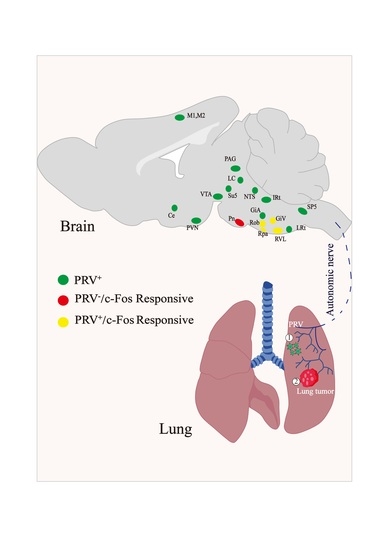
The Rostral Ventromedial and Lateral Medulla Are the Major Areas Responsive to Lung Cancer Progression among Brainstem Lung-Innervating Nuclei
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Lines
2.3. Establishment of the Murine Orthotopic Lung Cancer Model
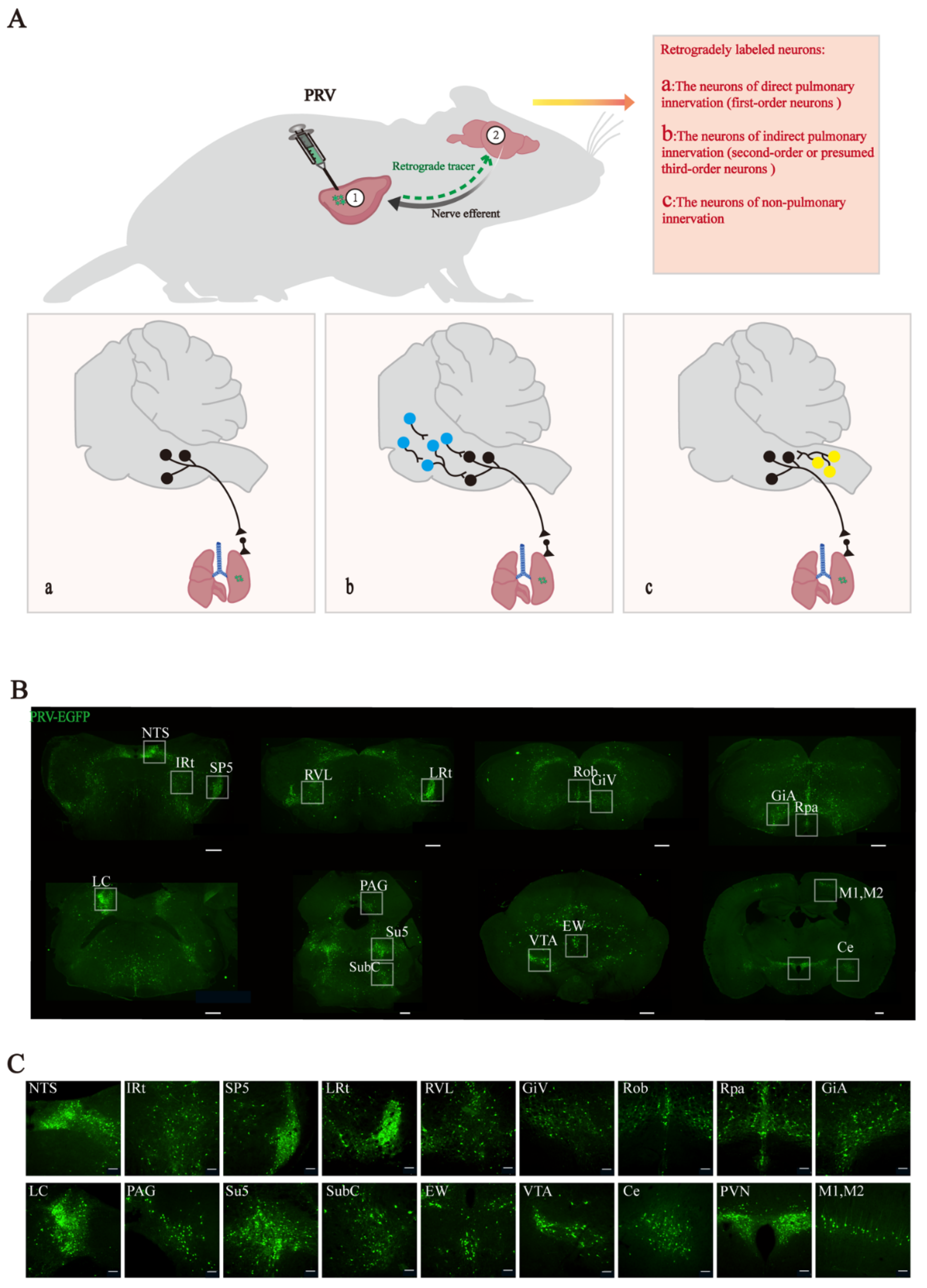
2.4. Pulmonary Orthotopic Injection of Pseudorabies Virus
2.5. Perfusion
2.6. Immunofluorescence Staining
2.7. Data and Statistical Analysis
3. Results
3.1. Retrograde Labeling of Lung-Associated Nuclei by Pseudorabies Virus
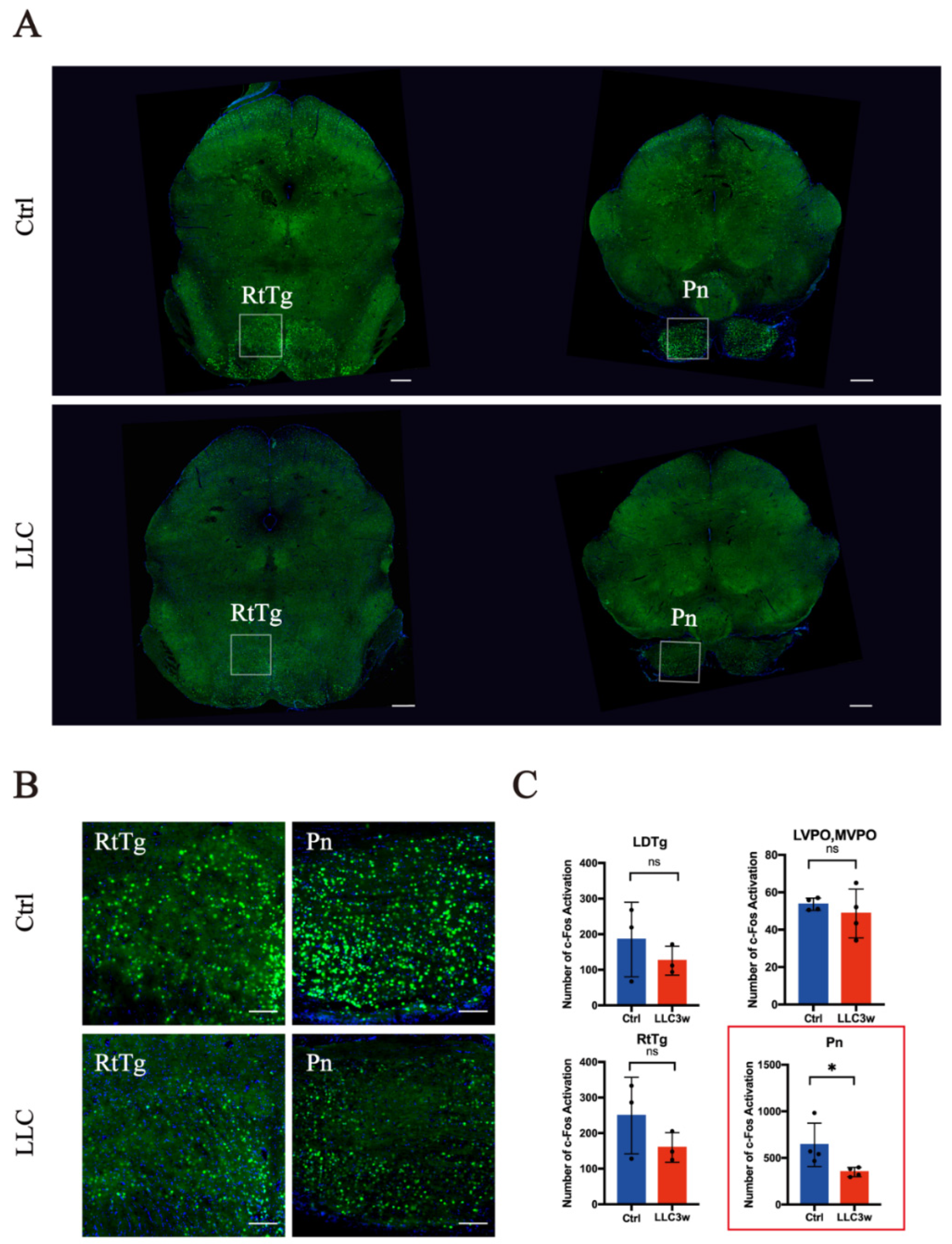
3.2. Activation of c-Fos in Lung-Associated Nuclei in the Brainstem Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Mao, Y.; Yang, D.; He, J.; Krasna, M.J. Epidemiology of lung cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shu, Q.; Fan, J. Neural regulation of interactions between group 2 innate lymphoid cells and pulmonary immune cells. Front. Immunol. 2020, 11, 576929. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Lai, D.; Cui, P.; Zhou, R.; Chen, Q.; Hou, J.; Su, Y.; Pan, L.; Ye, H.; Zhao, J.W.; et al. Selective Activation of Basal Forebrain Cholinergic Neurons Attenuates Polymicrobial Sepsis-Induced Inflammation via the Cholinergic Anti-Inflammatory Pathway. Crit. Care Med. 2017, 45, e1075–e1082. [Google Scholar] [CrossRef] [PubMed]
- Kalia, M. Brain stem localization of vagal preganglionic neurons. J. Auton. Nerv. Syst. 1981, 3, 451–481. [Google Scholar] [CrossRef]
- Haxhiu, M.A.; Jansen, A.S.; Cherniack, N.S.; Loewy, A.D. CNS innervation of airway-related parasympathetic preganglionic neurons: A transneuronal labeling study using pseudorabies virus. Brain Res. 1993, 618, 115–134. [Google Scholar] [CrossRef]
- Hadziefendic, S.; Haxhiu, M.A. CNS innervation of vagal preganglionic neurons controlling peripheral airways- a transneuronal labeling study using pseudorabies virus. J. Auton. Nerv. Syst. 1999, 76, 135–145. [Google Scholar] [CrossRef]
