The Role of Folate Deficiency as a Potential Risk Factor for Nontraumatic Anterior Spinal Artery Syndrome in an Adolescent Girl
Abstract
1. Introduction
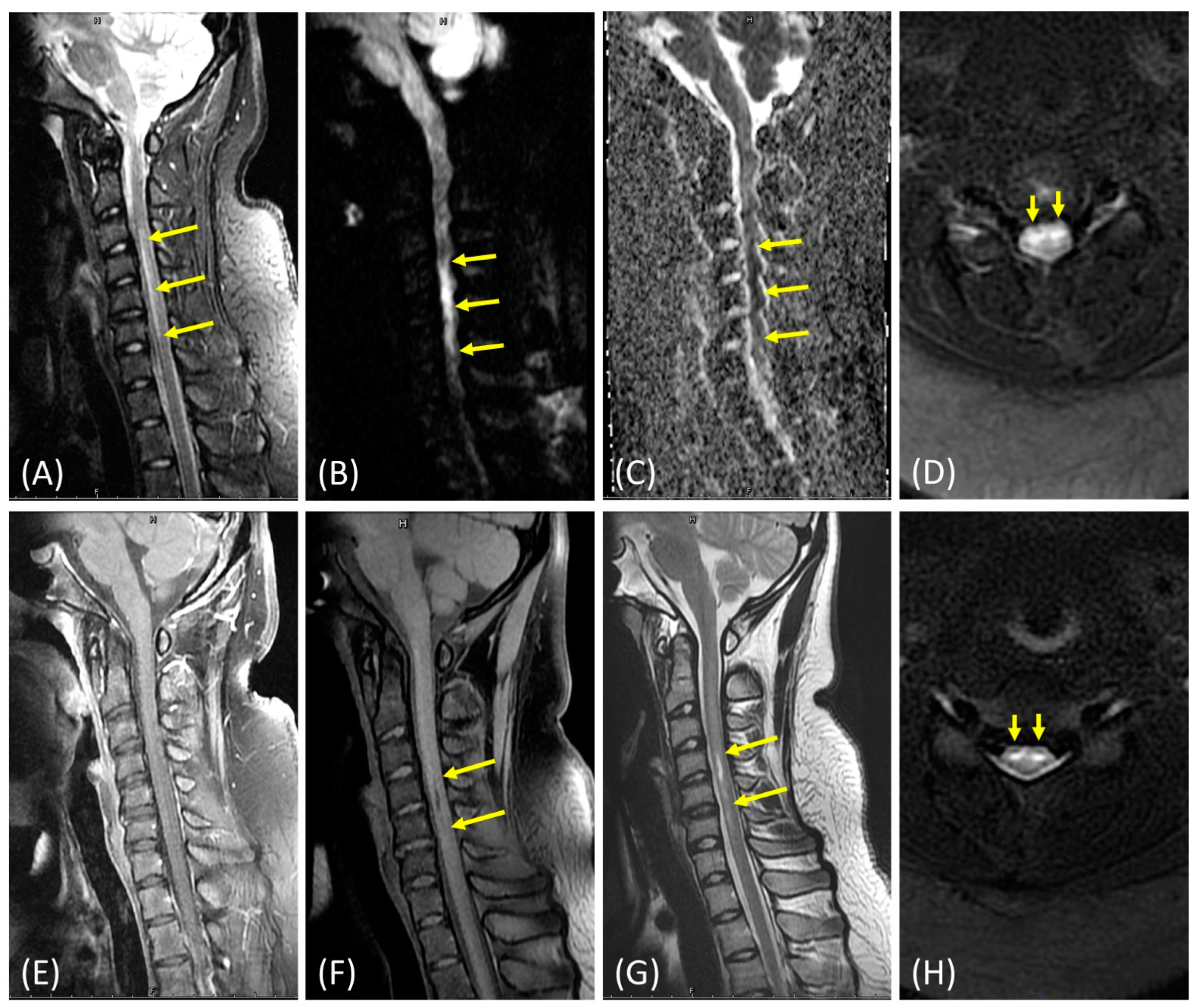
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, W.; Zhang, W.; Ma, G.; Ma, G.; He, J. Long-segment spinal cord infarction complicated with multiple cerebral infarctions: A case report. BMC Neurol. 2022, 22, 362. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhang, Z.F. Anterior Spinal Artery Syndrome in a Patient with Cervical Spondylosis Demonstrated by CT An-giography. Orthop Surg 2019, 11, 1220–1223. [Google Scholar] [CrossRef]
- Ergun, A.; Oder, W. Pediatric care report of spinal cord injury without radiographic abnormality (SCIWORA): Case report and literature review. Spinal Cord 2003, 41, 249–253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nance, J.R.; Golomb, M.R. Ischemic Spinal Cord Infarction in Children Without Vertebral Fracture. Pediatr. Neurol. 2007, 36, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Tykocki, T.; Poniatowski, Ł.A.; Czyz, M.; Wynne-Jones, G. Oblique corpectomy in the cervical spine. Spinal Cord 2018, 56, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kubaszewski, Ł.; Wojdasiewicz, P.; Rożek, M.; Słowińska, I.E.; Romanowska-Prochnicka, K.; Słowiński, R.; Poniatowski, Ł.A.; Gasik, R. Syndromes with chronic non-bacterial osteomyelitis in the spine. Reumatologia 2015, 53, 328–336. [Google Scholar] [CrossRef]
- Spencer, S.P.; Brock, T.D.; Matthews, R.R.; Stevens, W.K. Three Unique Presentations of Atraumatic Spinal Cord Infarction in the Pediatric Emergency Department. Pediatr. Emerg. Care 2014, 30, 354–357. [Google Scholar] [CrossRef]
- Lee, M.J.; Aronberg, R.; Manganaro, M.S.; Ibrahim, M.; Parmar, H.A. Diagnostic Approach to Intrinsic Abnormality of Spinal Cord Signal Intensity. RadioGraphics 2019, 39, 1824–1839. [Google Scholar] [CrossRef]
- Beslow, L.A.; Ichord, R.N.; Zimmerman, R.A.; Smith, S.E.; Licht, D.J. Role of Diffusion MRI in Diagnosis of Spinal Cord Infarction in Children. Neuropediatrics 2008, 39, 188–191. [Google Scholar] [CrossRef]
- Hsu, J.L.; Cheng, M.-Y.; Liao, M.-F.; Hsu, H.-C.; Weng, Y.-C.; Chang, K.-H.; Chang, H.-S.; Kuo, H.-C.; Huang, C.-C.; Lyu, R.-K.; et al. A comparison between spinal cord infarction and neuromyelitis optica spectrum disorders: Clinical and MRI studies. Sci. Rep. 2019, 9, 7435. [Google Scholar] [CrossRef]
