Improving Upper Limb and Gait Rehabilitation Outcomes in Post-Stroke Patients: A Scoping Review on the Additional Effects of Non-Invasive Brain Stimulation When Combined with Robot-Aided Rehabilitation
Abstract
1. Introduction
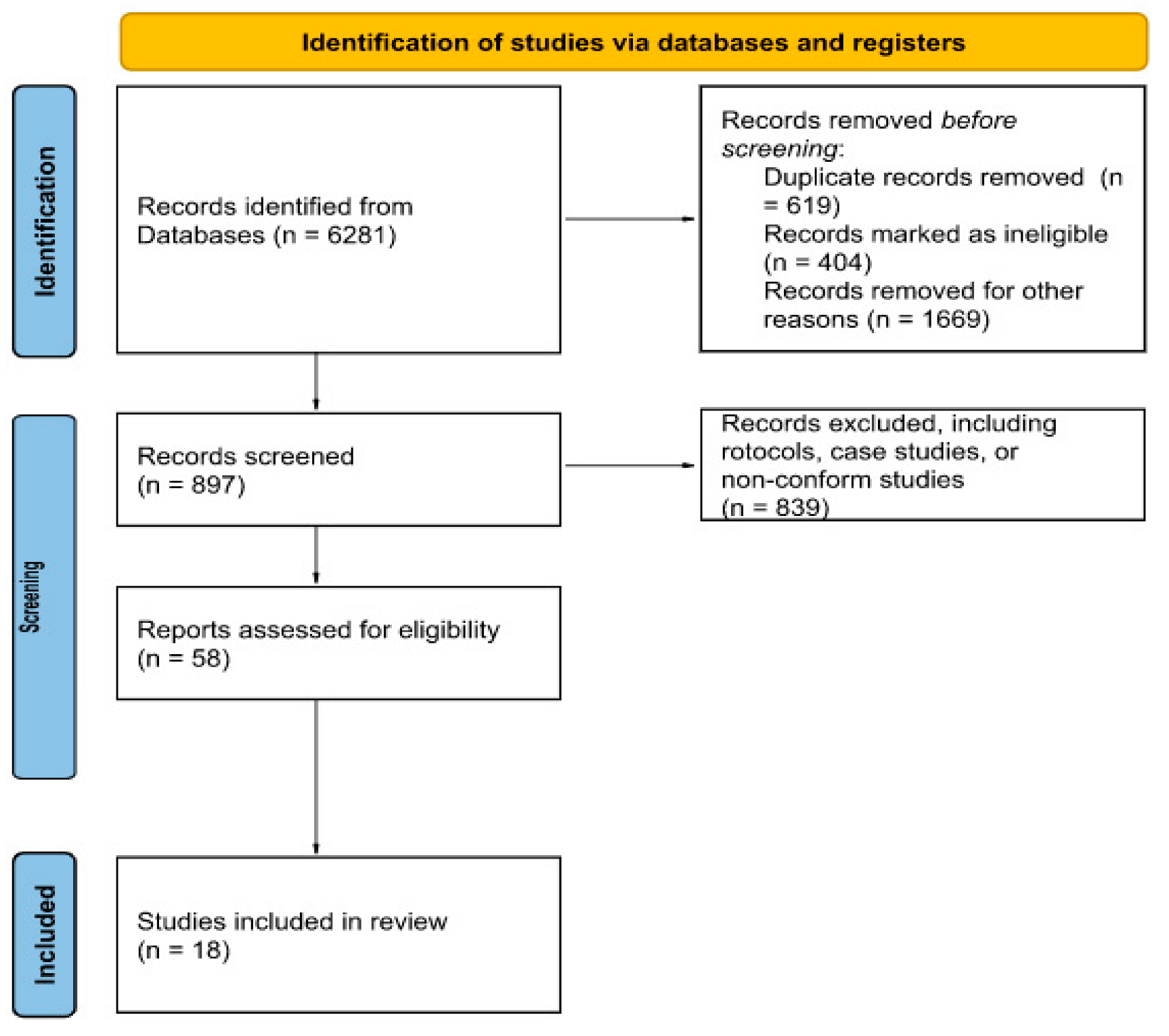
2. Materials and Methods
3. Results
4. Discussion
Strength and Weakness of NIBS-RAR Coupled Intervention
5. Final Remarks and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, X.; Ge, Y.; Chen, S.; Yan, Z.; Wang, Z.; Zhang, W.; Chen, Z.; Xue, T.; Wang, Z. Thrombectomy with or without thrombolysis in patients with acute ischemic stroke: A systematic review and meta-analysis. J. Neurol. 2022, 269, 1809–1816. [Google Scholar] [CrossRef]
- Veldema, J.; Gharabaghi, A. Non-invasive brain stimulation for improving gait, balance, and lower limbs motor function in stroke. J. Neuroeng. Rehabil. 2022, 19, 84. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.B.; Bernardo, W.M.; Oshiro, C.A.; Krebs, H.I.; Conforto, A.B. Effects of Robotic Therapy Associated With Noninvasive Brain Stimulation on Upper-Limb Rehabilitation After Stroke: Systematic Review and Meta-analysis of Randomized Clinical Trials. Neurorehabilit. Neural Repair 2021, 35, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, R.S.; Sorrentino, G.; Cassio, A.; Mazzoli, D.; Andrenelli, E.; Bizzarini, E.; Campanini, I.; Carmignano, S.M.; Cerulli, S.; Chisari, C.; et al. Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE) Robotic-assisted gait rehabilitation following stroke: A systematic review of current guidelines and practical clinical recommendations. Eur. J. Phys. Rehabil. Med. 2021, 57, 460–471. [Google Scholar] [CrossRef]
- Hatem, S.M.; Saussez, G.; Della Faille, M.; Prist, V.; Zhang, X.; Dispa, D.; Bleyenheuft, Y. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front. Hum. Neurosci. 2016, 10, 442. [Google Scholar] [CrossRef]
- Chien, W.T.; Chong, Y.Y.; Tse, M.K.; Chien, C.W.; Cheng, H.Y. Robot-assisted therapy for upper-limb rehabilitation in subacute stroke patients: A systematic review and meta-analysis. Brain Behav. 2020, 10, e01742. [Google Scholar] [CrossRef]
- Calafiore, D.; Negrini, F.; Tottoli, N.; Ferraro, F.; Ozyemisci-Taskiran, O.; de Sire, A. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: A systematic review. Eur. J. Phys. Rehabil. Med. 2022, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Bressi, F.; Cinnera, A.M.; Morone, G.; Campagnola, B.; Cricenti, L.; Santacaterina, F.; Miccinilli, S.; Zollo, L.; Paolucci, S.; Di Lazzaro, V.; et al. Combining Robot-Assisted Gait Training and Non-Invasive Brain Stimulation in Chronic Stroke Patients: A Systematic Review. Front. Neurol. 2022, 13, 795788. [Google Scholar] [CrossRef] [PubMed]
- Szelenberger, R.; Kostka, J.; Saluk-Bijak, J.; Miller, E. Pharmacological Interventions and Rehabilitation Approach for Enhancing Brain Self-repair and Stroke Recovery. Curr. Neuropharmacol. 2020, 18, 51–64. [Google Scholar] [CrossRef]
- Cirillo, C.; Brihmat, N.; Castel-Lacanal, E.; Le Friec, A.; Barbieux-Guillot, M.; Raposo, N.; Pariente, J.; Viguier, A.; Simonetta-Moreau, M.; Albucher, J.F.; et al. Post-stroke remodeling processes in animal models and humans. J. Cereb. Blood Flow Metab. 2020, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Grigoras, I.F.; Stagg, C.J. Recent advances in the role of excitation-inhibition balance in motor recovery post-stroke. Fac. Rev. 2021, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Chisari, C. Bottom-Up or Top-Down Approach? Understanding the Way to Reach the Milestone of Recovery in Stroke. Int. J. Neurorehabil. 2015, 2, 2. [Google Scholar]
