Greater Disability Is Associated with Worse Vestibular and Compensatory Oculomotor Functions in People Living with Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
2.3. Video Head Impulse Test
2.4. Identification of Compensatory Saccades
2.5. Variables of Interest
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Right–Left Lateral Canal Findings
3.2.1. Right Lateral Canal
3.2.2. Left Lateral Canal
3.3. Right Anterior–Left Posterior Canal Findings
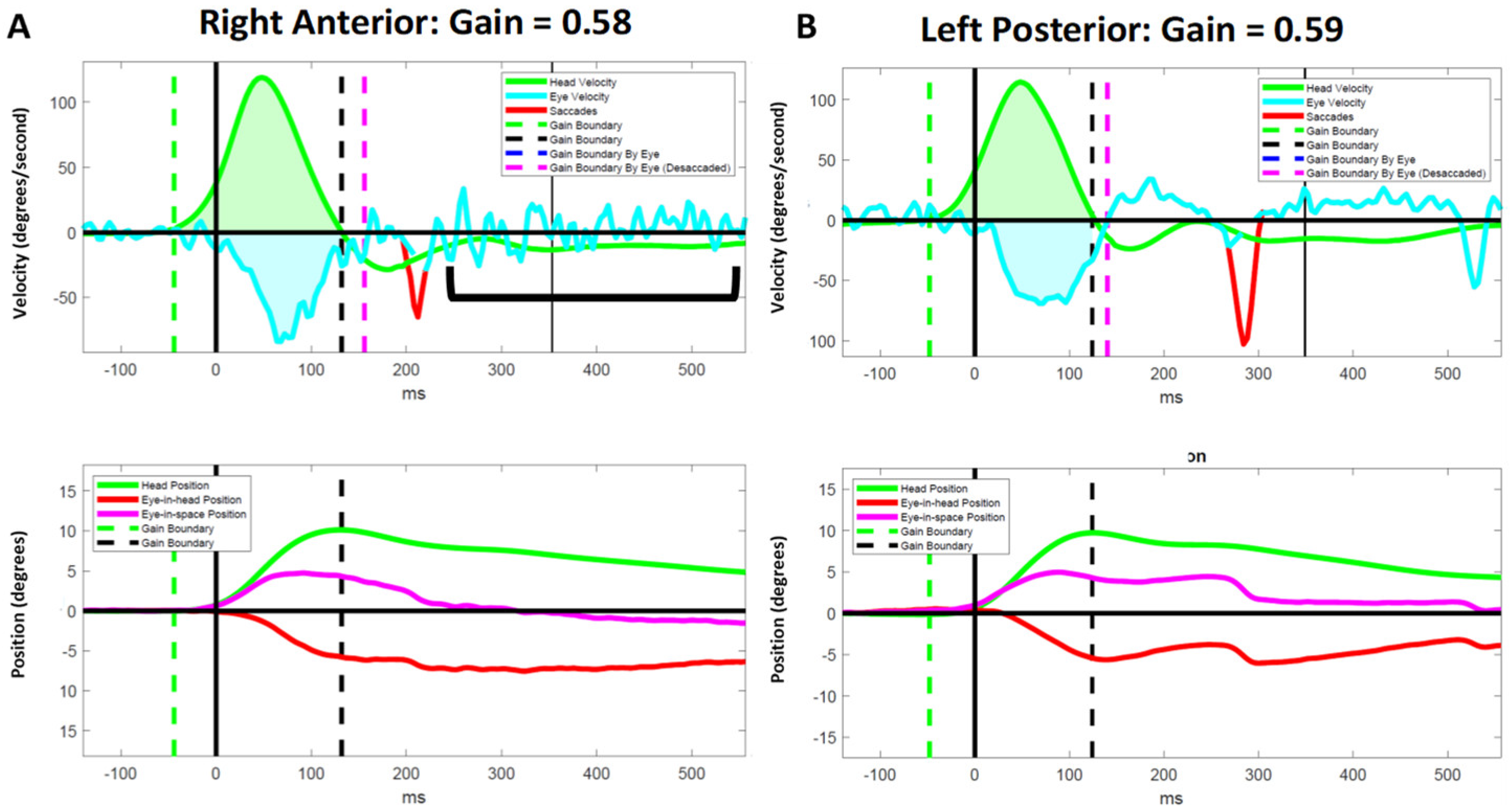
3.3.1. Right Anterior Canal
3.3.2. Left Posterior Canal
3.4. Left Anterior–Right Posterior Canal Findings
3.4.1. Left Anterior Canal
3.4.2. Right Posterior Canal
3.5. Atypical Compensatory Oculomotor Behavior
4. Discussion
4.1. Association between Measures of Gaze Stability and Disability
4.2. Effects of Internuclear Ophthalmoplegia on Video Head Impulse Test Results
4.3. Association between Compensatory Saccades and Disability
4.4. A Role for Personalized Vestibular Physical Therapy
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Amatya, B.; Khan, F.; Galea, M. Rehabilitation for people with multiple sclerosis: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2019, 1, CD012732. [Google Scholar] [CrossRef]
- Marrie, R.A.; Cutter, G.R.; Tyry, T. Substantial burden of dizziness in multiple sclerosis. Mult. Scler. Relat. Disord. 2013, 2, 21–28. [Google Scholar] [CrossRef]
- Mañago, M.M.; Schenkman, M.; Berliner, J.; Hebert, J.R. Gaze stabilization and dynamic visual acuity in people with multiple sclerosis. J. Vestib. Res. 2016, 26, 469–477. [Google Scholar] [CrossRef]
- Graves, J.; Balcer, L.J. Eye disorders in patients with multiple sclerosis: Natural history and management. Clin. Ophthalmol. 2010, 4, 1409–1422. [Google Scholar] [CrossRef]
- Ertugrul, G.; Konuskan, B.; Solmaz, I.; Anlar, B.; Aksoy, S. Vestibulo-ocular reflex involvement in childhood-onset multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 44, 102329. [Google Scholar] [CrossRef]
- Pavlović, I.; Ruška, B.; Pavičić, T.; Krbot Skorić, M.; Crnošija, L.; Adamec, I.; Habek, M. Video head impulse test can detect brainstem dysfunction in multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 14, 68–71. [Google Scholar] [CrossRef]
- Serra, A.; Derwenskus, J.; Downey, D.L.; Leigh, R.J. Role of eye movement examination and subjective visual vertical in clinical evaluation of multiple sclerosis. J. Neurol. 2003, 250, 569–575. [Google Scholar] [CrossRef]
- Schubert, M.C.; Hall, C.D.; Das, V.; Tusa, R.J.; Herdman, S.J. Oculomotor strategies and their effect on reducing gaze position error. Otol. Neurotol. 2010, 31, 228–231. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Curthoys, I.S. A clinical sign of canal paresis. Arch. Neurol. 1988, 45, 737–739. [Google Scholar] [CrossRef]
- MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S. The video head impulse test: Diagnostic accuracy in peripheral vestibulopathy. Neurology 2009, 73, 1134–1141. [Google Scholar] [CrossRef]
- Macdougall, H.G.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S.; Weber, K.P. The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS ONE 2013, 8, e61488. [Google Scholar] [CrossRef]
- Anson, E.R.; Bigelow, R.T.; Carey, J.P.; Xue, Q.L.; Studenski, S.; Schubert, M.C.; Agrawal, Y. VOR Gain Is Related to Compensatory Saccades in Healthy Older Adults. Front. Aging Neurosci. 2016, 8, 150. [Google Scholar] [CrossRef]
- Weber, K.P.; Aw, S.T.; Todd, M.J.; McGarvie, L.A.; Curthoys, I.S.; Halmagyi, G.M. Head impulse test in unilateral vestibular loss: Vestibulo-ocular reflex and catch-up saccades. Neurology 2008, 70, 454–463. [Google Scholar] [CrossRef]
- Blödow, A.; Pannasch, S.; Walther, L.E. Detection of isolated covert saccades with the video head impulse test in peripheral vestibular disorders. Auris Nasus Larynx 2013, 40, 348–351. [Google Scholar] [CrossRef]
- Halmagyi, G.M.; Chen, L.; MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Curthoys, I.S. The Video Head Impulse Test. Front. Neurol. 2017, 8, 258. [Google Scholar] [CrossRef]
- Chen, L.; Halmagyi, G.M. Video Head Impulse Testing: From Bench to Bedside. Semin. Neurol. 2020, 40, 5–17. [Google Scholar] [CrossRef]
- Grove, C.R.; Wagner, A.; Loyd, B.J.; Dibble, L.E.; Schubert, M.C. Unique compensatory oculomotor behavior in people living with multiple sclerosis. J. Neurol. Sci. 2022, 442, 120411. [Google Scholar] [CrossRef]
- Wagner, A.R.; Grove, C.R.; Loyd, B.J.; Dibble, L.E.; Schubert, M.C. Compensatory Saccades Differ for Those with Vestibular Hypofunction and Multiple Sclerosis Pointing to Unique Roles for Peripheral and Central Vestibular Inputs. J. Neurophysiol. 2022, 128, 934–945. [Google Scholar] [CrossRef]
- McGarvie, L.A.; MacDougall, H.G.; Halmagyi, G.M.; Burgess, A.M.; Weber, K.P.; Curthoys, I.S. The Video Head Impulse Test (vHIT) of Semicircular Canal Function-Age-Dependent Normative Values of VOR Gain in Healthy Subjects. Front. Neurol. 2015, 6, 154. [Google Scholar] [CrossRef]
- Loyd, B.J.; Agnew, L.; Fangman, A.; Thackeray, A.; Peterson, D.S.; Schubert, M.C.; Dibble, L. Characterizing gaze and postural stability deficits in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 55, 103205. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Dibble, L.E.; Schubert, M.C.; Sibthorp, J.; Foreman, K.B.; Gappmaier, E. Gaze Stability, Dynamic Balance and Participation Deficits in People with Multiple Sclerosis at Fall-Risk. Anat. Rec. 2018, 301, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Aw, S.T.; Chen, L.; Todd, M.J.; Barnett, M.H.; Halmagyi, G.M. Vestibulo-ocular reflex deficits with medial longitudinal fasciculus lesions. J. Neurol. 2017, 264, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.H.; Kim, S.S.; Kang, K.W.; Tarnutzer, A.A. Preferential Impairment of the Contralesional Posterior Semicircular Canal in Internuclear Ophthalmoplegia. Front. Neurol. 2017, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, G.D.; Christy, J.B.; Motl, R.W. Comprehensive Clinical Assessment of Vestibular Function in Multiple Sclerosis. J. Neurol. Phys. Ther. 2021, 45, 228–234. [Google Scholar] [CrossRef]
- Loyd, B.J.; Fangman, A.; Peterson, D.S.; Gappmaier, E.; Thackeray, A.; Schubert, M.C.; Dibble, L.E. Rehabilitation to Improve Gaze and Postural Stability in People with Multiple Sclerosis: A Randomized Clinical Trial. Neurorehabil. Neural Repair 2022, 15459683221124126. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Powell, L.E.; Myers, A.M. The Activities-specific Balance Confidence (ABC) Scale. J. Gerontol. Biol. Sci. Med. Sci. 1995, 50A, 28. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Translating Research into Clinical Practice, 4th ed.; Lippincott, Williams & Wilkins: Baltimore, MD, USA, 2011. [Google Scholar]
- Jacobson, G.P.; Newman, C.W. The development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Loyd, B.J.; Fangman, A.; Peterson, D.S.; Gappmaier, E.; Schubert, M.C.; Thackery, A.; Dibble, L. Rehabilitation to improve gaze and postural stability in people with multiple sclerosis: Study protocol for a prospective randomized clinical trial. BMC Neurol. 2019, 19, 119. [Google Scholar] [CrossRef]
- Anson, E.R.; Bigelow, R.T.; Carey, J.P.; Xue, Q.L.; Studenski, S.; Schubert, M.C.; Weber, K.P.; Agrawal, Y. Aging Increases Compensatory Saccade Amplitude in the Video Head Impulse Test. Front. Neurol. 2016, 7, 113. [Google Scholar] [CrossRef]
- Mantokoudis, G.; Schubert, M.C.; Tehrani, A.S.; Wong, A.L.; Agrawal, Y. Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol. Neurotol. 2014, 35, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Colagiorgio, P.; Versino, M.; Colnaghi, S.; Quaglieri, S.; Manfrin, M.; Zamaro, E.; Mantokoudis, G.; Zee, D.S.; Ramat, S. New insights into vestibular-saccade interaction based on covert corrective saccades in patients with unilateral vestibular deficits. J. Neurophysiol. 2017, 117, 2324–2338. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.R.; Schubert, M.C. Evidence a shared mechanism mediates ipsi- and contralesional compensatory saccades and gait after unilateral vestibular deafferentation. J. Neurophysiol. 