Confirmation Bias in the Course of Instructed Reinforcement Learning in Schizophrenia-Spectrum Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Clinical Assessment
2.2.2. General Neuropsychological Assessment
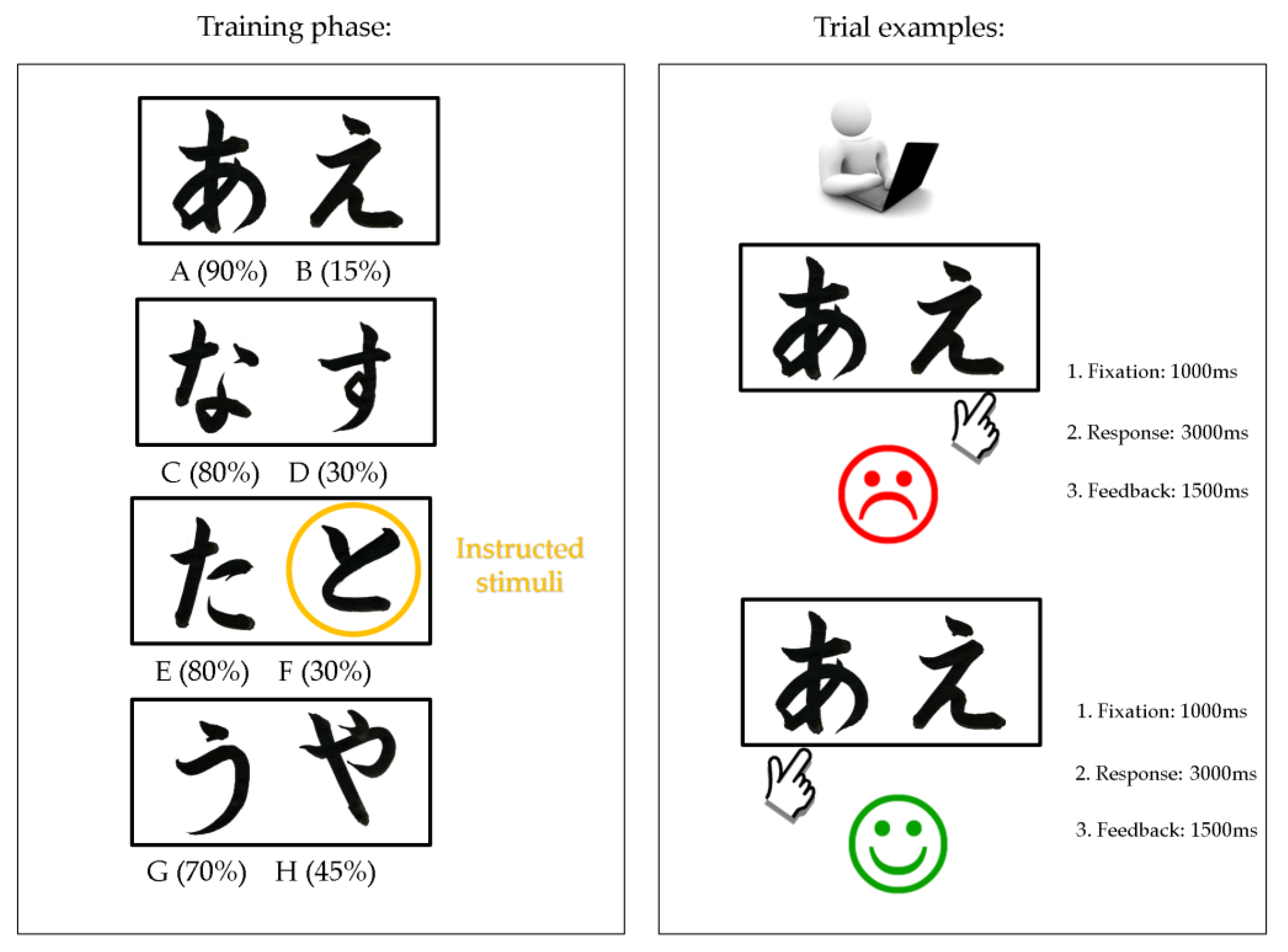
2.2.3. The Instructed Probabilistic Selection Task (IPST)
“Two black symbols will appear simultaneously on the computer screen. One symbol will be ‘correct’ and the other will be ‘incorrect’, but at first you will not know which is which. Try to guess the correct figure as quickly and accurately as possible. There is no absolute right answer, but some symbols will have a higher chance of being correct than others. Try to pick the symbol you find to have the highest chance of being correct. This symbol has the highest probability of being correct [symbol F is being shown]. You will have to figure out which of the other symbols you should select by trying them out. Now you will be tested on these instructions to make sure you have understood them fully”.
