Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications
Abstract
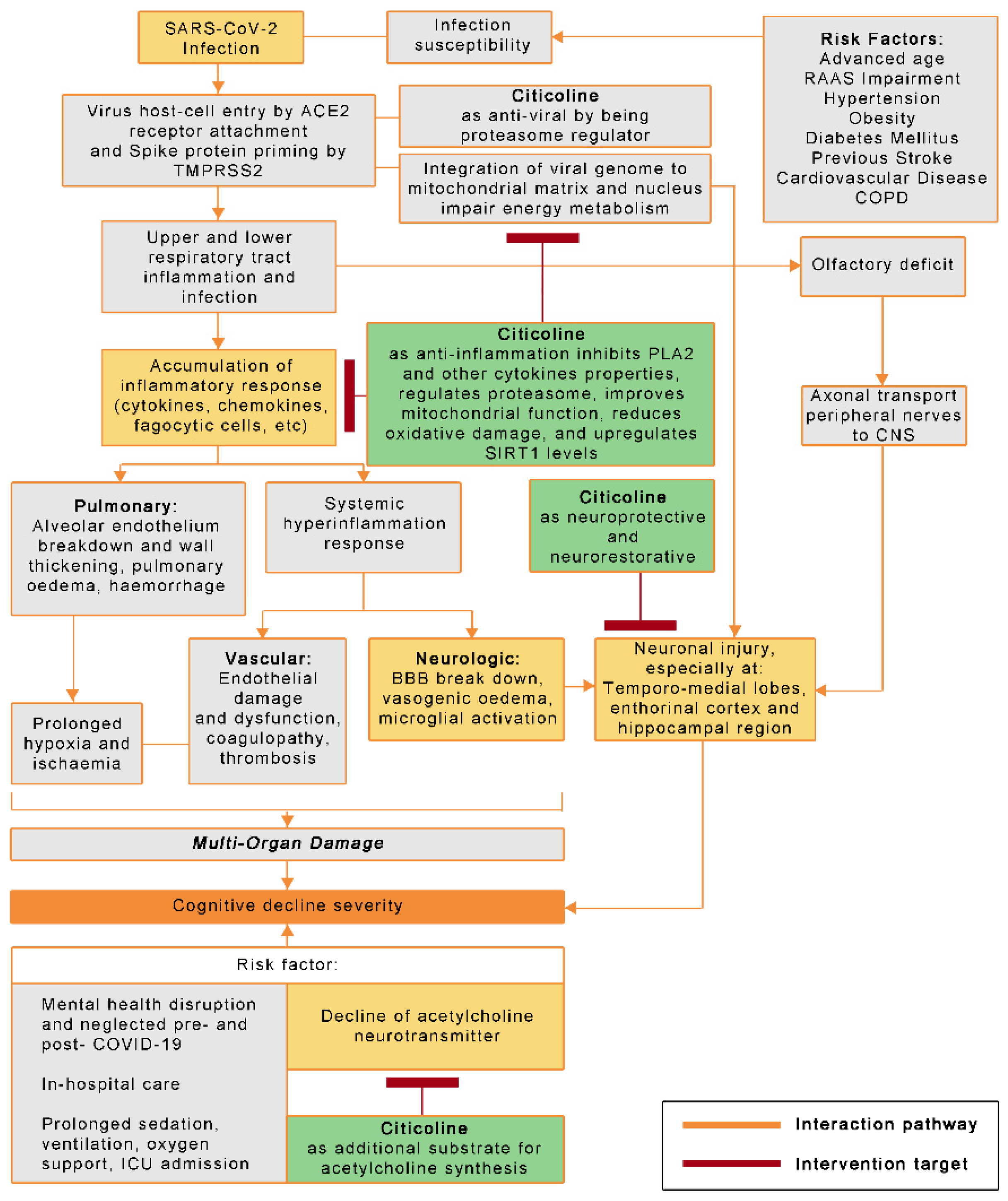
1. Introduction
2. COVID-19 and Cognitive Decline
2.1. SARS-CoV-2 Infection Pathophysiology
2.2. SARS-CoV-2 Infection and Sirtuin 1 (SIRT1)
2.3. SARS-CoV-2 Infection and the Role of Phospholipase A2 Cascade
2.4. SARS-CoV-2 Infection and the Role of Ubiquitin Protease System
2.5. SARS-CoV-2 Infection and Mitochondrial Dysfunction
2.6. SARS-CoV-2 Infection and Neurologic Complications
2.7. Cognitive and Functional Decline in SARS-CoV-2 Infection
2.8. Properties of Citicoline toward Cognitive Function
2.9. Profile of Citicoline
2.10. Citicoline Acts on Protective Mechanism by Increasing SIRT1 Expression
2.11. The Anti-Inflammatory Actions of Citicoline on Phospholipase A2 and Mitochondrial Dysfunction
2.12. The Anti-Inflammatory and Anti-Viral Actions of Citicoline on Ubiquitin Proteasome System
2.13. Effects of Citicoline on the Neurotransmitter System
2.14. Other Citicoline Anti-Inflammatory, Neuroprotective, and Neurorestorative Properties
2.15. The Potential Roles of Citicoline in COVID-19-Related Cognitive Decline and Other Neurologic Complications
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.-C.; Kuo, R.-L.; Shih, S.-R. COVID-19: The first documented coronavirus pandemic in history. Biomed. J. 2020, 43, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Popov, D. COVID-19 Toxicity, Role of phospholipases A2 in the development of Acute Severe Respiratory Distress Syndrome (ASRDS). Available online: https://doi.org/10.13140/RG.2.2.12006.65600 (accessed on 8 November 2021).
- Listings of WHO’s Response to COVID-19 [Internet]. Available online: https://www.who.int/news/item/29-06-2020-covidtimeline (accessed on 22 February 2021).
- Jasarevic, T.; Ghebreyesus, T.A.; Moussa; Ryan, M.; Chen; Van Kerkhove, M.; Helen; Isabelle; Christoph; Katrin; et al. WHO Virtual Press Conference on COVID-19—11 March 2020 [Internet]; World Health Organization (WHO): Geneva, Switzerland, 2020; Available online: https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2 (accessed on 6 March 2021).
- COVID-19 Vaccines [Internet]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed on 22 February 2021).