2020, 123, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Mantokoudis, G.; Tehrani, A.S.; Wozniak, A.; Eibenberger, K.; Kattah, J.C.; Guede, C.I.; Zee, D.S.; Newman-Toker, D.E. VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke. Otol. Neurotol. 2015, 36, 457–465. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Grossman, G.E.; Leigh, R.J.; Abel, L.A.; Lanska, D.J.; Thurston, S.E. Frequency and velocity of rotational head perturbations during locomotion. Exp. Brain Res. 1988, 70, 470–476. [Google Scholar] [CrossRef]
- Grossman, G.E.; Leigh, R.J.; Bruce, E.N.; Huebner, W.P.; Lanska, D.J. Performance of the human vestibuloocular reflex during locomotion. J. Neurophysiol. 1989, 62, 264–272. [Google Scholar] [CrossRef]
- Magnano, I.; Pes, G.M.; Pilurzi, G.; Cabboi, M.P.; Ginatempo, F.; Giaconi, E.; Tolu, E.; Achene, A.; Salis, A.; Rothwell, J.C.; et al. Exploring brainstem function in multiple sclerosis by combining brainstem reflexes, evoked potentials, clinical and MRI investigations. Clin. Neurophysiol. 2014, 125, 2286–2296. [Google Scholar] [CrossRef]
- Weier, K.; Penner, I.K.; Magon, S.; Amann, M.; Naegelin, Y.; Andelova, M.; Derfuss, T.; Stippich, C.; Radue, E.W.; Kappos, L.; et al. Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PLoS ONE 2014, 9, e86916. [Google Scholar] [CrossRef]
- Reulen, J.P.; Sanders, E.A.; Hogenhuis, L.A. Eye movement disorders in multiple sclerosis and optic neuritis. Brain 1983, 106 Pt 1, 121–140. [Google Scholar] [CrossRef]
- Di Stadio, A.; Ralli, M.; Altieri, M.; Greco, A.; Messineo, D.; Bernitsas, E. Audiovestibular symptoms in patients with multiple sclerosis: A correlation between self-reported symptomatology and MRI findings to monitor disease progression. Mult. Scler. Relat. Disord. 2020, 45, 102431. [Google Scholar] [CrossRef] [PubMed]
- Karatas, M. Internuclear and supranuclear disorders of eye movements: Clinical features and causes. Eur. J. Neurol. 2009, 16, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.A. Neuroophthalmology: Clinical Signs and Symptoms, 3rd ed.; Lea and Febiger: Philadelphia, PA, USA, 1992; pp. 525–543. [Google Scholar]
- McDonnell, M.N.; Hillier, S.L. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst. Rev. 2015, 1, CD005397. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Anson, E.R.; Carender, W.J.; Hoppes, C.W.; Cass, S.P.; Christy, J.B.; Cohen, H.S.; Fife, T.D.; et al. Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Updated Clinical Practice Guideline From the Academy of Neurologic Physical Therapy of the American Physical Therapy Association. J. Neurol. Phys. Ther. 2021, 46, 118–177. [Google Scholar] [CrossRef]