“It is time to test what you have learned. During this set of trials, you will not receive feedback (‘correct’ or ‘incorrect’) to your responses. If you see a new combination of symbols in the test, please choose the symbol that ‘feels’ more correct based on what you learned during the training sessions”.
2.3. Statistics
3. Results
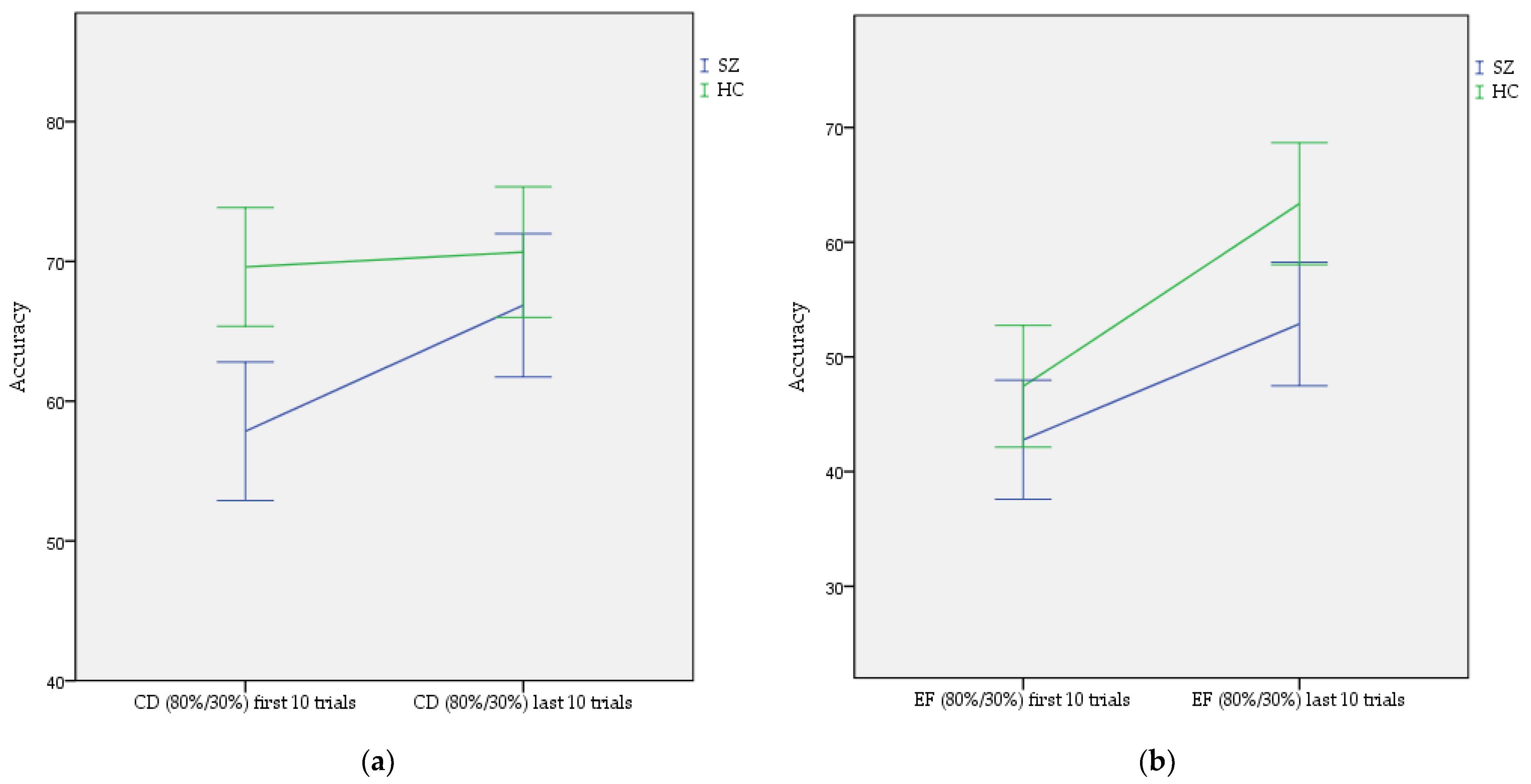
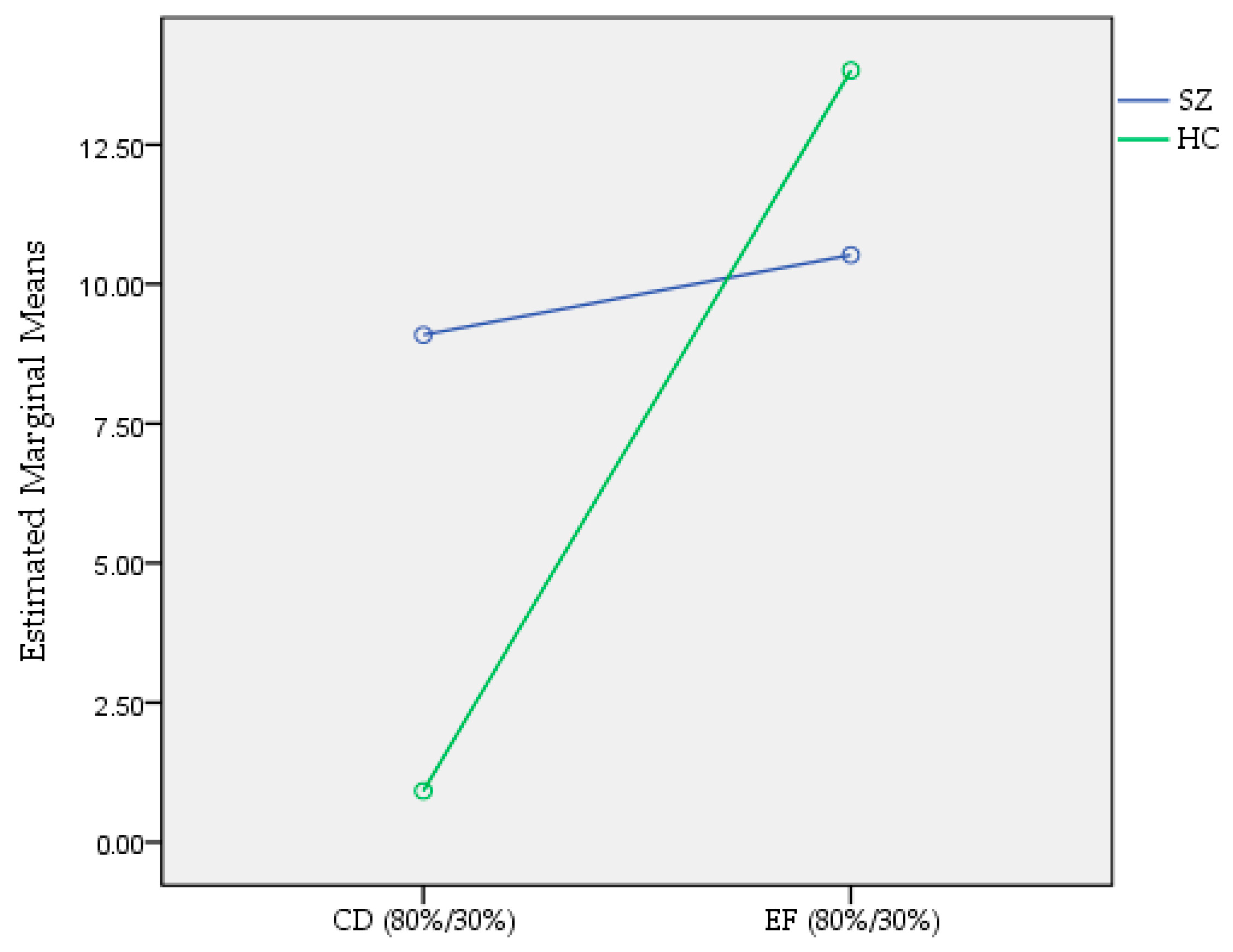
3.1. Reinforcement Learning in Instructed Probabilistic Task (IPST)
3.2. The Effect of Instruction in the Instructed Probabilistic Task (IPST)
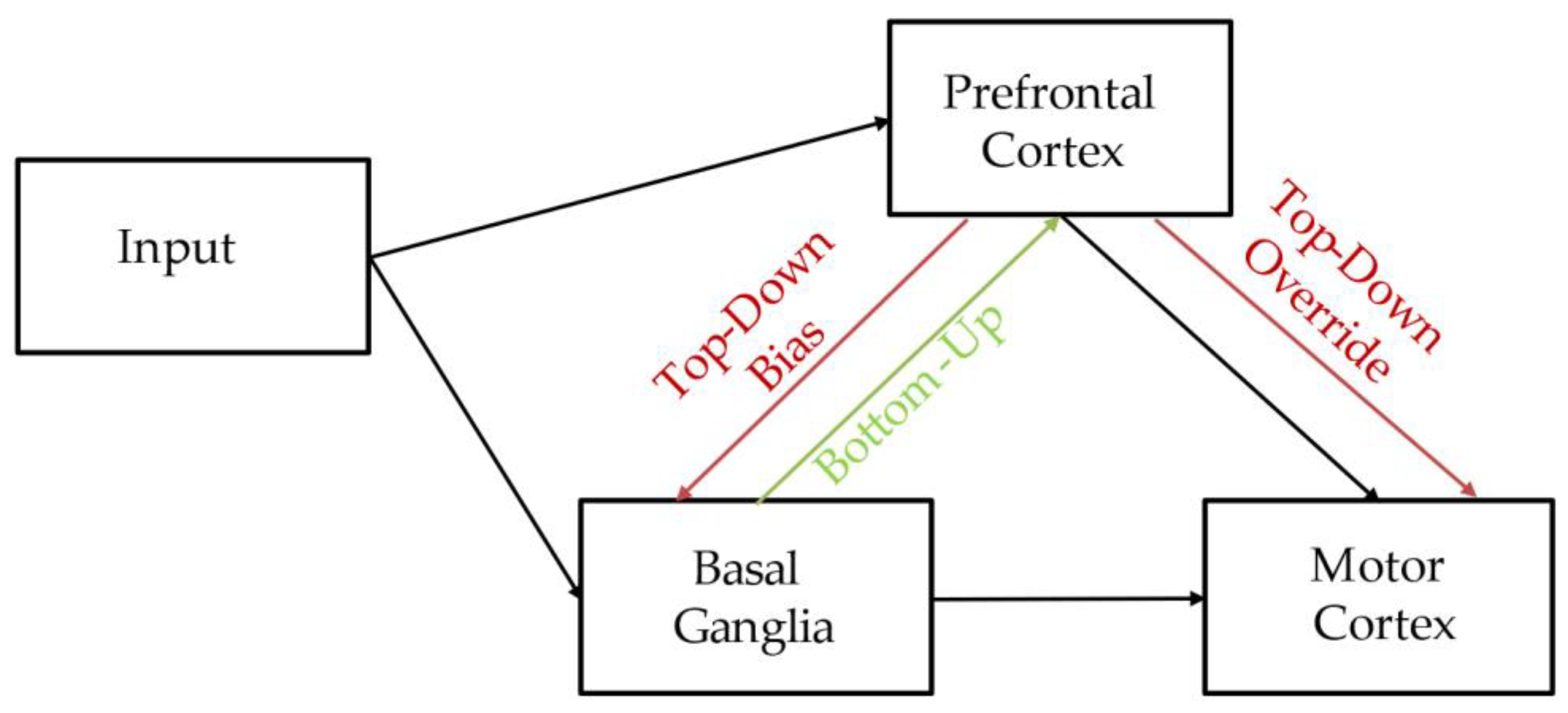
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahneman, D. A perspective on judgment and choice: Mapping bounded rationality. Am. Psychol. 2003, 58, 697–720. [Google Scholar] [CrossRef] [PubMed]
- Filoteo, J.V.; Maddox, W.T.; Simmons, A.N.; Ing, A.D.; Cagigas, X.E.; Matthews, S.; Paulus, M.P. Cortical and subcortical brain regions involved in rule-based category learning. Neuroreport 2005, 16, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Nomura, E.; Maddox, W.; Filoteo, J.; Ing, A.; Gitelman, D.; Parrish, T.; Mesulam, M.-M.; Reber, P. Neural Correlates of Rule-Based and Information-Integration Visual Category Learning. Cereb. Cortex 2006, 17, 37–43. [Google Scholar] [CrossRef]
- Bunge, S.A.; Kahn, I.; Wallis, J.; Miller, E.; Wagner, A.D. Neural Circuits Subserving the Retrieval and Maintenance of Abstract Rules. J. Neurophysiol. 2003, 90, 3419–3428. [Google Scholar] [CrossRef]
- O’Doherty, J.; Kringelbach, M.; Rolls, E.; Hornak, J.; Andrews, C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001, 4, 95–102. [Google Scholar] [CrossRef]
- Frank, M.J.; Moustafa, A.; Haughey, H.M.; Curran, T.; Hutchison, K.E. Genetic triple dissociation reveals multiple roles for dopamine in reinforcement learning. Proc. Natl. Acad. Sci. USA 2007, 104, 16311–16316. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci. 2007, 30, 259–288. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, V.D.; Eichele, T.; Pearlson, G.D. Functional brain networks in schizophrenia: A review. Front. Hum. Neurosci. 2009, 3, 17. [Google Scholar] [CrossRef]
