Therapeutic Effects of a Newly Developed 3D Magnetic Finger Rehabilitation Device in Subacute Stroke Patients: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
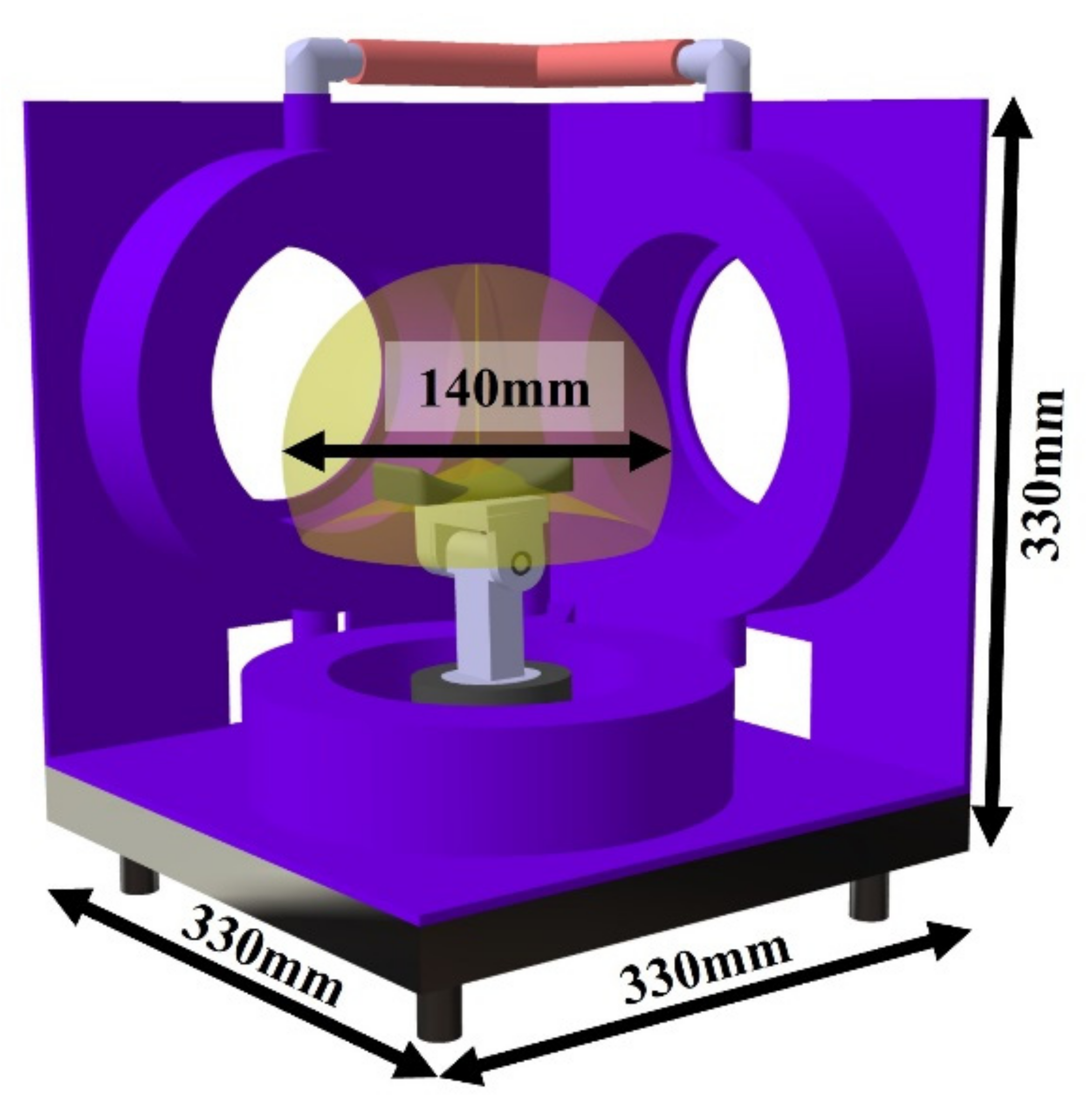
2.1. Development of the Device
2.2. Subjects
2.3. Magnetic Finger Rehabilitation Protocol
2.4. Outcome Measures
2.5. Statistics
3. Results
3.1. Safety Concerns
3.2. Demographic Characteristics
3.3. Primary Outcome Measures
3.4. Secondary Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 2014, Cd010820. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Zhang, X.; Wang, J. Hand Rehabilitation Robotics on Poststroke Motor Recovery. Behav. Neurol. 2017, 2017, 3908135. [Google Scholar] [CrossRef] [PubMed]
- Ben-Tzvi, P.; Ma, Z. Sensing and Force-Feedback Exoskeleton (SAFE) Robotic Glove. IEEE Trans. Neural Syst. Rehabil. Eng. A 2015, 23, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, S.B.; Holley, R.J.; Lum, P.S. Clinical effects of using HEXORR (Hand Exoskeleton Rehabilitation Robot) for movement therapy in stroke rehabilitation. Am. J. Phys. Med. Rehabil. 2013, 92, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ben-Tzvi, P.; Danoff, J. Hand Rehabilitation Learning System with an Exoskeleton Robotic Glove. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.Y.; Patterson, R.M. Soft robotic devices for hand rehabilitation and assistance: A narrative review. J. Neuroeng. Rehabil. 2018, 15, 9. [Google Scholar] [CrossRef] [Green Version]
- Radder, B.; Prange-Lasonder, G.; Kottink, A.I.R.; Melendez-Calderon, A.; Buurke, J.H.; Rietman, J.S. Feasibility of a wearable soft-robotic glove to support impaired hand function in stroke patients. J. Rehabil. Med. 2018, 50, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Bernocchi, P.; Mulè, C.; Vanoglio, F.; Taveggia, G.; Luisa, A.; Scalvini, S. Home-based hand rehabilitation with a robotic glove in hemiplegic patients after stroke: A pilot feasibility study. Top. Stroke Rehabil. 2018, 25, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Vanoglio, F.; Bernocchi, P.; Mulè, C.; Garofali, F.; Mora, C.; Taveggia, G.; Scalvini, S.; Luisa, A. Feasibility and efficacy of a robotic device for hand rehabilitation in hemiplegic stroke patients: A randomized pilot controlled study. Clin. Rehabil. 2017, 31, 351–360. [Google Scholar] [CrossRef]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst. Rev. 2018, 9, Cd006876. [Google Scholar] [CrossRef]