- Hebert, J.R.; Corboy, J.R.; Manago, M.M.; Schenkman, M. Effects of vestibular rehabilitation on multiple sclerosis-related fatigue and upright postural control: A randomized controlled trial. Phys. Ther. 2011, 91, 1166–1183. [Google Scholar] [CrossRef]
- Ozgen, G.; Karapolat, H.; Akkoc, Y.; Yuceyar, N. Is customized vestibular rehabilitation effective in patients with multiple sclerosis? A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 466–478. [Google Scholar]
- Tramontano, M.; Martino Cinnera, A.; Manzari, L.; Tozzi, F.F.; Caltagirone, C.; Morone, G.; Pompa, A.; Grasso, M.G. Vestibular rehabilitation has positive effects on balance, fatigue and activities of daily living in highly disabled multiple sclerosis people: A preliminary randomized controlled trial. Restor. Neurol. Neurosci. 2018, 36, 709–718. [Google Scholar] [CrossRef]


| Variable | PLW-min-MS (N = 8) | PLW-mild-MS (N = 23) | PLW-mod-MS (N = 6) |
|---|---|---|---|
| Age (years) a | 47.3 (12.2) | 55.5 (11.9) | 54.2 (13.8) |
| Female sex b | 6 (75.0%) | 18 (78.3%) | 4 (66.7%) |
| Age at onset (years) c | 41.0 (33.8–47.2) | 32.0 (28.0–42.5) | 38.5 (26.5–46.0) |
| MS duration (years) c | 2.0 (1.0–9.2) | 21.0 (11.0–28.5) | 18.5 (13.5–19.8) |
| Variable | PLW-min-MS (N = 8) | PLW-mild-MS (N = 23) | PLW-mod-MS (N = 6) | p-Value |
|---|---|---|---|---|
| Right lateral canal | ||||
| VOR gain | n = 66: 0.98 (0.93–1.02) | n = 275: 0.91 (0.79–0.99) | n = 74: 0.91 (0.79–1.03) | <0.001 |
| CS frequency | n = 66: 1.00 (0.00–1.75) | n = 275: 1.00 (1.00–2.00) | n = 74: 1.00 (1.00–2.00) | 0.002 |
| CS latency (ms) | n = 42: 230.00 (185.00–283.25) | n = 213: 220.00 (168.00–296.00) | n = 65: 200.00 (160.00–257.33) | 0.152 |
| GPE (°) | n = 66: 0.18 (−0.20–1.01) | n = 275: 1.16 (0.10–2.72) | n = 74: 0.26 (−0.95–1.57) | <0.001 |
| Left lateral canal | ||||
| VOR gain | n = 79: 0.92 (0.88–0.96) | n = 285: 0.86 (0.76–0.93) | n = 84: 0.92 (0.84–1.00) | <0.001 |
| CS frequency | n = 79: 1.00 (1.00–1.00) | n = 285: 1.00 (1.00–2.00) | n = 84: 1.00 (1.00–2.00) | 0.013 |
| CS latency (ms) | n = 63: 244.00 (210.33–308.00) | n = 231: 256.00 (213.00–312.33) | n = 76: 276.00 (223.50–324.00) | 0.085 |
| GPE (°) | n = 79: 1.30 (0.52–1.97) | n = 285: 2.09 (0.85–3.60) | n = 84: 0.84 (−0.12–1.81) | <0.001 |
| Right anterior canal | ||||
| VOR gain | n = 30: 0.80 (0.65–0.92) | n = 151: 0.81 (0.71–1.01) | n = 45: 0.66 (0.58–0.77) | <0.001 |
| CS frequency | n = 30: 2.00 (1.00–2.00) | n = 151: 1.00 (1.00–2.00) | n = 45: 0.00 (0.00–1.00) | <0.001 |
| CS latency (ms) | n = 27: 140.00 (90.00–206.00) | n = 127: 222.00 (140.00–280.00) | n = 22: 174.67 (100.00–213.50) | 0.001 |
| GPE (°) | n = 30: 1.43 (0.85–2.69) | n = 151: 1.72 (−0.19–3.44) | n = 45: 3.50 (2.33–4.31) | <0.001 |