- Baker, H.A.; Safavynia, S.A.; Evered, L.A. The ‘third wave’: Impending cognitive and functional decline in COVID-19 survivors. Brit. J. Anaesth. 2021, 126, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Miskowiak, K.; Johnsen, S.; Sattler, S.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Canoll, P.D.; Klein, R.S. How COVID-19 Affects the Brain. JAMA Psychiatry 2021, 78, 682. [Google Scholar] [CrossRef]
- Croall, I.D.; Hoggard, N.; Aziz, I.; Hadjivassiliou, M.; Sanders, D.S. Brain fog and non-coeliac gluten sensitivity: Proof of concept brain MRI pilot study. PLoS ONE 2020, 15, e0238283. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Ritchie, K.; Chan, D.; Watermeyer, T. The cognitive consequences of the COVID-19 epidemic: Collateral damage? Brain Commun. 2020, 2, fcaa069. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Clinical Management of COVID-19: Interim Guidance, 27 May 2020 [Internet]. Available online: https://apps.who.int/iris/handle/10665/332196 (accessed on 22 February 2021).
- Jaywant, A.; Vanderlind, W.M.; Alexopoulos, G.S.; Fridman, C.B.; Perlis, R.H.; Gunning, F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology 2021, 46, 2235–2240. [Google Scholar] [CrossRef]
- Weiss, S.B.; Smith, S.W.; Kennedy, E.P. The enzymatic formation of lecithin from cytidine diphosphate choline and d-1,2-diglyceride. J. Biol. Chem. 1958, 231, 53–64. [Google Scholar] [CrossRef]
- Farooqui, A.A.; A Horrocks, L.; Farooqui, T. Glycerophospholipids in brain: Their metabolism, incorporation into membranes, functions, and involvement in neurological disorders. Chem. Phys. Lipids 2000, 106, 1–29. [Google Scholar] [CrossRef]
- Goto, Y.; Okamoto, S.; Yonekawa, Y.; Taki, W.; Kikuchi, H.; Handa, H.; Kito, M. Degradation of phospholipid molecular species during experimental cerebral ischemia in rats. Stroke 1988, 19, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, K.; Uchida, K.; Fujikawa, R.; Nogami, A.; Nakamura, K.; Kawasaki, C.; Yamaguchi, K.; Morita, M.; Morishita, K.; Kubota, K.; et al. Neuroprotective effects of citidine-5-diphosphocholine on impaired spatial memory in a rat model of cerebrovascular dementia. J. Pharmacol. Sci. 2011, 116, 232–237. [Google Scholar] [CrossRef]
- Trimmel, H.; Majdan, M.; Wodak, A.; Herzer, G.; Csomor, D.; Brazinova, A. Citicoline in severe traumatic brain injury: Indications for improved outcome: A retrospective matched pair analysis from 14 Austrian trauma centers. Wien. Klin. Wochenschr. 2018, 130, 37–44. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Go, M.-L.; Wang, D.-Y.; Cheah, I.K.-M.; Halliwell, B. Effects of Antimalarial Drugs on Neuroinflammation-Potential Use for Treatment of COVID-19-Related Neurologic Complications. Mol. Neurobiol. 2021, 58, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Hemalika, D.V.D. Phospholipase enzymes as potential biomarker for SARS CoV-2 virus. Int. J. Sci. Res. Publ. 2020, 11, 189–197. [Google Scholar] [CrossRef]
- Longhitano, L.; Tibullo, D.; Giallongo, C.; Lazzarino, G.; Tartaglia, N.; Galimberti, S.; Li Volti, G.; Palumbo, G.A.; Liso, A. Proteasome Inhibitors as a Possible Therapy for SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 3622. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Ackermann, K.; Stuart, M.; Wex, C.; Protzer, U.; Schatzl, H.; Gilch, S. Severe Acute Respiratory Syndrome Coronavirus Replication Is Severely Impaired by MG132 due to Proteasome-Independent Inhibition of M-Calpain. J. Virol. 2012, 86, 10112–10122. [Google Scholar] [CrossRef] [PubMed]
- Barretto, N.; Jukneliene, D.; Ratia, K.; Chen, Z.; Mesecar, A.; Baker, S.C. The Papain-Like Protease of Severe Acute Respiratory Syndrome Coronavirus Has Deubiquitinating Activity. J. Virol. 2005, 79, 15189–15198. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Farooqui, T.; Kokotos, G.; Farooqui, A.A. Synthetic and Natural Inhibitors of Phospholipases A2: Their Importance for Understanding and Treatment of Neurological Disorders. ACS Chem. Neurosci. 2015, 6, 814–831. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Thanaraj, T.A.; Qaddoumi, M.G.; Hashem, A.; Abubaker, J.; Al-Mulla, F. The Role of Lipid Metabolism in COVID-19 Virus Infection and as a Drug Target. Int. J. Mol. Sci. 2020, 21, 3544. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Ong, W.-Y.; Horrocks, L.A. Inhibitors of Brain Phospholipase A2 Activity: Their Neuropharmacological Effects and Therapeutic Importance for the Treatment of Neurologic Disorders. Pharmacol. Rev. 2006, 58, 591–620. [Google Scholar] [CrossRef]
- Müller, C.; Hardt, M.; Schwudke, D.; Neuman, B.W.; Pleschka, S.; Ziebuhr, J. Inhibition of Cytosolic Phospholipase A2α Impairs an Early Step of Coronavirus Replication in Cell Culture. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Zafonte, R.D.; Bagiella, E.; Ansel, B.M.; Novack, T.A.; Friedewald, W.T.; Hesdorffer, D.C.; Timmons, S.D.; Jallo, J.; Eisenberg, H.; Hart, T.; et al. Effect of Citicoline on Functional and Cognitive Status among Patients with Traumatic Brain Injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA 2012, 308, 1993. [Google Scholar] [CrossRef] [PubMed]
- Secades Ruiz, J.J. Citicolina: Revisión farmacológica y clínica, actualización 2016. Rev. Neurol. 2016, 63, S1. [Google Scholar] [CrossRef]
- Overgaard, K. The Effects of Citicoline on Acute Ischemic Stroke: A Review. J. Stroke Cerebrovasc. Dis. 2014, 23, 1764–1769. [Google Scholar] [CrossRef]
- Álvarez-Sabín, J.; Román, G.C. The Role of Citicoline in Neuroprotection and Neurorepair in Ischemic Stroke. Brain Sci. 2013, 3, 1395–1414. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Khan, M.I.; Giri, R.; Kumar, L. Role of citicoline in improvement of cognition, memory and post stroke disability in stroke patients. Int. J. Adv. Med. 2019, 6, 429–434. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Valli, C.; Martí-Amarista, C.E.; Solà, I.; Martí-Fàbregas, J.; Bonfill Cosp, X. Citicoline for treating people with acute ischemic stroke. Cochrane Database Syst. Rev. 2020, 8, CD013066. [Google Scholar] [CrossRef]