- Boucher, A.A.; Taylor, J.M.; Luchtman-Jones, L. Aspirin in childhood acute ischemic stroke: The evidence for treatment and efficacy testing. Pediatr. Blood Cancer 2019, 66, e27665. [Google Scholar] [CrossRef] [PubMed]
- Golja, M.V.; Šmid, A.; Kuželički, N.K.; Trontelj, J.; Geršak, K.; Mlinarič-Raščan, I. Folate Insufficiency Due to MTHFR Deficiency Is Bypassed by 5-Methyltetrahydrofolate. J. Clin. Med. 2020, 9, 2836. [Google Scholar] [CrossRef]
- Poddar, R. Hyperhomocysteinemia is an emerging comorbidity in ischemic stroke. Exp. Neurol. 2021, 336, 113541. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.-C.; Yeh, W.-T.; Bai, C.-H.; Chen, H.-J.; Chuang, S.-Y.; Chang, H.-Y.; Lin, B.-F.; Chen, K.-J.; Pan, W.-H. Is Ischemic Stroke Risk Related to Folate Status or Other Nutrients Correlated With Folate Intake? Stroke 2008, 39, 3152–3158. [Google Scholar] [CrossRef] [PubMed]
- Bar, C.; Cheuret, E.; Bessou, P.; Pedespan, J.-M. Childhood idiopathic spinal cord infarction: Description of 7 cases and review of the literature. Brain Dev. 2017, 39, 818–827. [Google Scholar] [CrossRef]
- Zalewski, N.L.; Rabinstein, A.A.; Krecke, K.N.; Brown, R.D.; Wijdicks, E.F.M.; Weinshenker, B.G.; Kaufmann, T.J.; Morris, J.M.; Aksamit, A.J.; Bartleson, J.D.; et al. Characteristics of Spontaneous Spinal Cord Infarction and Proposed Diagnostic Criteria. JAMA Neurol. 2019, 76, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Nedeltchev, K.; Loher, T.J.; Stepper, F.; Arnold, M.; Schroth, G.; Mattle, H.P.; Sturzenegger, M. Long-Term Outcome of Acute Spinal Cord Ischemia Syndrome. Stroke 2004, 35, 560–565. [Google Scholar] [CrossRef]
- Sawada, D.; Ito, A.; Shiohama, T.; Tsukada, H.; Fujii, K. Spontaneous spinal cord infarction in a 10-year-old Japanese girl. Pediatr. Int. 2022, 64, e14909. [Google Scholar] [CrossRef]
- Seo, Z.W.; Huh, S.; Ko, H.-Y. Non-traumatic spinal cord infarction of the conus medullaris in a child: A case report. Spinal Cord Ser. Cases 2021, 7, 59. [Google Scholar] [CrossRef]
- Stettler, S.; El-Koussy, M.; Ritter, B.; Boltshauser, E.; Jeannet, P.-Y.; Kolditz, P.; Meyer-Heim, A.; Steinlin, M. Non-traumatic spinal cord ischaemia in childhood-clinical manifestation, neuroimaging and outcome. Eur. J. Paediatr. Neurol. 2013, 17, 176–184. [Google Scholar] [CrossRef]
