Elevated Level of Cerebrospinal Fluid Pyruvate Dehydrogenase Kinase 4 Is a Predictive Biomarker of Clinical Outcome after Subarachnoid Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
- (1)
- According to the Chinese Guidelines for the Diagnosis and Treatment of Subarachnoid Hemorrhage 2019 [17], all patients were diagnosed by brain CT, CT angiography (CTA) or whole cerebral angiography (DSA), and lumbar puncture;
- (2)
- The first onset, the age of onset, is 16–70 years old (including the critical value);
- (3)
- Signed consent from the subject or next of kin.
- (1)
- Failure to meet the inclusion criteria or being unfit for the experiment as determined by the responsible doctor;
- (2)
- Presence of severe cardiac insufficiency, renal dysfunction, diabetes, or other systemic diseases;
- (3)
- A history of traumatic brain injury, severe cerebral edema, or hydrocephaly.
- (1)
- Those who had been diagnosed with gonarthritis by a professional doctor requiring spinal anesthesia;
- (2)
- Patients aged 16–70 years (including cutoff values);
- (3)
- No current or pre-existing brain injuries, neurological diseases, or bleeding disorders, and subjects who needed surgery for knee arthritis;
- (4)
- Signed consent from the subject or next of kin.
2.2. Sample Collection
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Pyruvate Assay
2.5. Data Collection and Analysis
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics and an Overview of Upgrading
3.2. Clinical Data and Comparison of Patients in the Good and Poor Outcome Groups
3.3. Univariate and Multivariate Regression Analysis for Predicting Postoperative Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuette, A.J.; Barrow, D.L. Epidemiology and Long-Term Mortality in Subarachnoid Hemorrhage. World Neurosurg. 2013, 80, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Hop, J.W.; Rinkel, G.J.; Algra, A.; Van Gijn, J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: A systematic review. Stroke 1997, 28, 660–664. [Google Scholar] [CrossRef]
- Sehba, F.A.; Hou, J.; Pluta, R.M.; Zhang, J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012, 97, 14–37. [Google Scholar] [CrossRef]
- Rowland, M.; Hadjipavlou, G.; Kelly, M.; Westbrook, J.; Pattinson, K. Delayed cerebral ischaemia after subarachnoid haemorrhage: Looking beyond vasospasm. Br. J. Anaesth. 2012, 109, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011, 10, 618–625. [Google Scholar] [CrossRef]
- Maragkos, G.A.; Enriquez-Marulanda, A.; Salem, M.M.; Ascanio, L.C.; Chida, K.; Gupta, R.; Alturki, A.Y.; Kicielinski, K.P.; Ogilvy, C.S.; Moore, J.M.; et al. Proposal of a Grading System for Predicting Discharge Mortality and Functional Outcome in Patients with Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019, 121, e500–e510. [Google Scholar] [CrossRef] [PubMed]