- Fioravanti, M.; Yanagi, M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst. Rev. 2005, 2, CD000269. [Google Scholar] [CrossRef]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Qiu, Y.; Song, Y.; Feng, F.; Feng, J.; Song, Q.; Jia, Q.; Wang, J. Clinical characteristics of 82 cases of death from COVID-19. PLoS ONE 2020, 15, e0235458. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Abu-Izneid, T.; Olatunde, A.; Ahmed Khalil, A.; Alhumaydhi, F.A.; Tufail, T.; Shariati, M.A.; Rebezov, M.; Almarhoon, Z.M.; Mabkhot, Y.N.; et al. COVID-19 Pandemic: Epidemiology, Etiology, Conventional and Non-Conventional Therapies. Int. J. Environ. Res. Public Health 2020, 17, 8155. [Google Scholar] [CrossRef]
- Bayati, A.; Kumar, R.; Francis, V.; McPherson, P.S. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J. Biol. Chem. 2021, 296, 100306. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Shao, C.; Huang, J.; Gan, J.; Huang, X.; Bucci, E.; Piacentini, M.; Ippolito, G.; Melino, G. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020, 27, 1451–1454. [Google Scholar] [CrossRef]
- Tavasolian, F.; Rashidi, M.; Hatam, G.R.; Jeddi, M.; Hosseini, A.Z.; Mosawi, S.H.; Abdollahi, E.; Inman, R.D. HLA, Immune Response, and Susceptibility to COVID-19. Front. Immunol. 2021, 11, 601886. [Google Scholar] [CrossRef]
- Wang, S.-F.; Chen, K.-H.; Chen, M.; Li, W.-Y.; Chen, Y.-J.; Tsao, C.-H.; Yen, M.-Y.; Huang, J.C.; Chen, Y.-M.A. Human-Leukocyte Antigen Class I Cw 1502 and Class II DR 0301 Genotypes Are Associated with Resistance to Severe Acute Respiratory Syndrome (SARS) Infection. Viral Immunol. 2011, 24, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Mazas, A. HLA studies in the context of coronavirus outbreaks. Swiss Med. Wkly. 2020, 150, w20248. [Google Scholar] [CrossRef]
- Kangueane, P.; Sakharkar, M.K. HLA-Peptide Binding Prediction Using Structural and Modeling Principles. Methods Mol. Biol. 2007, 409, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Solis-Soto, M.T.; Paudel, D.; Marconi, P.; Gavioli, R.; Appay, V.; Caputo, A. Age-related decline of de novo T cell responsiveness as a cause of COVID-19 severity. GeroScience 2020, 42, 1015–1019. [Google Scholar] [CrossRef]
- Migliorini, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Tingart, M.; Maffulli, N. Association between HLA genotypes and COVID-19 susceptibility, severity and progression: A comprehensive review of the literature. Eur. J. Med. Res. 2021, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Mendes, K.L.; de Farias Lelis, D.; Santos, S.H.S. Nuclear sirtuins and inflammatory signaling pathways. Cytokine Growth Factor Rev. 2017, 38, 98–105. [Google Scholar] [CrossRef]
- Kwon, H.-S.; Ott, M. The ups and downs of SIRT1. Trends Biochem. Sci. 2008, 33, 517–525. [Google Scholar] [CrossRef]
- Ng, F.; Wijaya, L.; Tang, B.L. SIRT1 in the brain—Connections with aging-associated disorders and lifespan. Front. Cell. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Huarachi Olivera, R.E.; Lazarte Rivera, A. Coronavirus disease (COVID-19) and sirtuins. Rev. Fac. Cien. Med. Univ. Nac. Cordoba 2020, 77, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.G.G.; Oliveira, A.E.R.; Singh, Y.; Jimenez, L.; A Gonçalves, A.N.; Ogava, R.L.T.; Creighton, R.; Peron, J.P.S.; Nakaya, H.I. ACE2 Expression Is Increased in the Lungs of Patients with Comorbidities Associated with Severe COVID-19. J. Infect. Dis. 2020, 222, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Wentzel, A.R.; Richards, G.A. COVID-19: NAD+ deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Med. Hypotheses 2020, 144, 110044. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.M.; Wells, J.D.; Vachharajani, V.T.; Yoza, B.K.; McCall, C.E.; Hoth, J.J. SIRT1 mediates a primed response to immune challenge after traumatic lung injury. J. Trauma Acute Care Surg. 2015, 78, 1034–1038. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Pan, H.; Feldser, H.G.; Lainez, E.; Miller, C.; Leung, S.; Zhong, Z.; Zhao, S.; Sweitzer, S.; et al. SIRT1 activators suppress inflammatory responses through promotion of p65 deacetylation and inhibition of NF-κB activity. PLoS ONE 2012, 7, e46364. [Google Scholar] [CrossRef]
- Hussein, M.A.; Ismail, N.E.M.; Mohamed, A.H.; Borik, R.M.; Ali, A.A.; O Mosaad, Y. Plasma Phospholipids: A Promising Simple Biochemical Parameter to Evaluate COVID-19 Infection Severity. Bioinform. Biol. Insights 2021, 15. [Google Scholar] [CrossRef]
- Yan, B.; Chu, H.; Yang, D.; Sze, K.-H.; Lai, P.-M.; Yuan, S.; Shuai, H.; Wang, Y.; Kao, R.Y.-T.; Chan, J.F.-W.; et al. Characterization of the Lipidomic Profile of Human Coronavirus-Infected Cells: Implications for Lipid Metabolism Remodeling upon Coronavirus Replication. Viruses 2019, 11, 73. [Google Scholar] [CrossRef]
- Nakano, T.; Arita, H. Enhanced expression of group II phospholipase A2 gene in the tissues of endotoxin shock rats and its suppression by glucocorticoid. FEBS Lett. 1990, 273, 23–26. [Google Scholar] [CrossRef]
- Ohara, O.; Ishizaki, J.; Arita, H. Structure and function of phospholipase A2 receptor. Prog. Lipid Res. 1995, 34, 117–138. [Google Scholar] [CrossRef]
- Konieczkowski, M.; Sedor, J.R. Cell-specific regulation of type II phospholipase A2 expression in rat mesangial cells. J. Clin. Investig. 1993, 92, 2524–2532. [Google Scholar] [CrossRef]
- Triggiani, M.; Granata, F.; Oriente, A.; Gentile, M.; Petraroli, A.; Balestrieri, B.; Marone, G. Secretory phospholipases A2 induce cytokine release from blood and synovial fluid monocytes. Eur. J. Immunol. 2002, 32, 67–76. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine [Internet]; Oxford University Press: Oxford, UK, 2015; Available online: https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780198717478.001.0001/acprof-9780198717478 (accessed on 7 March 2021).