- Wolf, D.H.; Gur, R.C.; Valdez, J.N.; Loughead, J.; Elliott, M.A.; Gur, R.E.; Ragland, J.D. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. Neuroimaging 2007, 154, 221–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.I.; Manoach, D.S.; Mathalon, D.; Turner, J.; Mannell, M.; Brown, G.G.; Ford, J.M.; Gollub, R.L.; White, T.; Wible, C.; et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum. Brain Mapp. 2009, 30, 3795–3811. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Chica, M.; Rogers, B.; Damon, S.M.; Landman, B.A.; Woodward, N.D. Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia. Biol. Psychiatry 2017, 83, 509–517. [Google Scholar] [CrossRef]
- Kim, J.-J.; Seok, J.-H.; Park, H.-J.; Lee, D.S.; Lee, M.C.; Kwon, J.S. Functional disconnection of the semantic networks in schizophrenia. NeuroReport 2005, 16, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Diaconescu, A.O.; Jensen, J.; Wang, H.; Willeit, M.; Menon, M.; Kapur, S.; McIntosh, A.R. Aberrant Effective Connectivity in Schizophrenia Patients during Appetitive Conditioning. Front. Hum. Neurosci. 2011, 4, 239. [Google Scholar] [CrossRef] [PubMed]
- Schlagenhauf, F.; Sterzer, P.; Schmack, K.; Ballmaier, M.; Rapp, M.; Wrase, J.; Juckel, G.; Gallinat, J.; Heinz, A. Reward Feedback Alterations in Unmedicated Schizophrenia Patients: Relevance for Delusions. Biol. Psychiatry 2009, 65, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Del Fabro, L.; Schmidt, A.; Fortea, L.; Delvecchio, G.; D’Agostino, A.; Radua, J.; Borgwardt, S.; Brambilla, P. Functional brain network dysfunctions in subjects at high-risk for psychosis: A meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2021, 128, 90–101. [Google Scholar] [CrossRef]
- Barch, D.M.; Dowd, E.C. Goal Representations and Motivational Drive in Schizophrenia: The Role of Prefrontal-Striatal Interactions. Schizophr. Bull. 2010, 36, 919–934. [Google Scholar] [CrossRef]
- Kasanova, Z.; Ceccarini, J.; Frank, M.J.; Van Amelsvoort, T.; Booij, J.; Heinzel, A.; Mottaghy, F.M.; Myin-Germeys, I. Daily-life stress differentially impacts ventral striatal dopaminergic modulation of reward processing in first-degree relatives of individuals with psychosis. Eur. Neuropsychopharmacol. 2018, 28, 1314–1324. [Google Scholar] [CrossRef]