- Morone, G.; Spitoni, G.F.; De Bartolo, D.; Ghanbari Ghooshchy, S.; Di Iulio, F.; Paolucci, S.; Zoccolotti, P.; Iosa, M. Rehabilitative devices for a top-down approach. Expert Rev. Med. Devices 2019, 16, 187–195. [Google Scholar] [CrossRef]
- Molteni, F.; Gasperini, G.; Cannaviello, G.; Guanziroli, E. Exoskeleton and End-Effector Robots for Upper and Lower Limbs Rehabilitation: Narrative Review. PM R J. Inj. Funct. Rehabil. 2018, 10 (Suppl. 2), S174–S188. [Google Scholar] [CrossRef]
- Grefkes, C.; Fink, G.R. Recovery from stroke: Current concepts and future perspectives. Neurol. Res. Pract. 2020, 2, 17. [Google Scholar] [CrossRef]
- Koh, S.H. Mechanism of Recovery After Stroke. In Stroke Revisited: Pathophysiology of Stroke; Stroke Revisited; Lee, S.H., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Moggio, L.; de Sire, A.; Marotta, N.; Demeco, A.; Ammendolia, A. Exoskeleton versus end-effector robot-assisted therapy for finger-hand motor recovery in stroke survivors: Systematic review and meta-analysis. Top. Stroke Rehabil. 2021, 29, 539–550. [Google Scholar] [CrossRef]
- Mehrholz, J.; Thomas, S.; Kugler, J.; Pohl, M.; Elsner, B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst. Rev. 2020, 10, CD006185. [Google Scholar]
- Mehrholz, J.; Pollock, A.; Pohl, M.; Kugler, J.; Elsner, B. Systematic review with network meta-analysis of randomized controlled trials of robotic-assisted arm training for improving activities of daily living and upper limb function after stroke. J. Neuroeng. Rehabil. 2020, 17, 83. [Google Scholar] [CrossRef]
- Comino-Suárez, N.; Moreno, J.C.; Gómez-Soriano, J.; Megía-García, Á.; Serrano-Muñoz, D.; Taylor, J.; Alcobendas-Maestro, M.; Gil-Agudo, Á.; Del-Ama, A.J.; Avendaño-Coy, J. Transcranial direct current stimulation combined with robotic therapy for upper and lower limb function after stroke: A systematic review and meta-analysis of randomized control trials. J. Neuroeng. Rehabil. 2021, 18, 148. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, Y.; Li, C.; Luo, L.; Hua, Y.; Hu, J.; Bai, Y. Repetitive Transcranial Magnetic Stimulation of the Brain After Ischemic Stroke: Mechanisms from Animal Models. Cell. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Rosselin, C.; Amatya, B.; Hafezi, P.; Khan, F. Repetitive transcranial magnetic stimulation for management of post-stroke impairments: An overview of systematic reviews. J. Rehabil. Med. 2020, 52, jrm00015. [Google Scholar] [CrossRef] [PubMed]
- Elsner, B.; Kugler, J.; Pohl, M.; Mehrholz, J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst. Rev. 2020, 11, CD009645. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Guan, C.; Phua, K.S.; Wang, C.; Zhao, L.; Teo, W.P.; Chen, C.; Ng, Y.S.; Chew, E. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch. Phys. Med. Rehabil. 2015, 96, S79–S87. [Google Scholar] [CrossRef]
- Dehem, S.; Gilliaux, M.; Lejeune, T.; Delaunois, E.; Mbonda, P.; Vandermeeren, Y.; Detrembleur, C.; Stoquart, G. Effectiveness of a single session of dual-transcranial direct current stimulation in combination with upper limb robotic-assisted rehabilitation in chronic stroke patients: A randomized, double-blind, cross-over study. Int. J. Rehabil. Res. 2018, 41, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Capone, F.; Di Pino, G.; Pellegrino, G.; Florio, L.; Zollo, L.; Simonetti, D.; Ranieri, F.; Brunelli, N.; Corbetto, M.; et al. Combining Robotic Training and Non-Invasive Brain Stimulation in Severe Upper Limb-Impaired Chronic Stroke Patients. Front. Neurosci. 2016, 10, 88. [Google Scholar] [CrossRef]