- Sbardella, D.; Coletta, A.; Tundo, G.R.; Ahmed, I.M.; Bellia, F.; Oddone, F.; Manni, G.; Coletta, M. Structural and functional evidence for citicoline binding and modulation of 20S proteasome activity: Novel insights into its pro-proteostatic effect. Biochem. Pharmacol. 2020, 177, 113977. [Google Scholar] [CrossRef]
- Lindner, H.A.; Fotouhi-Ardakani, N.; Lytvyn, V.; Lachance, P.; Sulea, T.; Ménard, R. The Papain-Like Protease from the Severe Acute Respiratory Syndrome Coronavirus Is a Deubiquitinating Enzyme. J. Virol. 2005, 79, 15199–15208. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B.; Ptacek, R.; Ptackova, H.; Martin, A.; Kream, R.M. Selective Neuronal Mitochondrial Targeting in SARS-CoV-2 Infection Affects Cognitive Processes to Induce ‘Brain Fog’ and Results in Behavioral Changes That Favor Viral Survival. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e930886-1–e930886-4. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.E.; Fazal, F.M.; Parker, K.R.; Zou, J.; Chang, H.Y. RNA-GPS predicts SARS-CoV-2 RNA residency to host mitochondria and nucleolus. Cell Syst. 2020, 11, 102–108.e3. [Google Scholar] [CrossRef]
- Shenoy, S. Coronavirus (Covid-19) sepsis: Revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflamm. Res. 2020, 69, 1–9. [Google Scholar] [CrossRef]
- Singh, K.K.; Chaubey, G.; Chen, J.Y.; Suravajhala, P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am. J. Physiol.-Cell Physiol. 2020, 319, C258–C267. [Google Scholar] [CrossRef] [PubMed]
- Esch, T.; Stefano, G.B.; Ptacek, R.; Kream, R.M. Emerging Roles of Blood-Borne Intact and Respiring Mitochondria as Bidirectional Mediators of Pro- and Anti-Inflammatory Processes. Med. Sci. Monit. 2020, 26, e924337-1–e924337-3. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 Silent Hypoxemia Is Baffling to Physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, Q.; Li, Y.; Li, B.; Zhang, L. Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp. Neurol. 2018, 300, 41–50. [Google Scholar] [CrossRef]
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol. Lett. 2002, 23, 199–208. [Google Scholar]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Dahm, T.; Rudolph, H.; Schwerk, C.; Schroten, H.; Tenenbaum, T. Neuroinvasion and Inflammation in Viral Central Nervous System Infections. Mediat. Inflamm. 2016, 2016, 8562805. [Google Scholar] [CrossRef] [PubMed]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542-2020. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Bénézit, F.; Le Turnier, P.; Declerck, C.; Paillé, C.; Revest, M.; Dubée, V.; Tattevin, P.; for the RAN COVID Study Group. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020, 20, 1014–1015. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Radmard, S.; Epstein, S.E.; Roeder, H.J.; Michalak, A.J.; Shapiro, S.D.; Boehme, A.; Wilson, T.J.; Duran, J.C.; Bain, J.M.; Willey, J.Z.; et al. Inpatient Neurology Consultations During the Onset of the SARS-CoV-2 New York City Pandemic: A Single Center Case Series. Front. Neurol. 2020, 11, 805. [Google Scholar] [CrossRef]
- Kremer, S.; Lersy, F.; Anheim, M.; Merdji, H.; Schenck, M.; Oesterlé, H.; Bolognini, F.; Messie, J.; Khalil, A.; Gaudemer, A.; et al. Neurologic and neuroimaging findings in patients with COVID-19: A retrospective multicenter study. Neurology 2020, 95, e1868–e1882. [Google Scholar] [CrossRef] [PubMed]
- Studart-Neto, A.; Guedes, B.F.; e Tuma, R.d.L.; Camelo Filho, A.E.; Kubota, G.T.; Iepsen, B.D.; Moreira, G.P.; Rodrigues, J.C.; Ferrari, M.M.H.; Carra, R.B.; et al. Neurological consultations and diagnoses in a large, dedicated COVID-19 university hospital. Arq. Neuro-Psiquiatr. 2020, 78, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Oxley, T.J.; Mocco, J.; Majidi, S.; Kellner, C.P.; Shoirah, H.; Singh, I.P.; De Leacy, R.A.; Shigematsu, T.; Ladner, T.R.; Yaeger, K.A.; et al. Large-Vessel Stroke as a Presenting Feature of COVID-19 in the Young. N. Engl. J. Med. 2020, 382, e60. [Google Scholar] [CrossRef]
- Toscano, G.; Palmerini, F.; Ravaglia, S.; Ruiz, L.; Invernizzi, P.; Cuzzoni, M.G.; Franciotta, D.; Baldanti, F.; Daturi, R.; Postorino, P.; et al. Guillain–Barré Syndrome Associated with SARS-CoV-2. N. Engl. J. Med. 2020, 382, 2574–2576. [Google Scholar] [CrossRef]
- Caress, J.B.; Castoro, R.J.; Simmons, Z.; Scelsa, S.N.; Lewis, R.A.; Ahlawat, A.; Narayanaswami, P. COVID-19–associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve 2020, 62, 485–491. [Google Scholar] [CrossRef]
- Scullen, T.; Keen, J.; Mathkour, M.; Dumont, A.S.; Kahn, L. Coronavirus 2019 (COVID-19)–Associated Encephalopathies and Cerebrovascular Disease: The New Orleans Experience. World Neurosurg. 2020, 141, e437–e446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lu, S.; Chen, J.; Wei, N.; Wang, D.; Lyu, H.; Shi, C.; Hu, S. The landscape of cognitive function in recovered COVID-19 patients. J. Psychiatr. Res. 2020, 129, 98–102. [Google Scholar] [CrossRef]
- Lambert, N.; Survivor Corps. COVID-19 “Long Hauler” Symptoms Survey Report [Internet]; Indiana University School of Medicine: Indianapolis, IN, USA, 2020; Available online: https://scholarworks.iupui.edu/bitstream/handle/1805/25685/Lambert2020COVID-19.pdf (accessed on 27 June 2021). [CrossRef][Green Version]
- Shigemoto-Mogami, Y.; Hoshikawa, K.; Sato, K. Activated Microglia Disrupt the Blood-Brain Barrier and Induce Chemokines and Cytokines in a Rat in vitro Model. Front. Cell. Neurosci. 2018, 12, 494. [Google Scholar] [CrossRef]
