaptations in Coactivation after Isometric Resistance Training. J. Appl. Physiol. 1992, 73, 911–917. [Google Scholar] [CrossRef]
- Häkkinen, K.; Alen, M.; Kallinen, M.; Newton, R.U.; Kraemer, W.J. Neuromuscular Adaptation during Prolonged Strength Training, Detraining and Re-Strength-Training in Middle-Aged and Elderly People. Eur. J. Appl. Physiol. 2000, 83, 51–62. [Google Scholar] [CrossRef]
- Holtermann, A.; Roeleveld, K.; Vereijken, B.; Ettema, G. Changes in Agonist EMG Activation Level during MVC Cannot Explain Early Strength Improvement. Eur. J. Appl. Physiol. 2005, 94, 593–601. [Google Scholar] [CrossRef]
- Bru, B.; Amarantini, D. Influence of Sporting Expertise on the EMG—Torque Relationship during Isometric Contraction in Man. Comput. Methods Biomech. Biomed. Eng. 2008, 11, 43–44. [Google Scholar] [CrossRef]
- Baker, S.N. Oscillatory Interactions between Sensorimotor Cortex and the Periphery. Curr. Opin. Neurobiol. 2007, 17, 649–655. [Google Scholar] [CrossRef]
- Perez, M.A.; Lundbye-Jensen, J.; Nielsen, J.B. Changes in Corticospinal Drive to Spinal Motoneurones Following Visuo-Motor Skill Learning in Humans: Corticomuscular Coherence and Visuo-Motor Skill Learning. J. Physiol. 2006, 573, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, F.; Longcamp, M.; Cremoux, S.; Amarantini, D. Effect of Training Status on Beta-Range Corticomuscular Coherence in Agonist vs. Antagonist Muscles during Isometric Knee Contractions. Exp. Brain Res. 2017, 235, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Collet, C. Construction of the Motor Imagery Integrative Model in Sport: A Review and Theoretical Investigation of Motor Imagery Use. Int. Rev. Sport Exerc. Psychol. 2008, 1, 31–44. [Google Scholar] [CrossRef]
- Holmes, P.S.; Collins, D.J. The PETTLEP Approach to Motor Imagery: A Functional Equivalence Model for Sport Psychologists. J. Appl. Sport Psychol. 2001, 13, 60–83. [Google Scholar] [CrossRef]
- Perez, M.A.; Cohen, L.G. Mechanisms Underlying Functional Changes in the Primary Motor Cortex Ipsilateral to an Active Hand. J. Neurosci. 2008, 28, 5631–5640. [Google Scholar] [CrossRef]
| Dominant Arm | |||
| CONTROL | MI | OA | |
| Force (N) | 16.56 ± 3.97 | 16.51 ± 2.80 | 16.88 ± 3.72 |
| EMGRMS biceps brachii (mV) | 0.10 ± 0.03 | 0.08 ± 0.02 | 0.09 ± 0.03 |
| EMGRMS anterior deltoideus (mV) | 0.72 ± 0.25 | 0.73 ± 0.24 | 0.76 ± 0.28 |
| EMGRMS triceps brachii (mV) | 0.06 ± 0.01 | 0.11 ± 0.09 | 0.06 ± 0.01 |
| Non Dominant Arm | |||
| CONTROL | MI | OA | |
| Force (N) | 17.18 ± 5.39 | 15.03 ± 2.79 | 15.99 ± 3.44 |
| EMGRMS biceps brachii (mV) | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.06 ± 0.02 |
| EMGRMS anterior deltoideus (mV) | 0.55 ± 0.18 | 0.62 ± 0.19 | 0.59 ± 0.17 |
| EMGRMS triceps brachii (mV) | 0.05 ± 0.02 | 0.05 ± 0.02 | 0.10 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Anjos, T.; Guillot, A.; Kerautret, Y.; Daligault, S.; Di Rienzo, F. Corticomotor Plasticity Underlying Priming Effects of Motor Imagery on Force Performance. Brain Sci. 2022, 12, 1537. https://doi.org/10.3390/brainsci12111537
Dos Anjos T, Guillot A, Kerautret Y, Daligault S, Di Rienzo F. Corticomotor Plasticity Underlying Priming Effects of Motor Imagery on Force Performance. Brain Sciences. 2022; 12(11):1537. https://doi.org/10.3390/brainsci12111537
Chicago/Turabian StyleDos Anjos, Typhanie, Aymeric Guillot, Yann Kerautret, Sébastien Daligault, and Franck Di Rienzo. 2022. "Corticomotor Plasticity Underlying Priming Effects of Motor Imagery on Force Performance" Brain Sciences 12, no. 11: 1537. https://doi.org/10.3390/brainsci12111537
APA StyleDos Anjos, T., Guillot, A., Kerautret, Y., Daligault, S., & Di Rienzo, F. (2022). Corticomotor Plasticity Underlying Priming Effects of Motor Imagery on Force Performance. Brain Sciences, 12(11), 1537. https://doi.org/10.3390/brainsci12111537