- Edwards, D.J.; Cortes, M.; Rykman-Peltz, A.; Chang, J.; Elder, J.; Thickbroom, G.; Mariman, J.J.; Gerber, L.M.; Oromendia, C.; Krebs, H.I.; et al. Clinical improvement with intensive robot-assisted arm training in chronic stroke is unchanged by supplementary tDCS. Restor. Neurol. Neurosci. 2019, 37, 167–180. [Google Scholar] [CrossRef]
- Hesse, S.; Waldner, A.; Mehrholz, J.; Tomelleri, C.; Pohl, M.; Werner, C. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: An exploratory, randomized multicenter trial. Neurorehabilit. Neural Repair 2011, 25, 838–846. [Google Scholar] [CrossRef]
- Panker, M.; Nicole, S. The Effects of Robotic Training and Cortical Stimulation on Reaching Skill after Chronic Stroke. Ph.D. Dissertation, Georgetown University, Washington, DC, USA, 2011. [Google Scholar]
- Straudi, S.; Fregni, F.; Martinuzzi, C.; Pavarelli, C.; Salvioli, S.; Basaglia, N. tDCS and Robotics on Upper Limb Stroke Rehabilitation: Effect Modification by Stroke Duration and Type of Stroke. BioMed Res. Int. 2016, 2016, 5068127. [Google Scholar] [CrossRef]
- Tedesco Triccas, L.; Burridge, J.H.; Hughes, A.; Verheyden, G.; Desikan, M.; Rothwell, J. A double-blinded randomised controlled trial exploring the effect of anodal transcranial direct current stimulation and uni-lateral robot therapy for the impaired upper limb in sub-acute and chronic stroke. NeuroRehabilitation 2015, 37, 181–191. [Google Scholar] [CrossRef]
- Giacobbe, V.; Krebs, H.I.; Volpe, B.T.; Pascual-Leone, A.; Rykman, A.; Zeiarati, G.; Fregni, F.; Dipietro, L.; Thickbroom, G.W.; Edwards, D.J. Transcranial direct current stimulation (tDCS) and robotic practice in chronic stroke: The dimension of timing. NeuroRehabilitation 2013, 33, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Tran, V.D.; Dario, P.; Posteraro, F. Effects of Transcranial Direct Current Stimulation (tDCS) Combined With Wrist Robot-Assisted Rehabilitation on Motor Recovery in Subacute Stroke Patients: A Randomized Controlled Trial. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2019, 27, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Saeki, S.; Oda, T.; Matsushima, Y.; Hachisuka, K. Effects of anodal and cathodal transcranial direct current stimulation combined with robotic therapy on severely affected arms in chronic stroke patients. J. Rehabil. Med. 2013, 45, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Danzl, M.M.; Chelette, K.C.; Lee, K.; Lykins, D.; Sawaki, L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: A feasibility study. NeuroRehabilitation 2013, 33, 67–76. [Google Scholar] [CrossRef]
- Geroin, C.; Picelli, A.; Munari, D.; Waldner, A.; Tomelleri, C.; Smania, N. Combined transcranial direct current stimulation and robot-assisted gait training in patients with chronic stroke: A preliminary comparison. Clin. Rehabil. 2011, 25, 537–548. [Google Scholar] [CrossRef]
- Naro, A.; Billeri, L.; Manuli, A.; Balletta, T.; Cannavò, A.; Portaro, S.; Lauria, P.; Ciappina, F.; Calabrò, R.S. Breaking the ice to improve motor outcomes in patients with chronic stroke: A retrospective clinical study on neuromodulation plus robotics. Neurol. Sci. 2021, 42, 2785–2793. [Google Scholar] [CrossRef]
- Picelli, A.; Chemello, E.; Castellazzi, P.; Roncari, L.; Waldner, A.; Saltuari, L.; Smania, N. Combined effects of transcranial direct current stimulation (tDCS) and transcutaneous spinal direct current stimulation (tsDCS) on robot-assisted gait training in patients with chronic stroke: A pilot, double blind, randomized controlled trial. Restor. Neurol. Neurosci. 2015, 33, 357–368. [Google Scholar] [CrossRef]