- Helms, J.; Kremer, S.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Kummerlen, C.; Collange, O.; Boulay, C.; Fafi-Kremer, S.; Ohana, M.; et al. Neurologic Features in Severe SARS-CoV-2 Infection. N. Engl. J. Med. 2020, 382, 2268–2270. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.T.; Krishnan, V.; Chang, C.-Y.; Engle, S.M.; Casalini, G.; Rodgers, G.H.; Bivi, N.; Nickoloff, B.J.; Konrad, R.J.; de Bono, S.; et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2021, 147, 107–111. [Google Scholar] [CrossRef]
- Wijeratne, T.; Crewther, S. Post-COVID 19 Neurological Syndrome (PCNS); a novel syndrome with challenges for the global neurology community. J. Neurol. Sci. 2020, 419, 117179. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.S.; Medeiros, N.I.; Gomes, J.A. Immune response in COVID-19: What do we currently know? Microb. Pathog. 2020, 148, 104484. [Google Scholar] [CrossRef]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef]
- Manzo, C.; Serra-Mestres, J.; Isetta, M.; Castagna, A. Could COVID-19 anosmia and olfactory dysfunction trigger an increased risk of future dementia in patients with ApoE4? Med. Hypotheses 2021, 147, 110479. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef]
- Wang, F.; Kream, R.M.; Stefano, G.B. Long-Term Respiratory and Neurological Sequelae of COVID-19. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e928996-1–e928996-10. [Google Scholar] [CrossRef]
- Hampshire, A.; Trender, W.; Chamberlain, S.R.; Jolly, A.E.; Grant, J.E.; Patrick, F.; Mazibuko, N.; Williams, S.C.R.; Barnby, J.M.; Hellyer, P.; et al. Cognitive deficits in people who have recovered from COVID-19. EClinicalMedicine 2021, 39, 101044. [Google Scholar] [CrossRef] [PubMed]
- Beaud, V.; Crottaz-Herbette, S.; Dunet, V.; Vaucher, J.; Bernard-Valnet, R.; Du Pasquier, R.; Bart, P.-A.; Clarke, S. Pattern of cognitive deficits in severe COVID-19. J. Neurol. Neurosurg. Psychiatry 2021, 92, 567–568. [Google Scholar] [CrossRef]
- Hopkins, R.O.; Gale, S.D.; Weaver, L.K. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006, 20, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.O.; Weaver, L.K.; Collingridge, D.; Parkinson, R.B.; Chan, K.J.; Orme, J.F. Two-Year Cognitive, Emotional, and Quality-of-Life Outcomes in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 340–347. [Google Scholar] [CrossRef]
- Yerlikaya, D.; Emek-Savaş, D.D.; Kursun, B.B.; Oztura, I.; Yener, G.G. Electrophysiological and neuropsychological outcomes of severe obstructive sleep apnea: Effects of hypoxemia on cognitive performance. Cogn. Neurodyn. 2018, 12, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Rossi, S. COVID-19 pneumonia: ARDS or not? Crit. Care 2020, 24, 154. [Google Scholar] [CrossRef] [PubMed]
- Girard, T.D.; Ware, L.B.; Bernard, G.R.; Pandharipande, P.; Thompson, J.L.; Shintani, A.K.; Jackson, J.C.; Dittus, R.S.; Ely, E.W. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensiv. Care Med. 2012, 38, 1965–1973. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Safavynia, S.A.; Goldstein, P.A. The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving From Hypothesis to Treatment. Front. Psychiatry 2019, 9, 752. [Google Scholar] [CrossRef]
- Cunningham, C. Systemic inflammation and delirium: Important co-factors in the progression of dementia. Biochem. Soc. Trans. 2011, 39, 945–953. [Google Scholar] [CrossRef]
- Maity, S.; Saha, A. Therapeutic Potential of Exploiting Autophagy Cascade against Coronavirus Infection. Front. Microbiol. 2021, 12, 675419. [Google Scholar] [CrossRef]
- Li, Y.; Jones, J.W.; Choi, H.M.C.; Sarkar, C.; Kane, M.A.; Koh, E.Y.; Lipinski, M.M.; Wu, J. cPLA2 activation contributes to lysosomal defects leading to impairment of autophagy after spinal cord injury. Cell Death Dis. 2019, 10, 531. [Google Scholar] [CrossRef]
- Mancuso, D.J.; Kotzbauer, P.; Wozniak, D.F.; Sims, H.F.; Jenkins, C.M.; Guan, S.; Han, X.; Yang, K.; Sun, G.; Malik, I.; et al. Genetic Ablation of Calcium-independent Phospholipase A2γ Leads to Alterations in Hippocampal Cardiolipin Content and Molecular Species Distribution, Mitochondrial Degeneration, Autophagy, and Cognitive Dysfunction. J. Biol. Chem. 2009, 284, 35632–35644. [Google Scholar] [CrossRef]
- Lee, S.; Sato, Y.; Nixon, R.A. Lysosomal Proteolysis Inhibition Selectively Disrupts Axonal Transport of Degradative Organelles and Causes an Alzheimer’s-Like Axonal Dystrophy. J. Neurosci. 2011, 31, 7817–7830. [Google Scholar] [CrossRef]
- Gattaz, W.F.; Förstl, H.; Braus, D.F.; Maras, A.; Cairns, N.J.; Levy, R. Decreased phospholipase A2 activity in the brain and in platelets of patients with Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 1996, 246, 129–131. [Google Scholar] [CrossRef]
- Veloso, A.; Fernández, R.; Astigarraga, E.; Barreda-Gómez, G.; Manuel, I.; Giralt, M.T.; Ferrer, I.; Ochoa, B.; Rodríguez-Puertas, R.; Fernández, J.A. Distribution of lipids in human brain. Anal. Bioanal. Chem. 2011, 401, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Trumbore, M.W.; Pettegrew, J.W. Molecular Membrane Interactions of a Phospholipid Metabolite. Implications for Alzheimer’s Disease Pathophysiologya. Ann. N. Y. Acad. Sci. 1996, 777, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed Choline Plasmalogen and Phospholipid Fatty Acid Concentrations in Alzheimer’s Disease Prefrontal Cortex. J. Alzheimer’s Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Jacomy, H.; Fragoso, G.; Almazan, G.; Mushynski, W.E.; Talbot, P.J. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology 2006, 349, 335–346. [Google Scholar] [CrossRef]