- Márquez, J.C.; Granados, A.M.; Castillo, M. MRI of cervical spinal cord infarction in a patient with sickle cell disease. Clin. Imaging 2012, 36, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Sohal, A.S.; Sundaram, M.; Mallewa, M.; Tawil, M.; Kneen, R. Anterior Spinal Artery Syndrome in a Girl With Down Syndrome: Case Report and Literature Review. J. Spinal Cord Med. 2009, 32, 349–353. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hakimi, K.N.; Massagli, T.L. Anterior Spinal Artery Syndrome in Two Children With Genetic Thrombotic Disorders. J. Spinal Cord Med. 2005, 28, 69–73. [Google Scholar] [CrossRef]
- Ramelli, G.P.; Wyttenbach, R.; Giovanni, O.S.; Von Der Weid, N.; Ozdoba, C. Anterior Spinal Artery Syndrome in an Adolescent With Protein S Deficiency. J. Child Neurol. 2001, 16, 134–135. [Google Scholar] [CrossRef]
- Wilmshurst, J.; Walker, M.; E Pohl, K.R. Rapid onset transverse myelitis in adolescence: Implications for pathogenesis and prognosis. Arch. Dis. Child. 1999, 80, 137–142. [Google Scholar] [CrossRef][Green Version]
- Yousef, O.M.; Appenzeller, P.; Kornfeld, M. Fibrocartilagenous Embolism: An Unusual Cause of Spinal Cord Infarction. Am. J. Forensic Med. Pathol. 1998, 19, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Tosi, L.; Rigoli, G.; Beltramello, A. Fibrocartilaginous embolism of the spinal cord: A clinical and pathogenetic reconsidera-tion. J. Neurol. Neurosurg. Psychiatry 1996, 60, 55–60. [Google Scholar] [CrossRef]
- Toro, G.; Roman, G.C.; Navarro-Roman, L.; Cantillo, J.; Serrano, B.; Vergara, I. Natural History of Spinal Cord Infarction Caused by Nucleus Pulposus Embolism. Spine 1994, 19, 360–366. [Google Scholar] [CrossRef]
- Vandertop, W.P.; Elderson, A.; van Gijn, J.; Valk, J. Anterior spinal artery syndrome. AJNR Am. J. Neuroradiol. 1991, 12, 505–506. [Google Scholar]
| Category | Modified RS | S.E. | BH: 1.62 m | Evaluations | Prescriptions | Location | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Table | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 0-100 | BW (kg) | BMI (kg/m2) | Laboratory Study | Image/Electrophysiologic Study | Pulse Therapy | A | B | F | L | P.I | P.O | P | I | W | H | S | |
| Week 1 | 20 | 100 | 38.1 | D1:Young stroke panel (B12, folate, HC) D1:Lumbar puncture | D1: Brain CTA/CTV D2: Brain/Spine MRI, NCV/EMG, echocardiography | D2~D6 | TID | TID | QD | QD | QD | ||||||||||||||
| Week 2 | 50 | 96.2 | 36.66 | B12, folate, HC | TID | TID | 1/2 QD | QD | QD | ||||||||||||||||
| * Week 3 | 62 | 93.5 | 35.63 | D21: Carotid artery sonography | BID | TID | 1/4 QD | QD | |||||||||||||||||
| Week 4 | 71 | 87.4 | 33.3 | QD | TID | QD | |||||||||||||||||||