- Carr, M.J.; Undem, B.J. Bronchopulmonary afferent nerves. Respirology 2003, 8, 291–301. [Google Scholar] [CrossRef]
- Mazzone, S.B.; Undem, B.J. Vagal Afferent Innervation of the Airways in Health and Disease. Physiol. Rev. 2016, 96, 975–1024. [Google Scholar] [CrossRef]
- Su, X.; Matthay, M.A.; Malik, A.B. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis-induced acute lung inflammatory injury. J. Immunol. 2010, 184, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Fujii, T.; Moriwaki, Y.; Misawa, H.; Horiguchi, K. Non-neuronal cholinergic system in regulation of immune function with a focus on alpha7 nAChRs. Int. Immunopharmacol. 2015, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Halim, T.Y.; Krauss, R.H.; Sun, A.C.; Takei, F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012, 36, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.D.; Puybasset, L. The brain-lung-brain axis. Intensive Care Med. 2011, 37, 1054–1056. [Google Scholar] [CrossRef]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678.e623. [Google Scholar] [CrossRef]
- Hosang, L.; Canals, R.C.; van der Flier, F.J.; Hollensteiner, J.; Daniel, R.; Flugel, A.; Odoardi, F. The lung microbiome regulates brain autoimmunity. Nature 2022, 603, 138–144. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Lin, F.; Li, W.; Wang, P.; Liao, G.; Zhang, L. Functional Two-Way Crosstalk Between Brain and Lung: The Brain-Lung Axis. Cell. Mol. Neurobiol. 2022, 9, 1–13. [Google Scholar] [CrossRef]
- Belvisi, M.G. Overview of the innervation of the lung. Curr. Opin. Pharm. 2002, 2, 211–215. [Google Scholar] [CrossRef]
- Tanaka, M.; Toldi, J.; Vecsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef]