- Grande, X.; Berron, D.; Horner, A.J.; Bisby, J.A.; Düzel, E.; Burgess, N. Holistic Recollection via Pattern Completion Involves Hippocampal Subfield CA3. J. Neurosci. 2019, 39, 8100–8111. [Google Scholar] [CrossRef]
- Hosseini, S.; Wilk, E.; Michaelsen-Preusse, K.; Gerhauser, I.; Baumgärtner, W.; Geffers, R.; Schughart, K.; Korte, M. Long-Term Neuroinflammation Induced by Influenza A Virus Infection and the Impact on Hippocampal Neuron Morphology and Function. J. Neurosci. 2018, 38, 3060–3080. [Google Scholar] [CrossRef]
- Setti, S.E.; Hunsberger, H.C.; Reed, M.N. Alterations in hippocampal activity and Alzheimer’s disease. Transl. Issues Psychol. Sci. 2017, 3, 348–356. [Google Scholar] [CrossRef]
- Ostroumova, T.M.; Chernousov, P.A.; Kuznetsov, I.V. Cognitive impairment in COVID-19 survivors. Neurol. Neuropsychiatry Psychosom. 2021, 13, 126–130. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Almeria, M.; Cejudo, J.C.; Sotoca, J.; Deus, J.; Krupinski, J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain Behav. Immun. Health 2020, 9, 100163. [Google Scholar] [CrossRef]
- Jiloha, R.C. COVID-19 and Mental Health. Epidemiol. Int. 2020, 5, 7–9. [Google Scholar] [CrossRef]
- Grieb, P. Neuroprotective Properties of Citicoline: Facts, Doubts and Unresolved Issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lozano Fernández, R. Efficacy and safety of oral CDP-choline. Drug surveillance study in 2817 cases. Arzneimittelforschung 1983, 33, 1073–1080. [Google Scholar]
- Cacabelos, R.; Caamaño, J.; Gómez, M.J.; Fernández-Novoa, L.; Franco-Maside, A.; Alvarez, X.A. Therapeutic Effects of CDP-Choline in Alzheimer’s Disease-Cognition, Brain Mapping, Cerebrovascular Hemodynamics, and Immune Factorsa. Ann. N. Y. Acad. Sci. 1996, 777, 399–403. [Google Scholar] [CrossRef] [PubMed]
- A Alvarez, X.; Mouzo, R.; Pichel, V.; Pérez, P.; Laredo, M.; Fernández-Novoa, L.; Corzo, L.; Zas, R.; Alcaraz, M.; Secades, J.J.; et al. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find. Exp. Clin. Pharmacol. 1999, 21, 633–644. [Google Scholar] [PubMed]
- Jasielski, P.; Piędel, F.; Piwek, M.; Rocka, A.; Petit, V.; Rejdak, K. Application of Citicoline in Neurological Disorders: A Systematic Review. Nutrients 2020, 12, 3113. [Google Scholar] [CrossRef] [PubMed]
- Elibol, B.; Kilic, U. High Levels of SIRT1 Expression as a Protective Mechanism against Disease-Related Conditions. Front. Endocrinol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Y.; Cao, L.; Wang, D.; Guo, M.; Jiang, A.; Guo, D.; Hu, W.; Yang, J.; Tang, Z.; et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, V.; Tartaglia, E.; Sacchi, A.; Fimia, G.M.; Cimini, E.; Casetti, R.; Notari, S.; Grassi, G.; Marchioni, L.; Bibas, M.; et al. The unbalanced p53/SIRT1 axis may impact lymphocyte homeostasis in COVID-19 patients. Int. J. Infect. Dis. 2021, 105, 49–53. [Google Scholar] [CrossRef]
- Cardozo, C.M.; Hainaut, P. Viral strategies for circumventing p53: The case of severe acute respiratory syndrome coronavirus. Curr. Opin. Oncol. 2021, 33, 149–158. [Google Scholar] [CrossRef]
- Hemmat, N.; Asadzadeh, Z.; Ahangar, N.K.; Alemohammad, H.; Najafzadeh, B.; Derakhshani, A.; Baghbanzadeh, A.; Baghi, H.B.; Javadrashid, D.; Najafi, S.; et al. The roles of signaling pathways in SARS-CoV-2 infection; lessons learned from SARS-CoV and MERS-CoV. Arch. Virol. 2021, 166, 675–696. [Google Scholar] [CrossRef]
- Rizzi, L.; Roriz-Cruz, M. Sirtuin 1 and Alzheimer’s disease: An up-to-date review. Neuropeptides 2018, 71, 54–60. [Google Scholar] [CrossRef]
- Kilic, U.; Gok, O.; Erenberk, U.; Dundaroz, M.R.; Torun, E.; Kucukardali, Y.; Elibol-Can, B.; Uysal, O.; Dundar, T. A Remarkable Age-Related Increase in SIRT1 Protein Expression against Oxidative Stress in Elderly: SIRT1 Gene Variants and Longevity in Human. PLoS ONE 2015, 10, e0117954. [Google Scholar] [CrossRef]
- Braidy, N.; Jayasena, T.; Poljak, A.; Sachdev, P. Sirtuins in cognitive ageing and Alzheimer’s disease. Curr. Opin. Psychiatry 2012, 25, 226–230. [Google Scholar] [CrossRef]
- Hurtado, O.; Jiménez, M.H.; Zarruk, J.G.; Cuartero, M.; Ballesteros, I.; Camarero, G.; Moraga, A.; Pradillo, J.; Moro, M.A.; Lizasoain, I. Citicoline (CDP-choline) increases Sirtuin1 expression concomitant to neuroprotection in experimental stroke. J. Neurochem. 2013, 126, 819–826. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Zhang, Y.-L.; Wu, Y.-C. The Role of Sirt1 in Ischemic Stroke: Pathogenesis and Therapeutic Strategies. Front. Neurosci. 2018, 12, 833. [Google Scholar] [CrossRef]
- Secades, J.J. Citicoline in the Treatment of Cognitive Impairment. J. Neurol. Exp. Neurosci. 2019, 5. Available online: http://jneuroscience.com/2019/01/28/citicoline-in-the-treatment-of-cognitive-impairment/ (accessed on 22 February 2021). [CrossRef][Green Version]
- Wang, C.-S.; Lee, R.K. Choline plus cytidine stimulate phospholipid production, and the expression and secretion of amyloid precursor protein in rat PC12 cells. Neurosci. Lett. 2000, 283, 25–28. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Hatcher, J.F.; Dempsey, R.J. Citicoline: Neuroprotective mechanisms in cerebral ischemia. J. Neurochem. 2002, 80, 12–23. [Google Scholar] [CrossRef]