- Ji, D.-M.; Kim, M.-S.; Kim, S.-H. Multi-Link Magnet Device with Electromagnetic Manipulation System for Assisting Finger Movements with Wireless Operation. Appl. Sci. 2021, 11, 6762. [Google Scholar] [CrossRef]
- In-Chul, B.; Min Su, K.; Sung Hoon, K. A Novel Nonmechanical Finger Rehabilitation System Based on Magnetic Force Control. J. Magn. 2017, 22, 155–161. [Google Scholar]
- Kotteduwa Jayawarden, S.; Sandarage, R.; Farag, J.; Ganzert, C.; Winston, P.; Mills, P.; Reebye, R. Effect of treating elbow flexor spasticity with botulinum toxin injection and adjunctive casting on hemiparetic gait parameters: A prospective case series. J. Rehabil. Med. 2020, 52, jrm00110. [Google Scholar] [CrossRef] [PubMed]
- Colomer, C.; Noé, E.; Llorens, R. Mirror therapy in chronic stroke survivors with severely impaired upper limb function: A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 271–278. [Google Scholar]
- Hodics, T.M.; Nakatsuka, K.; Upreti, B.; Alex, A.; Smith, P.S.; Pezzullo, J.C. Wolf Motor Function Test for characterizing moderate to severe hemiparesis in stroke patients. Arch. Phys. Med. Rehabil. 2012, 93, 1963–1967. [Google Scholar] [CrossRef] [Green Version]
- Michimata, A.; Kondo, T.; Suzukamo, Y.; Chiba, M.; Izumi, S. The manual function test: Norms for 20- to 90-year-olds and effects of age, gender, and hand dominance on dexterity. Tohoku J. Exp. Med. 2008, 214, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, S.; Kondo, T.; Suzukamo, Y.; Michimata, A.; Izumi, S. Reliability and validity of the Manual Function Test in patients with stroke. Am. J. Phys. Med. Rehabil. 2009, 88, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Santisteban, L.; Térémetz, M.; Bleton, J.P.; Baron, J.C.; Maier, M.A.; Lindberg, P.G. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS ONE 2016, 11, e0154792. [Google Scholar] [CrossRef]
- Young, J.H.; Kyu, P.B.; Suk, S.H.; Kyoo, K.Y.; Bom, P.S.; Jong, P.N.; Hyun, K.S.; Hyun, K.T.; Ryoon, H.T. Development of the Korean Version of Modified Barthel Index (K-MBI): Multi-center Study for Subjects with Stroke. Ann. Rehabil. Med. 2007, 31, 283–297. [Google Scholar]