- Tanaka, M.; Vecsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry”. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shaanan, T.L.; Schiller, M.; Azulay-Debby, H.; Korin, B.; Boshnak, N.; Koren, T.; Krot, M.; Shakya, J.; Rahat, M.A.; Hakim, F.; et al. Modulation of anti-tumor immunity by the brain’s reward system. Nat. Commun. 2018, 9, 2723. [Google Scholar] [CrossRef] [PubMed]
- Mattia, M.; Spadacenta, S.; Pavone, L.; Quarato, P.; Esposito, V.; Sparano, A.; Sebastiano, F.; Di Gennaro, G.; Morace, R.; Cantore, G.; et al. Stop-event-related potentials from intracranial electrodes reveal a key role of premotor and motor cortices in stopping ongoing movements. Front. Neuroeng. 2012, 5, 12. [Google Scholar] [CrossRef]
- Battaglia, S.; Cardellicchio, P.; Di Fazio, C.; Nazzi, C.; Fracasso, A.; Borgomaneri, S. Stopping in (e)motion: Reactive action inhibition when facing valence-independent emotional stimuli. Front. Behav. Neurosci. 2022, 16, 998714. [Google Scholar] [CrossRef]
- Hanoun, M.; Maryanovich, M.; Arnal-Estape, A.; Frenette, P.S. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron 2015, 86, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Lv, X.; Zhao, L.; Wang, X. VLM catecholaminergic neurons control tumor growth by regulating CD8(+) T cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2103505118. [Google Scholar] [CrossRef]
- Chang, R.B.; Strochlic, D.E.; Williams, E.K.; Umans, B.D.; Liberles, S.D. Vagal sensory neuron subtypes that differentially control breathing. Cell 2015, 161, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Abdala, A.P.; Borgmann, A.; Rybak, I.A.; Paton, J.F. Brainstem respiratory networks: Building blocks and microcircuits. Trends Neurosci. 2013, 36, 152–162. [Google Scholar] [CrossRef]
- Travagli, R.A.; Hermann, G.E.; Browning, K.N.; Rogers, R.C. Brainstem circuits regulating gastric function. Annu. Rev. Physiol. 2006, 68, 279–305. [Google Scholar] [CrossRef]