- Picelli, A.; Brugnera, A.; Filippetti, M.; Mattiuz, N.; Chemello, E.; Modenese, A.; Gandolfi, M.; Waldner, A.; Saltuari, L.; Smania, N. Effects of two different protocols of cerebellar transcranial direct current stimulation combined with transcutaneous spinal direct current stimulation on robot-assisted gait training in patients with chronic supratentorial stroke: A single blind, randomized controlled trial. Restor. Neurol. Neurosci. 2019, 37, 97–107. [Google Scholar]
- Seo, H.G.; Lee, W.H.; Lee, S.H.; Yi, Y.; Kim, K.D.; Oh, B.M. Robotic-assisted gait training combined with transcranial direct current stimulation in chronic stroke patients: A pilot double-blind, randomized controlled trial. Restor. Neurol. Neurosci. 2017, 35, 527–536. [Google Scholar] [CrossRef]
- Leon, D.; Cortes, M.; Elder, J.; Kumru, H.; Laxe, S.; Edwards, D.J.; Tormos, J.M.; Bernabeu, M.; Pascual-Leone, A. tDCS does not enhance the effects of robot-assisted gait training in patients with subacute stroke. Restor. Neurol. Neurosci. 2017, 35, 377–384. [Google Scholar] [CrossRef]
- Di Pino, G.; Pellegrino, G.; Assenza, G.; Capone, F.; Ferreri, F.; Formica, D.; Ranieri, F.; Tombini, M.; Ziemann, U.; Rothwell, J.C.; et al. Modulation of brain plasticity in stroke: A novel model for neurorehabilitation. Nat. Rev. Neurol. 2014, 10, 597–608. [Google Scholar] [CrossRef]
- Ting, W.K.; Fadul, F.A.; Fecteau, S.; Ethier, C. Neurostimulation for Stroke Rehabilitation. Front. Neurosci. 2021, 15, 649459. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Mika, A.; Oleksy, Ł.; Stolarczyk, A.; Kielnar, R. Effects of Exoskeleton Gait Training on Balance, Load Distribution, and Functional Status in Stroke: A Randomized Controlled Trial. Front. Neurol. 2020, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Sankarasubramanian, V.; Machado, A.G.; Conforto, A.B.; Potter-Baker, K.A.; Cunningham, D.A.; Varnerin, N.M.; Wang, X.; Sakaie, K.; Plow, E.B. Inhibition versus facilitation of contralesional motor cortices in stroke: Deriving a model to tailor brain stimulation. Clin. Neurophysiol. 2017, 128, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Thickbroom, G.W.; Cortes, M.; Rykman, A.; Volpe, B.T.; Fregni, F.; Krebs, H.I.; Pascual-Leone, A.; Edwards, D.J. Stroke subtype and motor impairment influence contralesional excitability. Neurology 2015, 85, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Terranova, C.; Rizzo, V.; Cacciola, A.; Chillemi, G.; Calamuneri, A.; Milardi, D.; Quartarone, A. Is There a Future for Non-invasive Brain Stimulation as a Therapeutic Tool? Front. Neurol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Aleman, A.; Baeken, C.; Benninger, D.H.; Brunelin, J.; Di Lazzaro, V.; Filipović, S.R.; Grefkes, C.; Hasan, A.; Hummel, F.C.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 2020, 131, 474–528. [Google Scholar] [CrossRef]
- Liu, Y.; Mei, H.; Guo, L.; Liu, X.; Tao, X.; Ma, J. Non-invasive brain stimulation associated mirror therapy for upper-limb rehabilitation after stroke: Systematic review and meta-analysis of randomized clinical trials. Front. Neurol. 2022, 13, 918956. [Google Scholar]
- Ward, N.S. Non-invasive brain stimulation for stroke recovery: Ready for the big time? J. Neurol. Neurosurg. Psychiatry 2016, 87, 343–344. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J. Physiol. 2003, 553 Pt 1, 293–301. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Roth, A.; Kuo, M.F.; Fischer, A.K.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J. Neurosci. 2007, 27, 3807–3812. [Google Scholar] [CrossRef]
- Siebner, H.R.; Lang, N.; Rizzo, V.; Nitsche, M.A.; Paulus, W.; Lemon, R.N.; Rothwell, J.C. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: Evidence for homeostatic plasticity in the human motor cortex. J. Neurosci. 2004, 24, 3379–3385. [Google Scholar] [CrossRef]