| Left posterior canal | ||||
| VOR gain | n = 25: 0.89 (0.75–1.04) | n = 161: 0.67 (0.39–0.84) | n = 55: 0.52 (0.43–0.73) | <0.001 |
| CS frequency | n = 25: 1.00 (1.00–2.00) | n = 161: 1.00 (1.00–2.00) | n = 55: 1.00 (1.00–2.00) | 0.816 |
| CS latency (ms) | n = 19: 166.00 (103.00–210.00) | n = 138: 211.00 (172.00–275.50) | n = 44: 184.33 (92.00–255.50) | 0.001 |
| GPE (°) | n = 25: 0.79 (−0.36–1.77) | n = 161: 4.32 (2.02–6.75) | n = 55: 5.02 (2.77–6.31) | <0.001 |
| Left anterior canal | ||||
| VOR gain | n = 44: 0.85 (0.80–0.93) | n = 176: 0.75 (0.62–0.86) | n = 51: 0.67 (0.53–0.77) | <0.001 |
| CS frequency | n = 44: 1.00 (0.00–1.00) | n = 176: 1.00 (0.00–2.00) | n = 51: 1.00 (1.00–2.00) | 0.002 |
| CS latency (ms) | n = 28: 197.00 (115.50–294.00) | n = 123: 228.00 (152.00–316.00) | n = 42: 159.67 (100.00–214.00) | 0.003 |
| GPE (°) | n = 44: 1.65 (0.48–2.30) | n = 176: 3.00 (1.19–4.78) | n = 51: 3.18 (1.92–5.37) | <0.001 |
| Right posterior canal | ||||
| VOR gain | n = 48: 0.75 (0.71–0.81) | n = 191: 0.60 (0.29–0.74) | n = 56: 0.56 (0.45–0.70) | <0.001 |
| CS frequency | n = 48: 1.00 (1.00–2.00) | n = 191: 1.00 (1.00–2.00) | n = 56: 1.00 (1.00–2.00) | 0.03 |
| CS latency (ms) | n = 46: 220.00 (130.00–271.00) | n = 160: 209.33 (172.00–308.00) | n = 51: 200.00 (128.00–262.00) | 0.086 |
| GPE (°) | n = 48: 2.84 (1.83–3.61) | n = 191: 4.99 (2.50–8.53) | n = 56: 3.82 (2.57–5.18) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grove, C.R.; Wagner, A.; Yang, V.B.; Loyd, B.J.; Dibble, L.E.; Schubert, M.C. Greater Disability Is Associated with Worse Vestibular and Compensatory Oculomotor Functions in People Living with Multiple Sclerosis. Brain Sci. 2022, 12, 1519. https://doi.org/10.3390/brainsci12111519
Grove CR, Wagner A, Yang VB, Loyd BJ, Dibble LE, Schubert MC. Greater Disability Is Associated with Worse Vestibular and Compensatory Oculomotor Functions in People Living with Multiple Sclerosis. Brain Sciences. 2022; 12(11):1519. https://doi.org/10.3390/brainsci12111519
Chicago/Turabian StyleGrove, Colin R., Andrew Wagner, Victor B. Yang, Brian J. Loyd, Leland E. Dibble, and Michael C. Schubert. 2022. "Greater Disability Is Associated with Worse Vestibular and Compensatory Oculomotor Functions in People Living with Multiple Sclerosis" Brain Sciences 12, no. 11: 1519. https://doi.org/10.3390/brainsci12111519
APA StyleGrove, C. R., Wagner, A., Yang, V. B., Loyd, B. J., Dibble, L. E., & Schubert, M. C. (2022). Greater Disability Is Associated with Worse Vestibular and Compensatory Oculomotor Functions in People Living with Multiple Sclerosis. Brain Sciences, 12(11), 1519. https://doi.org/10.3390/brainsci12111519