- Iulia, C.; Ruxandra, T.; Costin, L.-B.; Liliana-Mary, V.; Chitu, I.; Tudosescu, R.; Leasu-Branet, C.; Voinea, L.-M. Citicoline—A neuroprotector with proven effects on glaucomatous disease. Rom. J. Ophthalmol. 2017, 61, 152–158. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F.; Dempsey, R.J. Cytidine-5′-diphosphocholine affects CTP-phosphocholine cytidylyltransferase and lyso-phosphatidylcholine after transient brain ischemia. J. Neurosci. Res. 2004, 76, 390–396. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F. Cytidine 5′-Diphosphocholine (CDP-Choline) in Stroke and Other CNS Disorders. Neurochem. Res. 2005, 30, 15–23. [Google Scholar] [CrossRef]
- Topuz, B.B.; Altinbas, B.; Ilhan, T.; Yilmaz, M.S.; Erdost, H.; Saha, S.; Savci, V.; Yalcin, M. Centrally administered CDP-choline induced cardiovascular responses are mediated by activation of the central phospholipase-prostaglandin signaling cascade. Brain Res. 2014, 1563, 61–71. [Google Scholar] [CrossRef]
- Oddone, F.; Rossetti, L.; Parravano, M.; Sbardella, D.; Coletta, M.; Ziccardi, L.; Roberti, G.; Carnevale, C.; Romano, D.; Manni, G.; et al. Citicoline in Ophthalmological Neurodegenerative Disease: A Comprehensive Review. Pharmaceuticals 2021, 14, 281. [Google Scholar] [CrossRef]
- Livneh, I.; Cohen-Kaplan, V.; Cohen-Rosenzweig, C.; Avni, N.; Ciechanover, A. The life cycle of the 26S proteasome: From birth, through regulation and function, and onto its death. Cell Res. 2016, 26, 869–885. [Google Scholar] [CrossRef]
- VerPlank, J.J.S.; Goldberg, A.L. Regulating protein breakdown through proteasome phosphorylation. Biochem. J. 2017, 474, 3355–3371. [Google Scholar] [CrossRef]
- Moutzouris, J.P.; Che, W.; Ramsay, E.E.; Manetsch, M.; Alkhouri, H.; Bjorkman, A.M.; Schuster, F.; Ge, Q.; Ammit, A.J. Proteasomal inhibition upregulates the endogenous MAPK deactivator MKP-1 in human airway smooth muscle: Mechanism of action and effect on cytokine secretion. Biochim. Biophys. Acta Bioenerg. 2010, 1803, 416–423. [Google Scholar] [CrossRef]
- Kennedy, M.; Helfand, B.K.I.; Gou, R.Y.; Gartaganis, S.L.; Webb, M.; Moccia, J.M.; Bruursema, S.N.; Dokic, B.; McCulloch, B.; Ring, H.; et al. Delirium in Older Patients with COVID-19 Presenting to the Emergency Department. JAMA Netw. Open 2020, 3, e2029540. [Google Scholar] [CrossRef]
- Yoon, B.-H.; Yoo, J.-I.; Youn, Y.; Ha, Y.-C. Cholinergic enhancers for preventing postoperative delirium among elderly patients after hip fracture surgery: A meta-analysis. Eur. Geriatr. Med. 2017, 8, 486–491. [Google Scholar] [CrossRef]
- Hshieh, T.T.; Fong, T.G.; Marcantonio, E.R.; Inouye, S.K. Cholinergic Deficiency Hypothesis in Delirium: A Synthesis of Current Evidence. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 764–772. [Google Scholar] [CrossRef]
- Tune, L.; Holland, A.; Folstein, M.; Damlouji, N.; Gardner, T.; Coyle, J. Association of postoperative delirium with raised serum levels of anticholinergic drugs. Lancet 1981, 318, 651–653. [Google Scholar] [CrossRef]
- Wengel, S.P.; Roccaforte, W.H.; Burke, W.J. Donepezil Improves Symptoms of Delirium in Dementia: Implications for Future Research. J. Geriatr. Psychiatry Neurol. 1998, 11, 159–161. [Google Scholar] [CrossRef]
- Díaz, V.; Rodríguez, J.; Barrientos, P.; Serra, M.; Salinas, H.; Toledo, C.; Kunze, S.; Varas, V.; Santelices, E.; Cabrera, C.; et al. Use of procholinergics in the prevention of postoperative delirium in hip fracture surgery in the elderly. A randomized controlled trial. Rev. Neurol. 2002, 33, 716–719. [Google Scholar]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Ikonomovic, M.D.; Styren, S.D.; Beckett, L.; Wisniewski, S.; Bennett, D.A.; Cochran, E.J.; Kordower, J.H.; Mufson, E.J. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann. Neurol. 2002, 51, 145–155. [Google Scholar] [CrossRef]
- Counts, S.E.; Mufson, E.J. The Role of Nerve Growth Factor Receptors in Cholinergic Basal Forebrain Degeneration in Prodromal Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2005, 64, 263–272. [Google Scholar] [CrossRef]
- Piamonte, B.L.C.; Espiritu, A.I.; Anlacan, V.M.M. Effects of Citicoline as an Adjunct Treatment for Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2020, 76, 725–732. [Google Scholar] [CrossRef]
- Tiwari, P.; Dwivedi, S.; Singh, M.P.; Mishra, R.; Chandy, A. Basic and modern concepts on cholinergic receptor: A review. Asian Pac. J. Trop. Dis. 2013, 3, 413–420. [Google Scholar] [CrossRef]
- Secades, J.J. Citicoline: Pharmacological and clinical review, 2010 update. Rev. Neurol. 2011, 52 (Suppl. 2), S1–S62. [Google Scholar] [PubMed]
- Secades, J.J. Citicoline: Pharmacological and clinical review, 2016 update. Rev. Neurol. 2016, 63 (Suppl. 3), S1–S73. [Google Scholar] [PubMed]
- Martinov, M.; Gusev, E. Current knowledge on the neuroprotective and neuroregenerative properties of citicoline in acute ischemic stroke. J. Exp. Pharmacol. 2015, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Plataras, C.; Tsakiris, S.; Angelogianni, P. Effect of CDP-choline on brain acetylcholinesterase and Na+,K+-ATPase in adult rats. Clin. Biochem. 2000, 33, 351–357. [Google Scholar] [CrossRef]
- Hurtado, O.; Moro, M.A.; Cárdenas, A.; Sánchez, V.; Fernández-Tomé, P.; Leza, J.C.; Lorenzo, P.; Secades, J.J.; Lozano, R.; Dávalos, A.; et al. Neuroprotection afforded by prior citicoline administration in experimental brain ischemia: Effects on glutamate transport. Neurobiol. Dis. 2005, 18, 336–345. [Google Scholar] [CrossRef]