- Jaja, B.N.; Cusimano, M.D.; Etminan, N.; Hanggi, D.; Hasan, D.; Ilodigwe, D.; Lantigua, H.; Le Roux, P.; Lo, B.; Louffat-Olivares, A.; et al. Clinical Prediction Models for Aneurysmal Subarachnoid Hemorrhage: A Systematic Review. Neurocritical Care 2013, 18, 143–153. [Google Scholar] [CrossRef]
- Kapapa, T.; Tjahjadi, M.; König, R.; Wirtz, C.R.; Woischneck, D. Which Clinical Variable Influences Health-Related Quality of Life the Most After Spontaneous Subarachnoid Hemorrhage? Hunt and Hess Scale, Fisher Score, World Federation of Neurosurgeons Score, Brussels Coma Score, and Glasgow Coma Score Compared. World Neurosurg. 2013, 80, 853–858. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Amuluru, K.; Smith, B.; Damodara, N.; El-Ghanem, M.; Singh, I.P.; Dangayach, N.; Gandhi, C.D. Emerging Markers of Early Brain Injury and Delayed Cerebral Ischemia in Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2017, 107, 148–159. [Google Scholar] [CrossRef]
- Fiskum, G.; Rosenthal, R.E.; Vereczki, V.; Martin, E.; Hoffman, G.E.; Chinopoulos, C.; Kowaltowski, A. Protection Against Ischemic Brain Injury by Inhibition of Mitochondrial Oxidative Stress. J. Bioenerg. Biomembr. 2004, 36, 347–352. [Google Scholar] [CrossRef]
- Jing, C.-H.; Wang, L.; Liu, P.-P.; Wu, C.; Ruan, D.; Chen, G. Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience 2012, 213, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J. Regulation of Mammalian Pyruvate Dehydrogenase Complex by a Phosphorylation–Dephosphorylation Cycle. Curr. Top. Cell. Regul. 1981, 18, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kark, R.A.; Rodriguez-Budelli, M.; Blass, J.P. Evidence for a primary defect of lipoamide dehydrogenase in Friedreich's ataxia. Adv. Neurol. 1978, 21, 163–180. [Google Scholar] [PubMed]
- Cortes, M.S. Re: Relationship between general movements in neonates who were growth restricted in utero and prenatal Doppler flow patterns. J. C. Tanis, D. M. Schmitz, M. R. Boelen, L. Casarella, P. P. van den Berg, C. M. Bilardo and A. F. Bos. Ultrasound Obstet Gynecol 2016; 48: 772–778. Ultrasound Obs. Gynecol. 2016, 48, 694. [Google Scholar] [CrossRef][Green Version]
- Kato, M.; Wynn, R.M.; Chuang, J.L.; Tso, S.-C.; Machius, M.; Li, J.; Chuang, D.T. Structural Basis for Inactivation of the Human Pyruvate Dehydrogenase Complex by Phosphorylation: Role of Disordered Phosphorylation Loops. Structure 2008, 16, 1849–1859. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Y.-Y.; Yan, H.-Y.; Liu, G.-J.; Zhou, Y.; Tao, T.; Yue, T.-T.; Pang, C.; Chen, X.-X.; Gao, S.; et al. PDK4 Decrease Neuronal Apoptosis via Inhibiting ROS-ASK1/P38 Pathway in Early Brain Injury After Subarachnoid Hemorrhage. Antioxidants Redox Signal. 2022, 36, 505–524. [Google Scholar] [CrossRef]