- Jocham, G.; Klein, T.A.; Ullsperger, M. Dopamine-Mediated Reinforcement Learning Signals in the Striatum and Ventromedial Prefrontal Cortex Underlie Value-Based Choices. J. Neurosci. 2011, 31, 1606–1613. [Google Scholar] [CrossRef]
- Reinen, J.M.; Whitton, A.E.; Pizzagalli, D.A.; Slifstein, M.; Abi-Dargham, A.; McGrath, P.J.; Iosifescu, D.V.; Schneier, F.R. Differential reinforcement learning responses to positive and negative information in unmedicated individuals with depression. Eur. Neuropsychopharmacol. 2021, 53, 89–100. [Google Scholar] [CrossRef]
- Doll, B.B.; Jacobs, W.J.; Sanfey, A.G.; Frank, M.J. Instructional control of reinforcement learning: A behavioral and neurocomputational investigation. Brain Res. 2009, 1299, 74–94. [Google Scholar] [CrossRef]
- Doll, B.B.; Hutchison, K.E.; Frank, M.J. Dopaminergic Genes Predict Individual Differences in Susceptibility to Confirmation Bias. J. Neurosci. 2011, 31, 6188–6198. [Google Scholar] [CrossRef]
- Doll, B.B.; Waltz, J.A.; Cockburn, J.; Brown, J.K.; Frank, M.J.; Gold, J.M. Reduced susceptibility to confirmation bias in schizophrenia. Cogn. Affect. Behav. Neurosci. 2014, 14, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, M.R.; Büchel, C. How initial confirmatory experience potentiates the detrimental influence of bad advice. NeuroImage 2013, 76, 125–133. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.P.; Dayan, P.; Friston, K.; Critchley, H.; Dolan, R. Temporal Difference Models and Reward-Related Learning in the Human Brain. Neuron 2003, 38, 329–337. [Google Scholar] [CrossRef]
- Schultz, W. Book Review: Reward Signaling by Dopamine Neurons. Neuroscientist 2001, 7, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Biele, G.; Rieskamp, J.; Krugel, L.K.; Heekeren, H. The neural basis of following advice. PLoS Biol. 2011, 9, e1001089. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Li, J.; Delgado, M.R.; Phelps, E.A. How instructed knowledge modulates the neural systems of reward learning. Proc. Natl. Acad. Sci. USA 2010, 108, 55–60. [Google Scholar] [CrossRef]
- Fouragnan, E.; Chierchia, G.; Greiner, S.; Neveu, R.; Avesani, P.; Coricelli, G. Reputational Priors Magnify Striatal Responses to Violations of Trust. J. Neurosci. 2013, 33, 3602–3611. [Google Scholar] [CrossRef]