- Wallace, A.C.; Talelli, P.; Crook, L.; Austin, D.; Farrell, R.; Hoad, D.; O’Keeffe, A.G.; Marsden, J.F.; Fitzpatrick, R.; Greenwood, R.; et al. Exploratory Randomized Double-Blind Placebo-Controlled Trial of Botulinum Therapy on Grasp Release After Stroke (PrOMBiS). Neurorehabilit. Neural Repair 2020, 34, 51–60. [Google Scholar] [CrossRef]
- Bessler, J.; Prange-Lasonder, G.B.; Schulte, R.V.; Schaake, L.; Prinsen, E.C.; Buurke, J.H. Occurrence and Type of Adverse Events During the Use of Stationary Gait Robots-A Systematic Literature Review. Front. Robot. AI 2020, 7, 557606. [Google Scholar] [CrossRef] [PubMed]
- Bessler, J.; Prange-Lasonder, G.B.; Schaake, L.; Saenz, J.F.; Bidard, C.; Fassi, I.; Valori, M.; Lassen, A.B.; Buurke, J.H. Safety Assessment of Rehabilitation Robots: A Review Identifying Safety Skills and Current Knowledge Gaps. Front. Robot. AI 2021, 8, 33. [Google Scholar] [CrossRef]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot assisted training for the upper limb after stroke (RATULS): A multicentre randomised controlled trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Rowe, J.B.; Chan, V.; Ingemanson, M.L.; Cramer, S.C.; Wolbrecht, E.T.; Reinkensmeyer, D.J. Robotic Assistance for Training Finger Movement Using a Hebbian Model: A Randomized Controlled Trial. Neurorehabilit. Neural Repair 2017, 31, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Kim, J.H.; Yong, S.Y.; Lee, Y.H.; Park, J.M.; Kim, S.H.; Lee, H.C. Effect of Task-Specific Lower Extremity Training on Cognitive and Gait Function in Stroke Patients: A Prospective Randomized Controlled Trial. Ann. Rehabil. Med. 2019, 43, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Radius (mm) | Wire Diameter (mm) | Resistance (Ω) | Number of Turns | |
|---|---|---|---|---|
| 3-axis coil | 115 | 2.0 | 1.65 | 405 |
| Patient No. | Group | Age (y) | Gender | Type | Affected Side | Lesion Location | Dominant Hand | Period after Onset (Days) | NIHSS | MoCA | Spasticity (MTS) † |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Intervention | 56 | M | Infarct | Right | BG | Right | 35 | 11 | 21 | 1 |
| 2 | Intervention | 62 | F | Hemorrhage | Left | BG | Right | 37 | 9 | 20 | 1 |
| 3 | Intervention | 63 | F | Infarct | Right | MCA | Right | 31 | 8 | 19 | 0 |
| 4 | Intervention | 59 | M | Infarct | Left | MCA | Right | 29 | 12 | 22 | 0 |
| 5 | Intervention | 60 | M | Infarct | Right | IC | Right | 33 | 11 | 23 | 0 |