- Cerebrovascular Disease and the Neurovascular Intervention Collaboration Group of the Chinese Medical Association Neurology Branch. Chinese guidelines for diagnosis and treatment of submental hemorrhage 2019. Chin. J. Neurol. 2019, 52, 1006–1021. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, Y.; Wang, Y.; Ma, W.; Han, X.; Wang, X.; Liu, N. HIF-1α/PDK4/autophagy pathway protects against advanced glycation end-products induced vascular smooth muscle cell calcification. Biochem. Biophys. Res. Commun. 2019, 517, 470–476. [Google Scholar] [CrossRef]
- Duan, L.; Ramachandran, A.; Akakpo, J.Y.; Woolbright, B.L.; Zhang, Y.; Jaeschke, H. Mice deficient in pyruvate dehydrogenase kinase 4 are protected against acetaminophen-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 2019, 387, 114849. [Google Scholar] [CrossRef]
- Li, G.; Li, M.; Hu, J.; Lei, R.; Xiong, H.; Ji, H.; Yin, H.; Wei, Q.; Hu, G. The microRNA-182-PDK4 axis regulates lung tumorigenesis by modulating pyruvate dehydrogenase and lipogenesis. Oncogene 2017, 36, 989–998. [Google Scholar] [CrossRef]
- Goldstein, J.T.; Berger, A.C.; Shih, J.; Duke, F.F.; Furst, L.; Kwiatkowski, D.J.; Cherniack, A.D.; Meyerson, M.; Strathdee, C.A. Genomic Activation of PPARG Reveals a Candidate Therapeutic Axis in Bladder Cancer. Cancer Res. 2017, 77, 6987–6998. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, J.; Zhao, M.; Han, Y.; Chen, P.; Mo, Q.; Wang, B.; Chen, G.; Fang, Y.; Tian, Y.; et al. Correction: Loss of the novel mitochondrial protein FAM210B promotes metastasis via PDK4-dependent metabolic reprogramming. Cell Death Dis. 2019, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; St Leger, R.J.; Fang, W. Pyruvate Accumulation Is the First Line of Cell Defense against Heat Stress in a Fungus. mBio 2017, 8, e01284-17. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Sun, L.; Sun, J.; Sun, B.; Gao, Y.; Shi, H. Ethyl pyruvate improves white matter remodeling in rats after traumatic brain injury. CNS Neurosci. Ther. 2021, 27, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Attenello, F.J.; Reid, P.; Wen, T.; Cen, S.; Kim-Tenser, M.; Sanossian, N.; Russin, J.; Amar, A.; Giannotta, S.; Mack, W.J.; et al. Evaluation of time to aneurysm treatment following subarachnoid hemorrhage: Comparison of patients treated with clipping versus coiling. J. Neurointerv. Surg. 2016, 8, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Cruz, N.F. Contributions of glycogen to astrocytic energetics during brain activation. Metab. Brain Dis. 2015, 30, 281–298. [Google Scholar] [CrossRef]
- Wang, K.-C.; Tang, S.-C.; Lee, J.-E.; Jeng, J.-S.; Lai, D.-M.; Huang, S.-J.; Hsieh, S.-T.; Tu, Y.-K. Intrathecal lactate predicting hydrocephalus after aneurysmal subarachnoid hemorrhage. J. Surg. Res. 2015, 199, 523–528. [Google Scholar] [CrossRef]