- Guan, Y.; Guo, W.; Zou, S.-P.; Dubner, R.; Ren, K. Inflammation-induced upregulation of AMPA receptor subunit expression in brain stem pain modulatory circuitry. Pain 2003, 104, 401–413. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Wouterlood, F.G. Neuroanatomical tract-tracing techniques that did go viral. Brain Struct. Funct. 2020, 225, 1193–1224. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.G.; Robertson, H.A. Activation of c-fos in the brain. Prog. Neurobiol. 1996, 50, 83–107. [Google Scholar] [CrossRef]
- Weiss, I.D.; Ella, E.; Dominsky, O.; Smith, Y.; Abraham, M.; Wald, H.; Shlomai, Z.; Zamir, G.; Feigelson, S.W.; Shezen, E.; et al. In the hunt for therapeutic targets: Mimicking the growth, metastasis, and stromal associations of early-stage lung cancer using a novel orthotopic animal model. J. Thorac. Oncol. 2015, 10, 46–58. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Lin, F.; Song, T.; Zhu, X.; Lei, H. Mapping accumulative whole-brain activities during environmental enrichment with manganese-enhanced magnetic resonance imaging. Neuroimage 2020, 210, 116588. [Google Scholar] [CrossRef] [PubMed]
- Luyck, K.; Scheyltjens, I.; Nuttin, B.; Arckens, L.; Luyten, L. c-Fos expression following context conditioning and deep brain stimulation in the bed nucleus of the stria terminalis in rats. Sci. Rep. 2020, 10, 20529. [Google Scholar] [CrossRef] [PubMed]
- Madden, C.J.; Sved, A.F. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell. Mol. Neurobiol. 2003, 23, 739–749. [Google Scholar] [CrossRef]
- Dampney, R.A. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef]
- da Silva, G.S.; Giusti, H.; Benedetti, M.; Dias, M.B.; Gargaglioni, L.H.; Branco, L.G.; Glass, M.L. Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflug. Arch. 2011, 462, 407–418. [Google Scholar] [CrossRef]