- Lang, N.; Siebner, H.R.; Ernst, D.; Nitsche, M.A.; Paulus, W.; Lemon, R.N.; Rothwell, J.C. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol. Psychiatry 2004, 56, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.L.; Edwards, D.; Dunning, K.; Fregni, F.; Stein, J.; Laine, J.; Rogers, L.M.; Vox, F.; Durand-Sanchez, A.; Bockbrader, M.; et al. Randomized Sham-Controlled Trial of Navigated Repetitive Transcranial Magnetic Stimulation for Motor Recovery in Stroke. Stroke 2018, 49, 2138–2146. [Google Scholar] [CrossRef]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot assisted training for the upper limb after stroke (RATULS): A multicentre randomised controlled trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef]
- Krebs, H.I.; Krams, M.; Agrafiotis, D.K.; DiBernardo, A.; Chavez, J.C.; Littman, G.S.; Yang, E.; Byttebier, G.; Dipietro, L.; Rykman, A.; et al. Robotic measurement of arm movements after stroke establishes biomarkers of motor recovery. Stroke 2014, 45, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.S.; Wittenberg, G.F.; Krebs, H.I.; Zhan, M.; Bever, C.T.; Whitall, J. Robot-Assisted Arm Training in Chronic Stroke: Addition of Transition-to-Task Practice. Neurorehabilit. Neural Repair 2019, 33, 751–761. [Google Scholar] [CrossRef]
- Powell, E.S.; Carrico, C.; Westgate, P.M.; Chelette, K.C.; Nichols, L.; Reddy, L.; Salyers, E.; Ward, A.; Sawaki, L. Time configuration of combined neuromodulation and motor training after stroke: A proof-of-concept study. NeuroRehabilitation 2016, 39, 439–449. [Google Scholar] [CrossRef]
- Oldrati, V.; Colombo, B.; Antonietti, A. Combination of a short cognitive training and tDCS to enhance visuospatial skills: A comparison between online and offline neuromodulation. Brain Res. 2018, 1678, 32–39. [Google Scholar] [CrossRef]
- Yang, J.F.; Gorassini, M. Spinal and brain control of human walking: Implications for retraining of walking. Neuroscientist 2006, 12, 379–389. [Google Scholar] [CrossRef]
- Bocci, T.; Vannini, B.; Torzini, A.; Mazzatenta, A.; Vergari, M.; Cogiamanian, F.; Priori, A.; Sartucci, F. Cathodal transcutaneous spinal direct current stimulation (tsDCS) improves motor unit recruitment in healthy subjects. Neurosci. Lett. 2014, 578, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Lu, M.K.; Antal, A.; Classen, J.; Nitsche, M.; Ziemann, U.; Ridding, M.; Hamada, M.; Ugawa, Y.; Jaberzadeh, S.; et al. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2017, 128, 2318–2329. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Bonnì, S.; Casula, E.P.; Iosa, M.; Paolucci, S.; Pellicciari, M.C.; Cinnera, A.M.; Ponzo, V.; Maiella, M.; Picazio, S.; et al. Effect of Cerebellar Stimulation on Gait and Balance Recovery in Patients With Hemiparetic Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Wessel, M.J.; Hummel, F.C. Non-invasive Cerebellar Stimulation: A Promising Approach for Stroke Recovery? Cerebellum 2018, 17, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Cantone, M.; Lanza, G.; Ranieri, F.; Opie, G.M.; Terranova, C. Editorial: Non-invasive Brain Stimulation in the Study and Modulation of Metaplasticity in Neurological Disorders. Front. Neurol. 2021, 12, 721906. [Google Scholar] [CrossRef] [PubMed]
- Turi, Z.; Lenz, M.; Paulus, W.; Mittner, M.; Vlachos, A. Selecting stimulation intensity in repetitive transcranial magnetic stimulation studies: A systematic review between 1991 and 2020. Eur. J. Neurosci. 2021, 53, 3404–3415. [Google Scholar] [CrossRef]