- Radolf, S.; Smoll, N.; Drenckhahn, C.; Dreier, J.P.; Vajkoczy, P.; Sarrafzadeh, A.S. Cerebral Lactate Correlates with Early Onset Pneumonia after Aneurysmal SAH. Transl. Stroke Res. 2014, 5, 278–285. [Google Scholar] [CrossRef][Green Version]
- Zahra, K.; Gopal, N.; Freeman, W.D.; Turnbull, M.T. Using Cerebral Metabolites to Guide Precision Medicine for Subarachnoid Hemorrhage: Lactate and Pyruvate. Metabolites 2019, 9, 245. [Google Scholar] [CrossRef]
- Gao, X.; Gao, Y.-Y.; Wu, L.-Y.; Peng, Z.; Liu, X.-Z.; Chen, X.-X.; Gao, S.; Zhang, H.-S.; Lu, Y.; Hang, C.-H.; et al. High Expression of PDK4 Could Play a Potentially Protective Role by Attenuating Oxidative Stress after Subarachnoid Hemorrhage. J. Clin. Med. 2022, 11, 3974. [Google Scholar] [CrossRef]
- Lilla, N.; Füllgraf, H.; Stetter, C.; Köhler, S.; Ernestus, R.-I.; Westermaier, T. First Description of Reduced Pyruvate Dehydrogenase Enzyme Activity Following Subarachnoid Hemorrhage (SAH). Front. Neurosci. 2017, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Agoston, D.V.; Shutes-David, A.; Peskind, E.R. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017, 31, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
| Items | Control | SAH (n = 34) | ||||
|---|---|---|---|---|---|---|
| All | p Value | Good Outcome | Poor Outcome | p Value | ||
| Participants (n) | 6 | 34 | - | 26 | 8 | - |
| Age (years) | 56.5 (42.5–66.0) | 59.5 (51.5–66.5) | 0.544 | 59.5 (51.5–66.5) | 59.5 (49.75–66.5) | 0.935 |
| Gender | 0.792 | 0.961 | ||||
| male | 3 (50%) | 13 (38.2%) | 10 (38.5%) | 3 (37.5%) | ||
| female | 3 (50%) | 21 (61.8%) | 16 (61.5%) | 5 (62.5%) | ||
| Hypertension | - | 0.161 | ||||
| <140/90 | - | 14 (41.2%) | 9 (34.6%) | 5 (62.5%) | ||
| ≥140/90 | - | 20 (58.8%) | 17 (65.4%) | 3 (37.5%) | ||
| Aneurysm | - | 0.840 | ||||
| No | - | 5 (14.7%) | 4 (15.4%) | 1 (12.5%) | ||
| Yes | - | 29 (85.3%) | 22 (84.6%) | 7 (87.5%) | ||
| Time to surgery (h) | - | 18.8 (12.0–24.0) | - | 18.3 (12.0–23.3) | 20.0 (14.3–24.5) | 0.046 |
| Initial GCS | - | 0.008 | ||||
| >12 | - | 25 (73.5%) | 22 (84.6%) | 3 (37.5%) | ||
| ≤12 | - | 9 (26.5%) | 4 (15.4%) | 5 (62.5%) | ||
| Initial Hunt-Hess | - | 0.317 | ||||
| 3–4 | - | 16 (47.1%) | 11 (42.3%) | 5 (62.5%) | ||
| 1–2 | - | 18 (52.9%) | 15 (57.7%) | 3 (37.5%) | ||
| CSF (days, after hemorrhage) | - | 0.664 | ||||
| 1 | - | 8 (23.5%) | 5 (19.2%) | 3 (37.5%) | ||
| 2 | - | 14 (41.2%) | 12 (46.2%) | 2 (25%) | ||
| 3 | - | 12 (35.3%) | 9 (34.6%) | 3 (37.5%) | ||
| White cells in CSF (×106) | - | 177.0 (72.0–351.0) | - | 182.5 (76.5–416.0) | 131.5 (25.3–313.3) | 0.118 |
| Red cells in CSF (×106) | - | 83500.0 (29750.0–204000.0) | - | 83500.0 (33250.0–246250.0) | 65000.00 (25250.00–155000.00) | 0.761 |
| PDK4 in CSF (ng/mL) | 3.5 (3.3–3.9) | 7.2 (5.6–8.6) | 0.003 | 7.7 (6.2–8.8) | 5.2 (4.1–6.7) | 0.002 |