- Holtman, J.R., Jr.; Anastasi, N.C.; Norman, W.P.; Dretchen, K.L. Effect of electrical and chemical stimulation of the raphe obscurus on phrenic nerve activity in the cat. Brain Res. 1986, 362, 214–220. [Google Scholar] [CrossRef]
- Bou-Flores, C.; Lajard, A.M.; Monteau, R.; De Maeyer, E.; Seif, I.; Lanoir, J.; Hilaire, G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: Possible role of a serotonin excess. J. Neurosci. 2000, 20, 4646–4656. [Google Scholar] [CrossRef]
- Cao, Y.; Fujito, Y.; Matsuyama, K.; Aoki, M. Effects of electrical stimulation of the medullary raphe nuclei on respiratory movement in rats. J. Comp. Physiol. A 2006, 192, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.; Stornetta, R.L.; Stornetta, D.S.; Abbott, S.B.G.; Guyenet, P.G. Differential contribution of the retrotrapezoid nucleus and C1 neurons to active expiration and arousal in rats. J. Neurosci. 2020, 40, 8683–8697. [Google Scholar] [CrossRef] [PubMed]
- Malheiros-Lima, M.R.; Silva, J.N.; Souza, F.C.; Takakura, A.C.; Moreira, T.S. C1 neurons are part of the circuitry that recruits active expiration in response to the activation of peripheral chemoreceptors. Elife 2020, 9, e52572. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Nattie, E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J. Physiol. 2006, 570, 385–396. [Google Scholar] [CrossRef]
- Kuo, S.J.; Hwa, Y.; Chai, C.Y. Cardio-inhibitory mechanism in the gigantocellular reticular nucleus of the medulla oblongata. Brain Res. 1979, 178, 221–232. [Google Scholar] [CrossRef]
- Achari, N.K.; Downman, C.B.; Weber, W.V. A cardio-inhibitory pathway in the brain stem of the cat. J. Physiol. 1968, 197, 35P. [Google Scholar]
- Zhao, Z.; Wang, L.; Gao, W.; Hu, F.; Zhang, J.; Ren, Y.; Lin, R.; Feng, Q.; Cheng, M.; Ju, D.; et al. A central catecholaminergic circuit controls blood glucose levels during stress. Neuron 2017, 95, 138–152.e135. [Google Scholar] [CrossRef]