- Waltz, J.A.; Frank, M.J.; Wiecki, T.V.; Gold, J.M. Altered probabilistic learning and response biases in schizophrenia: Behavioral evidence and neurocomputational modeling. Neuropsychology 2011, 25, 86–97. [Google Scholar] [CrossRef]
- Waltz, J.A.; Gold, J.M. Probabilistic reversal learning impairments in schizophrenia: Further evidence of orbitofrontal dysfunction. Schizophr. Res. 2007, 93, 296–303. [Google Scholar] [CrossRef]
- Strauss, G.P.; Frank, M.J.; Waltz, J.A.; Kasanova, Z.; Herbener, E.S.; Gold, J.M. Deficits in Positive Reinforcement Learning and Uncertainty-Driven Exploration Are Associated with Distinct Aspects of Negative Symptoms in Schizophrenia. Biol. Psychiatry 2011, 69, 424–431. [Google Scholar] [CrossRef]
- Waltz, J.A.; Frank, M.J.; Robinson, B.M.; Gold, J.M. Selective Reinforcement Learning Deficits in Schizophrenia Support Predictions from Computational Models of Striatal-Cortical Dysfunction. Biol. Psychiatry 2007, 62, 756–764. [Google Scholar] [CrossRef]
- McGuffin, P.; Farner, A.; Harvey, I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch. Gen. Psychiatry 1991, 48, 764–770. [Google Scholar] [CrossRef]
- Andreasen, N.C. Negative v Positive Schizophrenia. Arch. Gen. Psychiatry 1982, 39, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Åsberg, M. A New Depression Scale Designed to be Sensitive to Change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.W. Chlorpromazine Equivalent Doses for the Newer Atypical Antipsychotics. J. Clin. Psychiatry 2003, 64, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Schutte, I.; Slagter, H.; Collins, A.G.E.; Frank, M.J.; Kenemans, J.L. Stimulus discriminability may bias value-based probabilistic learning. PLoS ONE 2017, 12, e0176205. [Google Scholar] [CrossRef] [PubMed]
- Tardiff, N.; Graves, K.N.; Thompson-Schill, S.L. The Role of Frontostriatal Systems in Instructed Reinforcement Learning: Evidence from Genetic and Experimentally-Induced Variation. Front. Hum. Neurosci. 2018, 12, 472. [Google Scholar] [CrossRef]