| Week 5 | 75 | 89.9 | 34.26 | D29: SSEP U/L limbs | QD | TID | QD | ||||||||||||||||||
| * Week 6 | 84 | 89.4 | 34.06 | QD | TID | QD | |||||||||||||||||||
| * Week 9 | NA | NA | NA | QD | TID | QD | |||||||||||||||||||
| Week 10 | 88 | 86.9 | 33.11 | QD | BID | ||||||||||||||||||||
| Week 16 | NA | NA | NA | D96: Spine MRI follow-up | QD | BID | QD | ||||||||||||||||||
| Week 20 | 90 | 93 | 35.44 | B12, folate, HC | D120: NCV/EMG follow-up | QD | QD | QD | |||||||||||||||||
| Week 24 | 92 | 90.5 | 34.48 | QD | QD | 1/4 QD | |||||||||||||||||||
| Study/ Year | No. case | Age/ Gender | Underlying diseases | Clinical Manifestations | Radiological lesions | Potential Etiology | Outcome at the latest FU (mRS) | ||
|---|---|---|---|---|---|---|---|---|---|
| Sensory impairment | Motor impairment | Bladder/Bowel dysfunction | |||||||
| Our Case 2022 | 1 | 14Y/F | Obesity BMI:38.1kg/m2 | Dysesthesia, temp., pain | Paresis: RU, LL | +/+ | C3─C6 | Low serum folate level | 1/6 |
| Sawada [17] 2022 | 1 | 10Y/F | Nil | Temp., pain | Paraparesis | +/− | C7─T2 | Unknown | 1/6 |
| Seo [18] 2021 | 1 | 12Y/F | Nil | Nil | Paraparesis | +/+ | T12─CM | Unknown | 1/6 |
| Bar [15] 2017 | 1 | 15Y/F | Asthma | Nil | Plegia: RL Paresis: LL | +/+ | T11─L1 | Unknown | 3/6 |
| 2 | 14Y/F | Nil | Temp., pain | Paraplegia | +/− | T7─T11 | Unknown | 1/6 | |
| 3 | 13Y/F | Nil | All modalities | Paraplegia | +/+ | T8─T12 | Unknown | 3/6 | |
| 4 | 13Y/F | Nil | Nil | Paraplegia | +/+ | CM | Unknown | 3/6 | |
| 5 | 14Y/F | Early puberty | Temp., pain, LT | Plegia: LL Paresis: RL | +/− | CM | Unknown | 2/6 | |
| Spencer [7] 2014 | 1 | 12Y/F | Nil | All modalities | Paraplegia | +/+ | T1─CM | Unknown | 3/6 |
| 2 | 14Y/M | Nil | Temp., pain | Paraplegia | +/− | T7─T12 | Unknown | 1/6 | |
| Stettler [19] 2013 | 1 | 13Y/F | S/P spinal surgery | Dysesthesia, temp., pain | Plegia: RL Paresis: LL | +/+ | T7─T9 | MTHFR, Ho, c.677C>T | 1/6 |
| 2 | 13Y/F | Nil | Dysesthesia, temp., pain | Plegia: RL Paresis: LL | +/+ | T3─T5 | Unknown | 1/6 | |
| Márquez [20] 2012 | 1 | 19Y/M | Sickle cell disease | Nil | Quadriparesis | +/− | C2─C7 | Hemoglobi-nopathy | 2/6 |
| Sohal [21] 2009 | 1 | 16Y/F | Down syndrome | Not applicable1 | Paraplegia | +/− | T5─T12 | Unknown | 2/6 |
| Nance [4] 2007 | 1 | 14Y/F | Nil | Dysesthesia, pain | Quadriplegia | +/+ | Low medulla, C1─C7, T3 | Unknown | 5/6 |
| 2 | 17Y/M | Back pain, palpitations | All modalities | Paraparesis | −/− | C2, T5─T9 | MTHFR, CH, c.677C>T and 1298A>C | 2/6 | |