- Abe, C.; Inoue, T.; Inglis, M.A.; Viar, K.E.; Huang, L.; Ye, H.; Rosin, D.L.; Stornetta, R.L.; Okusa, M.D.; Guyenet, P.G. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 2017, 20, 700–707. [Google Scholar] [CrossRef]
- Madden, C.J.; Stocker, S.D.; Sved, A.F. Attenuation of homeostatic responses to hypotension and glucoprivation after destruction of catecholaminergic rostral ventrolateral medulla neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R751–R759. [Google Scholar] [CrossRef]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Marmol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Furness, J.B.; Rivera, L.R.; Cho, H.J.; Bravo, D.M.; Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 729–740. [Google Scholar] [CrossRef]
- Nagao, S. Pontine nuclei-mediated cerebello-cerebral interactions and its functional role. Cerebellum 2004, 3, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.I.; Tsukahara, N. Cerebrocerebellar communication systems. Physiol. Rev. 1974, 54, 957–1006. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.E. Subcortical projections to the pontine nuclei in the cat. J. Comp. Neurol. 1989, 282, 331–354. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Guo, L.; Cheng, X.; Guo, N.; Shi, M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J. Pathol. 2018, 244, 49–60. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Romeo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Andersson, U.; Tracey, K.J. Reflex principles of immunological homeostasis. Annu. Rev. Immunol. 2012, 30, 313–335. [Google Scholar] [CrossRef]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.; Foster, S.L.; Woolf, C.J. Neuroimmunity: Physiology and Pathology. Annu. Rev. Immunol. 2016, 34, 421–447. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Tracey, K.J. Essential Neuroscience in Immunology. J. Immunol. 2017, 198, 3389–3397. [Google Scholar] [CrossRef] [PubMed]