| Pyruvate in CSF (mM) | 3.9 (1.9–4.4) | 11.6 (8.6–11.9) | 0.001 | 11.7 (11.4–12.5) | 7.9 (6.3–8.2) | 0.000 |
| GOS (after surgery) | - | 0.213 | ||||
| 5 | - | 15 (44.1%) | 13 (50%) | 2 (25%) | ||
| <5 | - | 19 (55.9%) | 13 (50%) | 6 (75%) | ||
| Parameters | Univariable Logistic Regression | Multivariable Logistic Regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (years) | 0.994 | 0.915–1.081 | 0.894 | |||
| Gender (male/female) | 1.042 | 0.203–5.343 | 0.961 | |||
| Hypertension | 3.148 | 0.608–16.289 | 0.171 | |||
| <140/90 | ||||||
| ≥140/90 | ||||||
| Aneurysm | 0.786 | 0.075–8.243 | 0.841 | |||
| No | ||||||
| Yes | ||||||
| Time to surgery (h) | 0.876 | 0.773–0.992 | 0.037 | 0.795 | 0.646–0.977 | 0.029 |
| Initial GCS | 9.167 | 1.539–54.592 | 0.015 | 2.758 | 0.177–43.106 | 0.469 |
| >12 | ||||||
| ≤12 | ||||||
| Initial Hunt-Hess | 0.44 | 0.086–2.244 | 0.323 | |||
| 3–4 | ||||||
| 1–2 | ||||||
| CSF (days, after hemorrhage) | 1.306 | 0.459–3.713 | 0.617 | |||
| 1 | ||||||
| 2 | ||||||
| 3 | ||||||
| White cells in CSF (×106) | 1.003 | 0.998–1.008 | 0.237 | |||
| Red cells in CSF (×106) | 1 | 1.000–1.000 | 0.507 | |||
| PDK4 in CSF (ng/mL) | 2.998 | 1.254–7.166 | 0.014 | 4.525 | 1.135–18.038 | 0.032 |
| Pyruvate in CSF (mM) | 11462 | 0.000–1.384E12 | 0.325 | |||
| GOS (after surgery) | 3.296 | 0.508–17.708 | 0.035 | |||
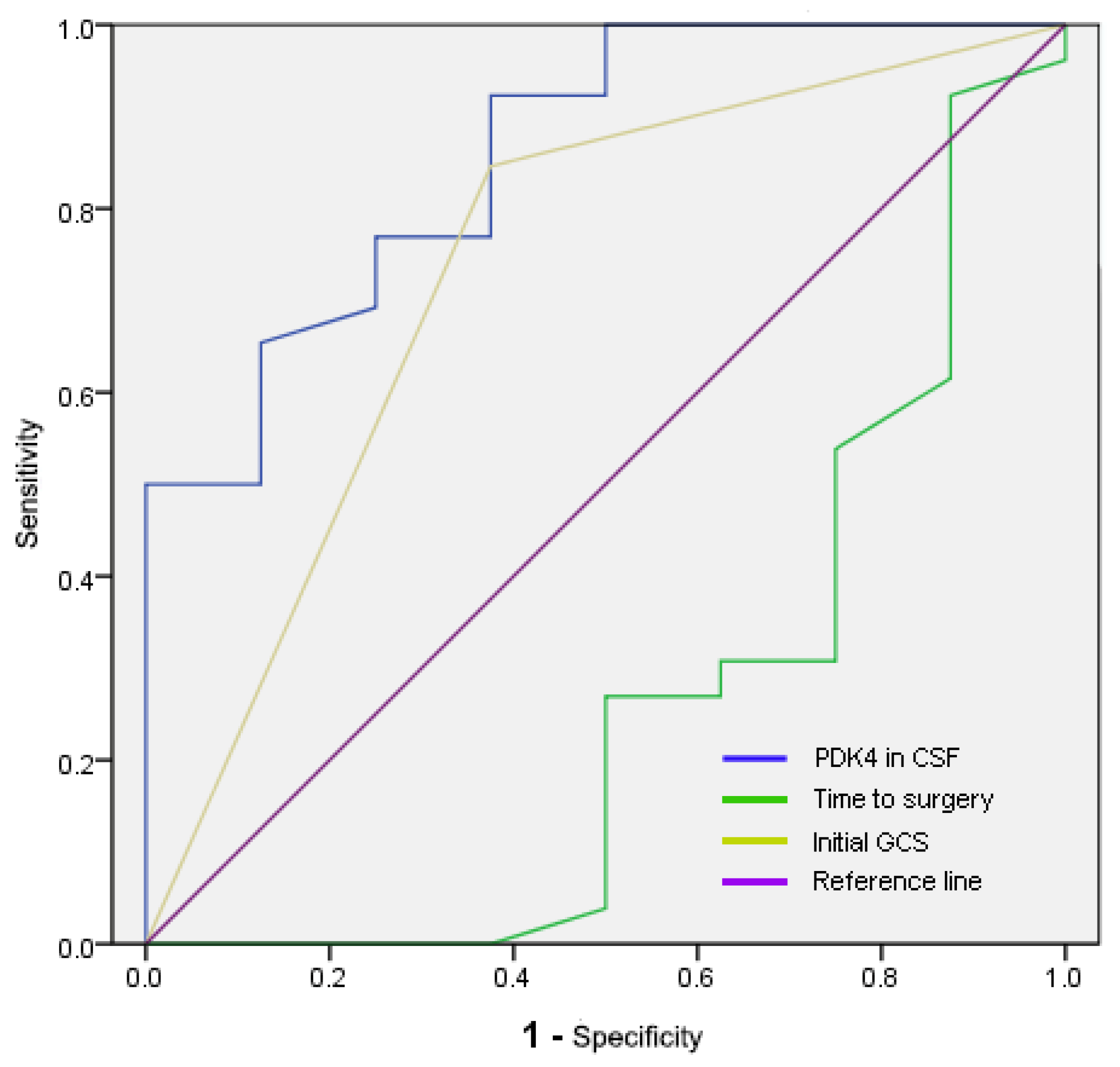
| Parameters | AUC (95% CI) | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|
| PDK4 in CSF (ng/mL) | 0.858 (0.714–1) | 5.54183 | 0.923 | 0.625 |
| Time to surgery (h) | 0.264 (0.03–0.499) | 6.75 | 0.923 | 0.125 |
| Initial GCS | 0.736 (0.517–0.954) | 0.6 | 0.846 | 0.625 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Zhang, H.; Peng, Z.; Zhuang, Z.; Li, W. Elevated Level of Cerebrospinal Fluid Pyruvate Dehydrogenase Kinase 4 Is a Predictive Biomarker of Clinical Outcome after Subarachnoid Hemorrhage. Brain Sci. 2022, 12, 1507. https://doi.org/10.3390/brainsci12111507
Gao X, Zhang H, Peng Z, Zhuang Z, Li W. Elevated Level of Cerebrospinal Fluid Pyruvate Dehydrogenase Kinase 4 Is a Predictive Biomarker of Clinical Outcome after Subarachnoid Hemorrhage. Brain Sciences. 2022; 12(11):1507. https://doi.org/10.3390/brainsci12111507
Chicago/Turabian StyleGao, Xuan, Huasheng Zhang, Zheng Peng, Zong Zhuang, and Wei Li. 2022. "Elevated Level of Cerebrospinal Fluid Pyruvate Dehydrogenase Kinase 4 Is a Predictive Biomarker of Clinical Outcome after Subarachnoid Hemorrhage" Brain Sciences 12, no. 11: 1507. https://doi.org/10.3390/brainsci12111507
APA StyleGao, X., Zhang, H., Peng, Z., Zhuang, Z., & Li, W. (2022). Elevated Level of Cerebrospinal Fluid Pyruvate Dehydrogenase Kinase 4 Is a Predictive Biomarker of Clinical Outcome after Subarachnoid Hemorrhage. Brain Sciences, 12(11), 1507. https://doi.org/10.3390/brainsci12111507