| Hakimi [22] 2005 | 1 | 15Y/M | Nil | Pain, LT | Paraplegia | +/+ | Midthoracic level─CM | Prothrombin variant, He | 3/6 |
| 2 | 12Y/F | S/P spinal surgery | Temp., pain, PC | Paraplegia | +/+ | Not performed2 | Protein S deficiency | 3/6 | |
| Ramelli [23] 2001 | 1 | 15Y/M | Nil | Temp., pain | Paraplegia | +/+ | T5─T6 | Protein S deficiency | 3/6 |
| Wilmshur st [24] 1999 | 1 | 14Y/F | Nil | All modalities | Paraplegia | +/− | T9─CM | Unknown | 4/6 |
| 2 | 15Y/F | Nil | Temp., pain, PC | Paraplegia | +/− | Anterior+Right posterior cord | Unknown | 3/6 | |
| 3 | 14Y/M | Nil | Dysesthesia, temp., pain | Paraplegia | +/− | Anterior distal thoracic cord | Unknown | 3/6 | |
| 4 | 16Y/M | Nil | Pain | Paraparesis | +/− | T3─T6 | Unknown | 3/6 | |
| 5 | 15Y/F | Nil | Temp., pain | Paraplegia | +/+ | T9─CM | Unknown | 4/6 | |
| Yousef [25] 1998 | 1 | 14Y/F | Obesity BMI:33.6kg/m2 | Pain, LT | Paraplegia | +/− | Unspecified | FCE | 6/63 |
| Tosi [26] 1996 | 1 | 16Y/F | Nil | Temp., pain, LT, PC | Paraplegia | +/− | T11-L1 | FCE | No description |
| Toro [27] 1994 | 1 | 16Y/F | Nil | All modalities | Paraplegia | +/− | Not performed | FCE | 6/64 |
| Vandertop [28] 1991 | 1 | 17Y/M | Nil | Dysesthesia, temp., pain | Quadriparesis | +/− | C1-T3 | Unknown | 2/6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.-C.; Yang, Y.-Y.; Luxton, G.W.G.; Lin, Y.-P.; Hung, K.-S.; Hu, C.-F. The Role of Folate Deficiency as a Potential Risk Factor for Nontraumatic Anterior Spinal Artery Syndrome in an Adolescent Girl. Brain Sci. 2022, 12, 1470. https://doi.org/10.3390/brainsci12111470
Hu C-C, Yang Y-Y, Luxton GWG, Lin Y-P, Hung K-S, Hu C-F. The Role of Folate Deficiency as a Potential Risk Factor for Nontraumatic Anterior Spinal Artery Syndrome in an Adolescent Girl. Brain Sciences. 2022; 12(11):1470. https://doi.org/10.3390/brainsci12111470
Chicago/Turabian StyleHu, Chun-Chieh, Yung-Yu Yang, G. W. Gant Luxton, Yu-Pang Lin, Kuo-Sheng Hung, and Chih-Fen Hu. 2022. "The Role of Folate Deficiency as a Potential Risk Factor for Nontraumatic Anterior Spinal Artery Syndrome in an Adolescent Girl" Brain Sciences 12, no. 11: 1470. https://doi.org/10.3390/brainsci12111470
APA StyleHu, C.-C., Yang, Y.-Y., Luxton, G. W. G., Lin, Y.-P., Hung, K.-S., & Hu, C.-F. (2022). The Role of Folate Deficiency as a Potential Risk Factor for Nontraumatic Anterior Spinal Artery Syndrome in an Adolescent Girl. Brain Sciences, 12(11), 1470. https://doi.org/10.3390/brainsci12111470