- Gold, J.M.; Waltz, J.A.; Matveeva, T.M.; Kasanova, Z.; Strauss, G.P.; Herbener, E.S.; Collins, A.G.E.; Frank, M.J. Negative symptoms and the failure to represent the expected reward value of actions: Behavioral and computational modeling evidence. Arch. Gen. Psychiatry 2012, 69, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Cicero, D.C.; Martin, E.A.; Becker, T.M.; Kerns, J.G. Reinforcement learning deficits in people with schizophrenia persist after extended trials. Psychiatry Res. 2014, 220, 760–764. [Google Scholar] [CrossRef][Green Version]
- Strauss, G.P.; Thaler, N.S.; Matveeva, T.M.; Vogel, S.J.; Sutton, G.P.; Lee, B.G.; Allen, D.N. Predicting psychosis across diagnostic boundaries: Behavioral and computational modeling evidence for impaired reinforcement learning in schizophrenia and bipolar disorder with a history of psychosis. J. Abnorm. Psychol. 2015, 124, 697–708. [Google Scholar] [CrossRef]
- Kapur, S. Psychosis as a state of aberrant salience: A framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psychiatry 2003, 160, 13–23. [Google Scholar] [CrossRef]
- Waltz, J.A.; Gold, J.M. Motivational Deficits in Schizophrenia and the Representation of Expected Value. Curr. Top. Behav. Neurosci. 2016, 27, 375–410. [Google Scholar] [CrossRef]
- Riceberg, J.S.; Shapiro, M.L. Reward Stability Determines the Contribution of Orbitofrontal Cortex to Adaptive Behavior. J. Neurosci. 2012, 32, 16402–16409. [Google Scholar] [CrossRef]
- Schiffer, A.-M.; Siletti, K.; Waszak, F.; Yeung, N. Adaptive behaviour and feedback processing integrate experience and instruction in reinforcement learning. NeuroImage 2016, 146, 626–641. [Google Scholar] [CrossRef]
- Walsh, M.M.; Anderson, J.R. Modulation of the feedback-related negativity by instruction and experience. Proc. Natl. Acad. Sci. USA 2011, 108, 19048–19053. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.P.; Waltz, J.A.; Gold, J.M. A Review of Reward Processing and Motivational Impairment in Schizophrenia. Schizophr. Bull. 2013, 40, S107–S116. [Google Scholar] [CrossRef]
- Kéri, S.; Kelemen, O.; Szekeres, G.; Bagóczky, N.; Erdélyi, R.; Antal, A.; Benedek, G.; Janka, Z. Schizophrenics know more than they can tell: Probabilistic classification learning in schizophrenia. Psychol. Med. 2000, 30, 149–155. [Google Scholar] [CrossRef]
- Dominey, P.F.; Georgieff, N. Schizophrenics learn surface but not abstract structure in a serial reaction time task. NeuroReport 1997, 8, 2877–2882. [Google Scholar] [CrossRef][Green Version]