- Tofts, P.S. The distribution of induced currents in magnetic stimulation of the nervous system. Phys. Med. Biol. 1990, 35, 1119–1128. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Ziemann, U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front. Neural Circuits 2013, 7, 18. [Google Scholar] [CrossRef]
- Henneman, E.; Mendell, L.M. Functional Organization of Motoneuron Pool and its Inputs. Compr. Physiol. 2011, 1, 423–507. [Google Scholar]
- Yamashita, A.; Murakami, T.; Hattori, N.; Miyai, I.; Ugawa, Y. Intensity dependency of peripheral nerve stimulation in spinal LTP induced by paired associative corticospinal-motoneuronal stimulation (PCMS). PLoS ONE 2021, 16, e0259931. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Kwon, M.; Fernández, J.R.; Zegarek, G.F.; Lo, S.B.; Firestein, B.L. BDNF-promoted increases in proximal dendrites occur via CREB-dependent transcriptional regulation of cypin. J. Neurosci. 2011, 31, 9735–9745. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.; Lindberg, D.R.; Makowiecki, K.; Gray, A.; Asp, A.J.; Rodger, J.; Choi, D.S.; Croarkin, P.E. Medium- and high-intensity rTMS reduces psychomotor agitation with distinct neurobiologic mechanisms. Transl. Psychiatry 2018, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Zhang, R.G.; Xue, F.; Wang, H.N.; Chen, Y.C.; Hu, G.T.; Peng, Y.; Peng, Z.W.; Tan, Q.R. Quetiapine and repetitive transcranial magnetic stimulation ameliorate depression-like behaviors and up-regulate the proliferation of hippocampal-derived neural stem cells in a rat model of depression: The involvement of the BDNF/ERK signal pathway. Pharmacol. Biochem. Behav. 2015, 136, 39–46. [Google Scholar] [CrossRef]
- Feng, S.F.; Shi, T.Y.; Fan, Y.; Wang, W.N.; Chen, Y.C.; Tan, Q.R. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behav. Brain Res. 2012, 232, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng. 2011, 8, 046011. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Boggio, P.S.; Mansur, C.G.; Wagner, T.; Ferreira, M.J.; Lima, M.C.; Rigonatti, S.P.; Marcolin, M.A.; Freedman, S.D.; Nitsche, M.A.; et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport 2005, 16, 1551–1555. [Google Scholar] [CrossRef]
- Hummel, F.; Celnik, P.; Giraux, P.; Floel, A.; Wu, W.-H.; Gerloff, C.; Cohen, L.G. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain 2005, 128, 490–499. [Google Scholar] [CrossRef]
- Lindenberg, R.; Renga, V.; Zhu, L.L.; Nair, D.; Schlaug, G.M.D.P. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 2010, 75, 2176–2184. [Google Scholar] [CrossRef]
- Duque, J.; Hummel, F.; Celnik, P.; Murase, N.; Mazzocchio, R.; Cohen, L.G. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 2005, 28, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Hummel, F.C.; Cohen, L.G. Non-invasive brain stimulation: A new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006, 5, 708–712. [Google Scholar] [CrossRef]
- Tazoe, T.; Endoh, T.; Kitamura, T.; Ogata, T. Polarity specific effects of transcranial direct current stimulation on interhemispheric inhibition. PLoS ONE 2014, 9, e11424. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Johansen-Berg, H. Studying the effects of transcranial direct-current stimulation in stroke recovery using magnetic resonance imaging. Front. Hum. Neurosci. 2013, 7, 857. [Google Scholar] [CrossRef]
- Cha, T.H.; Hwang, H.S. Rehabilitation Interventions Combined with Noninvasive Brain Stimulation on Upper Limb Motor Function in Stroke Patients. Brain Sci. 2022, 12, 994. [Google Scholar] [CrossRef]