- Hurtado, O.; Pradillo, J.; Fernández-López, D.; Morales, J.; Sobrino, T.; Castillo, J.; Alborch, E.; Moro, M.; Lizasoain, I. Delayed post-ischemic administration of CDP-choline increases EAAT2 association to lipid rafts and affords neuroprotection in experimental stroke. Neurobiol. Dis. 2008, 29, 123–131. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J. Citicoline mechanisms and clinical efficacy in cerebral ischemia. J. Neurosci. Res. 2002, 70, 133–139. [Google Scholar] [CrossRef]
- Adibhatla, R.M.; Hatcher, J.F.; Dempsey, R.J. Effects of Citicoline on Phospholipid and Glutathione Levels in Transient Cerebral Ischemia. Stroke 2001, 32, 2376–2381. [Google Scholar] [CrossRef]
- Rao, A.M.; Hatcher, J.F.; Dempsey, R.J. Lipid Alterations in Transient Forebrain Ischemia: Possible New Mechanisms of CDP-Choline Neuroprotection. J. Neurochem. 2008, 75, 2528–2535. [Google Scholar] [CrossRef]
- Sobrado, M.; López, M.; Carceller, F.; García, A.; Roda, J. Combined nimodipine and citicoline reduce infarct size, attenuate apoptosis and increase bcl-2 expression after focal cerebral ischemia. Neuroscience 2003, 118, 107–113. [Google Scholar] [CrossRef]
- Herskovits, A.Z.; Guarente, L. SIRT1 in Neurodevelopment and Brain Senescence. Neuron 2014, 81, 471–483. [Google Scholar] [CrossRef]
- Krupinski, J.; Ferrer, I.; Barrachina, M.; Secades, J.; Mercadal, J.; Lozano, R. CDP-choline reduces pro-caspase and cleaved caspase-3 expression, nuclear DNA fragmentation, and specific PARP-cleaved products of caspase activation following middle cerebral artery occlusion in the rat. Neuropharmacology 2002, 42, 846–854. [Google Scholar] [CrossRef]
- Schäbitz, W.-R.; Weber, J.; Takano, K.; Sandage, B.W.; Locke, K.W.; Fisher, M. The effects of prolonged treatment with citicoline in temporary experimental focal ischemia. J. Neurol. Sci. 1996, 138, 21–25. [Google Scholar] [CrossRef]
- Wolf, R. Effects of CDP-Choline on Macrophages and Oligodendrocytes in Neuroinflammation [Internet]; Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU): Erlangen, Germany, 2016; Available online: https://opus4.kobv.de/opus4-fau/frontdoor/index/index/docId/7141 (accessed on 23 February 2021).
- Conant, R.; Schauss, A.G. Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: A review of the literature. Altern. Med. Rev. J. Clin. Ther. 2004, 9, 17–31. [Google Scholar]
- López-Coviella, I.; Agut, J.; Savci, V.; Ortiz, J.A.; Wurtman, R.J. Evidence that 5′-Cytidinediphosphocholine Can Affect Brain Phospholipid Composition by Increasing Choline and Cytidine Plasma Levels. J. Neurochem. 2002, 65, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Zazueta, C.; Buelna-Chontal, M.; Macías-López, A.; Román-Anguiano, N.G.; Gonzalez-Pacheco, H.; Pavon, N.; Springall, R.; Aranda-Frausto, A.; Bojalil, R.; Silva-Palacios, A.; et al. Cytidine-5’-Diphosphocholine Protects the Liver From Ischemia/Reperfusion Injury Preserving Mitochondrial Function and Reducing Oxidative Stress. Liver Transplant. 2018, 24, 1070–1083. [Google Scholar] [CrossRef]
- Zhiliuk, V.I.; Mamchur, V.I.; Pavlov, S.V. Role of functional state of neuronal mitochondria of cerebral cortex in mechanisms of nootropic activity of neuroprotectors in rats with alloxan hyperglycemia. Eksp. Klin. Farmakol. 2015, 78, 10–14. [Google Scholar]
- Arrigoni, E.; Averet, N.; Cohadon, F. Effects of CDP-choline on phospholipase A2 and cholinephosphotransferase activities following a cryogenic brain injury in the rabbit. Biochem. Pharmacol. 1987, 36, 3697–3700. [Google Scholar] [CrossRef]
- Najjar, S.; Najjar, A.; Chong, D.J.; Pramanik, B.K.; Kirsch, C.; Kuzniecky, R.I.; Pacia, S.V.; Azhar, S. Central nervous system complications associated with SARS-CoV-2 infection: Integrative concepts of pathophysiology and case reports. J. Neuroinflamm. 2020, 17, 231. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.L.; Gupta, K.; Climer, J.R.; Monaghan, C.K.; Hasselmo, M.E. Cholinergic modulation of cognitive processing: Insights drawn from computational models. Front. Behav. Neurosci. 2012, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Gareri, P.; Castagna, A.; Cotroneo, A.M.; Putignano, D.; Conforti, R.; Santamaria, F.; Marino, S.; Putignano, S. The Citicholinage Study: Citicoline Plus Cholinesterase Inhibitors in Aged Patients Affected with Alzheimer’s Disease Study. J. Alzheimer’s Dis. 2017, 56, 557–565. [Google Scholar] [CrossRef]
- Chen, X.; Wang, K. The fate of medications evaluated for ischemic stroke pharmacotherapy over the period 1995–2015. Acta Pharm. Sin. B 2016, 6, 522–530. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turana, Y.; Nathaniel, M.; Shen, R.; Ali, S.; Aparasu, R.R. Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications. Brain Sci. 2022, 12, 59. https://doi.org/10.3390/brainsci12010059
Turana Y, Nathaniel M, Shen R, Ali S, Aparasu RR. Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications. Brain Sciences. 2022; 12(1):59. https://doi.org/10.3390/brainsci12010059
Chicago/Turabian StyleTurana, Yuda, Michael Nathaniel, Robert Shen, Soegianto Ali, and Rajender R. Aparasu. 2022. "Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications" Brain Sciences 12, no. 1: 59. https://doi.org/10.3390/brainsci12010059
APA StyleTurana, Y., Nathaniel, M., Shen, R., Ali, S., & Aparasu, R. R. (2022). Citicoline and COVID-19-Related Cognitive and Other Neurologic Complications. Brain Sciences, 12(1), 59. https://doi.org/10.3390/brainsci12010059