- Pratt, D.N.; Barch, D.M.; Carter, C.S.; Gold, J.M.; Ragland, J.D.; Silverstein, S.M.; MacDonald, A.W. Reliability and Replicability of Implicit and Explicit Reinforcement Learning Paradigms in People with Psychotic Disorders. Schizophr. Bull. 2020, 47, 731–739. [Google Scholar] [CrossRef]
- Knowlton, B.J.; Mangels, J.A.; Squire, L.R. A Neostriatal Habit Learning System in Humans. Science 1996, 273, 1399–1402. [Google Scholar] [CrossRef]
- Reber, P.J.; Knowlton, B.J.; Squire, L.R. Dissociable properties of memory systems: Differences in the flexibility of declarative and nondeclarative knowledge. Behav. Neurosci. 1996, 110, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Gras-Vincendon, A.; Danion, J.-M.; Grangé, D.; Bilik, M.; Willard-Schroeder, D.; Sichel, J.-P.; Singer, L. Explicit memory, repetition priming and cognitive skill learning in schizophrenia. Schizophr. Res. 1994, 13, 117–126. [Google Scholar] [CrossRef]
- Clare, L.; McKenna, P.; Mortimer, A.; Baddeley, A. Memory in schizophrenia: What is impaired and what is preserved? Neuropsychologia 1993, 31, 1225–1241. [Google Scholar] [CrossRef]
- Vita, A.; Barlati, S.; Ceraso, A.; Nibbio, G.; Ariu, C.; Deste, G.; Wykes, T. Effectiveness, Core Elements, and Moderators of Response of Cognitive Remediation for Schizophrenia: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2021, 78, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Gold, J.M.; Strauss, G.P.; Waltz, J.A.; Robinson, B.M.; Brown, J.K.; Frank, M.J. Negative Symptoms of Schizophrenia Are Associated with Abnormal Effort-Cost Computations. Biol. Psychiatry 2013, 74, 130–136. [Google Scholar] [CrossRef] [PubMed]

| Category | Variable | SZ | HC | p-Value |
|---|---|---|---|---|
| Demographic information | Age (years) | 38.19 ± 13.81 | 36.80 ± 13.34 | 0.528 1 |
| Sex (M/F) | 5712/45 | 45/75 | 0.005 | |
| Educational level (%) | - | - | <0.001 | |
| Primary | 19.8% | 11.7% | ||
| Vocational | 9.9% | 0.0% | ||
| Secondary | 44% | 48.7% | ||
| Higher | 26.4% | 39.6% | ||
| Neurocognition (mean ± SD) | RBANS—immediate memory | 38.78 ± 11.20 | 50.66 ± 7.08 | <0.001 2 |
| RBANS—visuospatial and constructional | 33.06 ± 6.51 | 37.24 ± 3.27 | <0.001 2 | |