- Triccas, L.T.; Burridge, J.H.; Hughes, A.M.; Pickering, R.M.; Desikan, M.; Rothwell, J.C.; Verheyden, G. Multiple sessions of transcranial direct current stimulation and upper extremity rehabilitation in stroke: A review and meta-analysis. Clin. Neurophysiol. 2016, 127, 946–955. [Google Scholar] [CrossRef]
- Nudo, R.J.; Jenkins, W.M.; Merzenich, M.M. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosens. Mot. Res. 1990, 7, 463–483. [Google Scholar] [CrossRef]
- Kanno, M.; Matsumoto, M.; Togashi, H.; Yoshioka, M.; Mano, Y. Effects of acute repetitive transcranial magnetic stimulation on dopamine release in the rat dorsolateral striatum. J. Neurol. Sci. 2004, 217, 73–81. [Google Scholar] [CrossRef]
- Webster, B.R.; Celnik, P.A.; Cohen, L.G. Noninvasive brain stimulation in stroke rehabilitation. NeuroRx J. Am. Soc. Exp. NeuroTher. 2006, 3, 474–481. [Google Scholar] [CrossRef]
- Kubis, N. Non-Invasive Brain Stimulation to Enhance Post-Stroke Recovery. Front. Neural Circuits 2016, 10, 56. [Google Scholar] [CrossRef]
- Gerges, A.; Hordacre, B.; Pietro, F.D.; Moseley, G.L.; Berryman, C. Do Adults with Stroke have Altered Interhemispheric Inhibition? A Systematic Review with Meta-Analysis. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2022, 31, 106494. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Bai, Y. A Review of Exercise-Induced Neuroplasticity in Ischemic Stroke: Pathology and Mechanisms. Mol. Neurobiol. 2020, 57, 4218–4231. [Google Scholar] [CrossRef]
- Joy, M.T.; Carmichael, S.T. Learning and Stroke Recovery: Parallelism of Biological Substrates. Semin. Neurol. 2021, 41, 147–156. [Google Scholar] [CrossRef]
- Ada, L.; Dean, C.M.; Lindley, R.; Lloyd, G. Improving community ambulation after stroke: The AMBULATE Trial. BMC Neurol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
| Authors | Active-Control Group n | Device Type | Joint Task | NIBS | Outcomes # |
|---|---|---|---|---|---|
| NIBS + RAR vs. RAR + sham NIBS comparison | |||||
| Ang 2015 [25] | 10-9 | MIT Manus (EE) 1 | shoulder/elbow unimanual tasks | a/c-tDCS 10 sessions, 20-min before RAR for 2 weeks | active > sham in AL active = sham in BFS |
| Dehem 2018 [26] | 11-10 | REAplan robot (EE) | shoulder/elbow unimanual tasks | a/c-tDCS 1 session, 20-min during RAR (20-min) 1 day | active > sham in AL |
| Di Lazzaro 2016 [27] | 8-9 | InMotion2 * (EE) 2 | shoulder/elbow unimanual tasks | continuous theta-burst stimulation 10 sessions on affected hemisphere before RAR for 2 weeks | active > sham in BFS |
| Edwards 2019 [28] | 41-41 | MIT Manus (EE) 3 | entire arm unimanual tasks | a-tDCS 36 sessions, 20-min before RAR for 12 weeks | active = sham in BFS and AL |
| Hesse 2011 [29] | 32-32 | Bi-Manu Track (EE) 4 | wrist/hand bimanual tasks | a-tDCS 30 sessions, 20-min at RAR beginning (400 movements) for 6 weeks | active = sham in BFS |
| 32-32 | Bi-Manu Track (EE) 5 | wrist/hand bimanual tasks | c-tDCS 30 sessions, 20-min at RAR beginning (400 movements) for 6 weeks | active = sham in BFS | |
| Panker 2011 [30] | 9-9 | ReoGo(EE) | shoulder/elbow unimanual tasks | a-tDCS 22 sessions, 20-min at RAR beginning (60 min) for 2.5 weeks | active > sham in BFS sham > active in AL |
| Straudi 2016 [31] | 12-11 | ReoGo (EE) | shoulder/elbow unimanual tasks | a/c-tDCS 10 sessions, 30-min during the 30 min of RAR for 2 weeks | active = sham in BFS sham > active in AL |
| Tedesco Triccas 2015 [32] | 12-11 | Armeo®Spring (Ex) | whole arm unimanual tasks | a-tDCS 18 sessions, 25-min during the first 25 min of 75 min RAR for 8 weeks | sham > active in BFS sham > active in AL |
| Mazzoleni 2019 [34] | 20-19 | InMotion (EE) | wrist unimanual tasks | a-tDCS 30 sessions, 20-min during the treatment for 6 weeks | active = sham in BSF active > sham in AL |