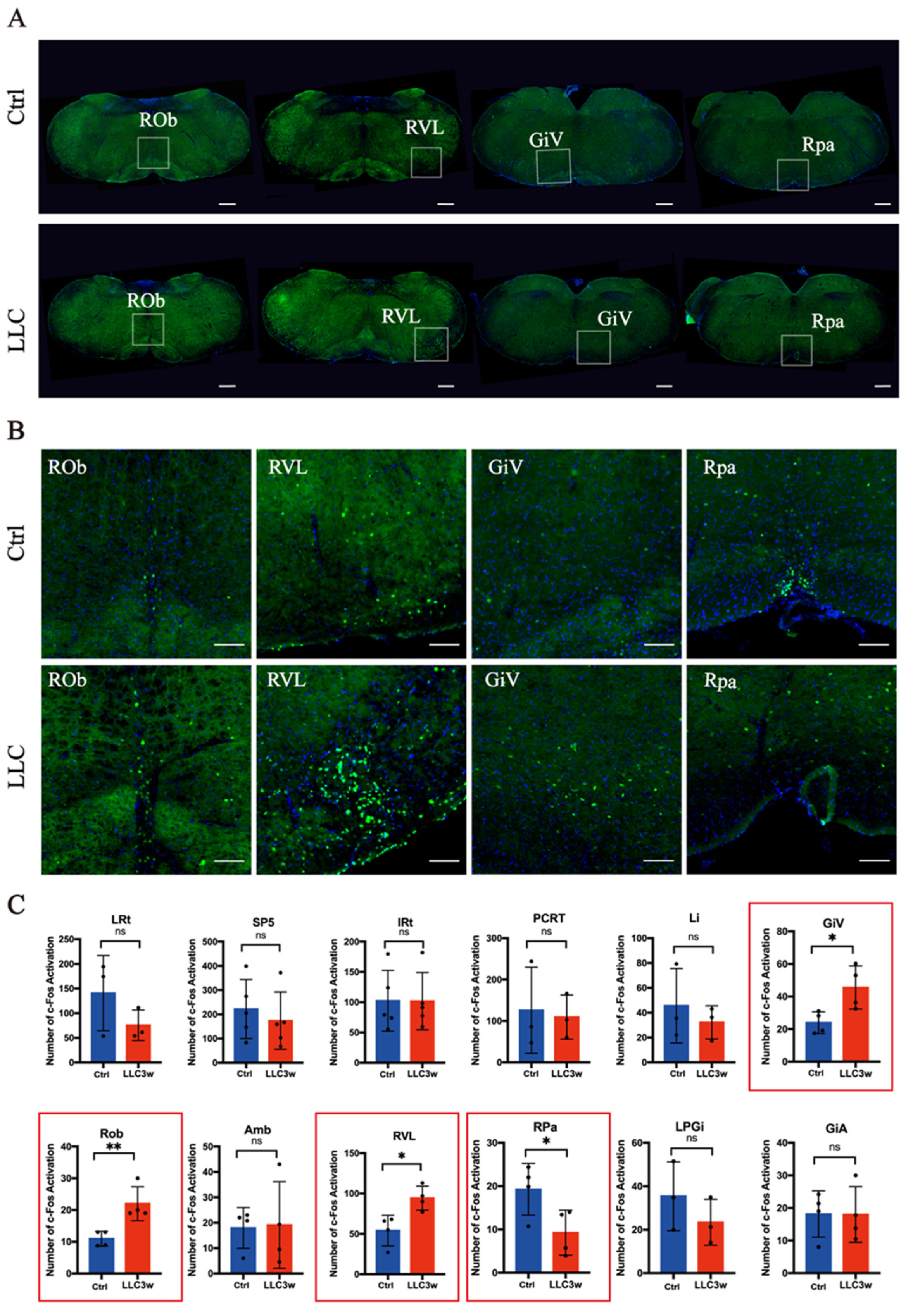

| LRt | SP5 | IRt | PCRT | Li | GiV | ROb | Amb | RVL | RPa | |
| Number of mice | 3 | 5 | 5 | 3 | 3 | 4 | 4 | 4 | 4 | 4 |
| p value | 0.2414 | 0.5443 | 0.9815 | 0.8220 | 0.5186 | 0.0271 | 0.0092 | 0.9060 | 0.0156 | 0.0450 |
| LPGi | GiA | LDTg | LVPO, MVPO | RtTg | Pn | DR | PL | PAG | EW | |
| Number of mice | 3 | 4 | 3 | 4 | 3 | 4 | 3 | 3 | 4 | 3 |
| p value | 0.3363 | 0.9806 | 0.4133 | 0.4963 | 0.2493 | 0.0492 | 0.2872 | 0.4515 | 0.2123 | 0.6226 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Jiao, Y.; Shi, Y.; Xu, S.; Tang, D.; Chen, S.; Gao, P.; Zhang, X.; Zhao, X.; Cai, M.; et al. The Rostral Ventromedial and Lateral Medulla Are the Major Areas Responsive to Lung Cancer Progression among Brainstem Lung-Innervating Nuclei. Brain Sci. 2022, 12, 1486. https://doi.org/10.3390/brainsci12111486
Chen M, Jiao Y, Shi Y, Xu S, Tang D, Chen S, Gao P, Zhang X, Zhao X, Cai M, et al. The Rostral Ventromedial and Lateral Medulla Are the Major Areas Responsive to Lung Cancer Progression among Brainstem Lung-Innervating Nuclei. Brain Sciences. 2022; 12(11):1486. https://doi.org/10.3390/brainsci12111486
Chicago/Turabian StyleChen, Mo, Yingfu Jiao, Yumiao Shi, Saihong Xu, Dan Tang, Sihan Chen, Po Gao, Xindi Zhang, Xiaojing Zhao, Mengmeng Cai, and et al. 2022. "The Rostral Ventromedial and Lateral Medulla Are the Major Areas Responsive to Lung Cancer Progression among Brainstem Lung-Innervating Nuclei" Brain Sciences 12, no. 11: 1486. https://doi.org/10.3390/brainsci12111486
APA StyleChen, M., Jiao, Y., Shi, Y., Xu, S., Tang, D., Chen, S., Gao, P., Zhang, X., Zhao, X., Cai, M., Yu, W., & Xie, K. (2022). The Rostral Ventromedial and Lateral Medulla Are the Major Areas Responsive to Lung Cancer Progression among Brainstem Lung-Innervating Nuclei. Brain Sciences, 12(11), 1486. https://doi.org/10.3390/brainsci12111486