| RBANS—language | 28.55 ± 6.73 | 34.61 ± 6.71 | <0.001 1 | |
| RBANS—attention | 43.99 ± 13.40 | 63.66 ± 14.10 | <0.001 1 | |
| RBANS—delayed memory | 42.38 ± 11.57 | 54.21 ± 5.86 | <0.001 2 | |
| RBANS—total score | 187.25 ± 39.88 | 240.67 ± 29.17 | <0.001 2 | |
| Clinical ratings (mean ± SD) | Age of onset | 24.82 ± 7.42 | - | - |
| Illness duration | 12.13 ± 10.56 | - | - | |
| BPRS | 40.02 ± 10.48 | - | - | |
| PANSS—positive symptoms | 13.34 ± 4.71 | - | - | |
| PANSS—negative symptoms | 21.04 ± 9.60 | - | - | |
| PANSS—general symptoms | 29.35 ± 7.97 | - | - | |
| SANS | 33.46 ± 22.84 | - | - | |
| SAPS | 20.09 ± 20.19 | - | - | |
| MADRS | 8.33 ± 9.00 | - | - | |
| GAF | 47.08 ± 20.54 | - | - | |
| Antipsychotic medication (mean ± SD) | CPZeq | 501.98 ± 340.52 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frydecka, D.; Piotrowski, P.; Bielawski, T.; Pawlak, E.; Kłosińska, E.; Krefft, M.; Al Noaimy, K.; Rymaszewska, J.; Moustafa, A.A.; Drapała, J.; et al. Confirmation Bias in the Course of Instructed Reinforcement Learning in Schizophrenia-Spectrum Disorders. Brain Sci. 2022, 12, 90. https://doi.org/10.3390/brainsci12010090
Frydecka D, Piotrowski P, Bielawski T, Pawlak E, Kłosińska E, Krefft M, Al Noaimy K, Rymaszewska J, Moustafa AA, Drapała J, et al. Confirmation Bias in the Course of Instructed Reinforcement Learning in Schizophrenia-Spectrum Disorders. Brain Sciences. 2022; 12(1):90. https://doi.org/10.3390/brainsci12010090
Chicago/Turabian StyleFrydecka, Dorota, Patryk Piotrowski, Tomasz Bielawski, Edyta Pawlak, Ewa Kłosińska, Maja Krefft, Kamila Al Noaimy, Joanna Rymaszewska, Ahmed A. Moustafa, Jarosław Drapała, and et al. 2022. "Confirmation Bias in the Course of Instructed Reinforcement Learning in Schizophrenia-Spectrum Disorders" Brain Sciences 12, no. 1: 90. https://doi.org/10.3390/brainsci12010090
APA StyleFrydecka, D., Piotrowski, P., Bielawski, T., Pawlak, E., Kłosińska, E., Krefft, M., Al Noaimy, K., Rymaszewska, J., Moustafa, A. A., Drapała, J., & Misiak, B. (2022). Confirmation Bias in the Course of Instructed Reinforcement Learning in Schizophrenia-Spectrum Disorders. Brain Sciences, 12(1), 90. https://doi.org/10.3390/brainsci12010090