| 6 | Intervention | 61 | F | Infarct | Left | IC | Right | 32 | 11 | 24 | 0 |
| 7 | Control | 63 | F | Infarct | Left | MCA | Right | 35 | 8 | 22 | 0 |
| 8 | Control | 63 | M | Infarct | Right | MCA | Right | 36 | 8 | 19 | 1 |
| 9 | Control | 61 | M | Hemorrhage | Right | BG | Right | 30 | 13 | 18 | 0 |
| 10 | Control | 60 | F | Hemorrhage | Right | BG | Right | 27 | 12 | 24 | 1 |
| 11 | Control | 58 | M | Infarct | Left | IC | Right | 28 | 11 | 24 | 0 |
| 12 | Control | 60 | F | Infarct | Left | IC | Right | 33 | 10 | 23 | 0 |
| Intervention Group | Control Group | MW-U | p–Value † | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ Post-Pre | Pre | Post | Δ Post-Pre | |||
| WMFT score | 13.4 (13.1) | 20.9 (19.5) | 7.5 | 13.1 (12.9) | 15.2 (14.8) | 2.1 | 3.500 | 0.016 * |
| WMFT time (sec) | 88 (84) | 67 (65) | 21 | 89 (85) | 73 (71) | 16 | 5.500 | 0.042 * |
| MFT | 22.5 (20.9) | 39.3 (38.6) | 16.8 | 23.1 (21.3) | 31.7 (29.6) | 8.6 | 4.500 | 0.038 * |
| FMA_U | 23.8 (22.8) | 33.0 (32.1) | 9.2 | 22.9 (21.1) | 26.8 (25.2) | 4.1 | 5.500 | 0.042 * |
| K-MBI | 46 (43) | 68 (66) | 22 | 47 (44) | 60 (58) | 14 | 5.500 | 0.042 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Ji, D.-M.; Kim, C.-Y.; Choi, S.-B.; Joo, M.-C.; Kim, M.-S. Therapeutic Effects of a Newly Developed 3D Magnetic Finger Rehabilitation Device in Subacute Stroke Patients: A Pilot Study. Brain Sci. 2022, 12, 113. https://doi.org/10.3390/brainsci12010113
Kim S-H, Ji D-M, Kim C-Y, Choi S-B, Joo M-C, Kim M-S. Therapeutic Effects of a Newly Developed 3D Magnetic Finger Rehabilitation Device in Subacute Stroke Patients: A Pilot Study. Brain Sciences. 2022; 12(1):113. https://doi.org/10.3390/brainsci12010113
Chicago/Turabian StyleKim, Sung-Hoon, Dong-Min Ji, Chan-Yong Kim, Sung-Bok Choi, Min-Cheol Joo, and Min-Su Kim. 2022. "Therapeutic Effects of a Newly Developed 3D Magnetic Finger Rehabilitation Device in Subacute Stroke Patients: A Pilot Study" Brain Sciences 12, no. 1: 113. https://doi.org/10.3390/brainsci12010113
APA StyleKim, S.-H., Ji, D.-M., Kim, C.-Y., Choi, S.-B., Joo, M.-C., & Kim, M.-S. (2022). Therapeutic Effects of a Newly Developed 3D Magnetic Finger Rehabilitation Device in Subacute Stroke Patients: A Pilot Study. Brain Sciences, 12(1), 113. https://doi.org/10.3390/brainsci12010113