| Timing of NIBS delivery during RAR | |||||
| Giacobbe 2013 [33] | 12-12-12 | InMotion3 (EE) | wrist unimanual tasks | a/c-tDCS 1 session, 20-min before vs. during vs. after training (with sham during the training) 1 day |
|
| Different NIBSs’ comparison | |||||
| Ochi 2013 [35] | 18-18 | Bi-Manu Track (EE) | elbow/wrist bimanual tasks | a-tDCS vs. c-tDCS 5 sessions, 10-min during the treatment for 5 days | a-tDCS=c-tDCS in BFS and spasticity no effects on MAL |
| Authors | Active-Control Group n | Device | NIBS | Outcomes # |
|---|---|---|---|---|
| NIBS + RAR vs. RAR + sham NIBS comparison | ||||
| Danzl 2013 [36] | 4-4 | Lokomat (Ex) | c/a-tDCS (ref. supraorbit) 3 days/week sessions, 20-min before the training (20–40 min) for 4 weeks | active = sham in gait speed and balance |
| Geroin 2011 [37] | 10-10-10 | Gait Trainer 1 (Ex) | c/a-tDCS (ref. contralateral orbit) 5 days/week sessions, 7-min real vs. sham vs. no NIBS during the training (50-min) 1 for 2 weeks | active = sham > no NIBS in gait endurance active = sham > no NIBS in gait speed |
| Seo 2017 [41] | 10-11 | Walkbot_S (Ex) | c-tDCS (ref. contralateral orbit) 5 days/week sessions, 20-min during the training (45 min) for 2 weeks | active > sham in gait endurance |
| Timing of NIBS delivery during RAR | ||||
| Naro 2020 [38] | 9-15-18 | Lokomat®Pro(Ex) | c/a-tDCS (ref. contralateral CMA) 6 days/week sessions, 10-min before vs. during vs. after training (60-min) 2 for 8 weeks | during = after > before in gait endurance, fall risk, and gait performance during = after = before in gait speed, disability burden, and gait performance |
| Different NIBSs’ comparison | ||||
| Picelli 2015 [39] | 10-10-10 | G-EO System (EE) |
during the training (20 min) for 2 weeks | active > sham in gait endurance up to two weeks but not up to one month after treatment |
| 10-10 | G-EO System (EE) |
during the training (20 min) for 2 weeks | active > sham in gait endurance up to two weeks but not up to one month after treatment | |
| Picelli 2019 [40] | 20-20 | G-EO System (EE) |
during the training (20 min) for 2 weeks | active = sham in gait endurance |
| Leon 2017 [42] | 9-17-23 | Gait Trainer or Lokomat (Ex) |
during the training (30–45 min) for 4 weeks | tDCSLEG = tDCSHAND = tDCSNO in improving gait speed and gait performance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naro, A.; Calabrò, R.S. Improving Upper Limb and Gait Rehabilitation Outcomes in Post-Stroke Patients: A Scoping Review on the Additional Effects of Non-Invasive Brain Stimulation When Combined with Robot-Aided Rehabilitation. Brain Sci. 2022, 12, 1511. https://doi.org/10.3390/brainsci12111511
Naro A, Calabrò RS. Improving Upper Limb and Gait Rehabilitation Outcomes in Post-Stroke Patients: A Scoping Review on the Additional Effects of Non-Invasive Brain Stimulation When Combined with Robot-Aided Rehabilitation. Brain Sciences. 2022; 12(11):1511. https://doi.org/10.3390/brainsci12111511
Chicago/Turabian StyleNaro, Antonino, and Rocco Salvatore Calabrò. 2022. "Improving Upper Limb and Gait Rehabilitation Outcomes in Post-Stroke Patients: A Scoping Review on the Additional Effects of Non-Invasive Brain Stimulation When Combined with Robot-Aided Rehabilitation" Brain Sciences 12, no. 11: 1511. https://doi.org/10.3390/brainsci12111511
APA StyleNaro, A., & Calabrò, R. S. (2022). Improving Upper Limb and Gait Rehabilitation Outcomes in Post-Stroke Patients: A Scoping Review on the Additional Effects of Non-Invasive Brain Stimulation When Combined with Robot-Aided Rehabilitation. Brain Sciences, 12(11), 1511. https://doi.org/10.3390/brainsci12111511






