Abstract
Although Electroacupuncture (EA) has been reported to be potentially effective for cognitive disorders, there is limited information about which domains of cognitive function can be improved by EA treatment. Sixty patients with MCI were randomly assigned (1:1:1) to groups to receive 24 sessions over 12 weeks of EA, sham EA, or usual care. In the EA group, electric stimulation was applied at bilateral PC6 and HT7. Various cognitive tests included in the Seoul Neuropsychological Screening Battery II (SNSB-II) were performed at baseline and post-treatment to explore effects of EA on five cognitive domains: attention, language, visuospatial function, memory, and frontal/executive function. Among 60 randomized participants (63.7 ± 7.1 years, 89.7% females), 45 (75%) completed the study. Of the five cognitive function domains of SNSB-II, the T score of visuospatial function showed a tendency to be higher in the EA group than in the usual care group at post-treatment assessment (mean difference: 10.16 (95% CI, 1.14, 19.18), Cohen’s d = 0.72, p = 0.0283). According to the results of this pilot study, the estimated effect size of EA on the visuospatial function of MCI patients compared to usual care was medium. Large-scale clinical trials are needed to confirm effects of EA on cognitive functions.
1. Introduction
Electroacupuncture (EA) is defined as the application of electric stimulation through acupuncture needles on acupoints. In general, EA has been thought to produce a higher intensity of stimulation on acupoints than manual acupuncture [1]. EA is commonly used for relieving pain [2]. Through neuroprotective mechanisms, acupuncture-related treatment has the potential to improve cognitive functions in animal studies [3]. Systematic reviews of randomized controlled trials have shown controversial but potential EA effects for improving the cognitive function of patients with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) compared to anti-dementia drugs including nimodipine and donepezil [4,5,6,7].
MCI is a preclinical state of early dementia, which is defined by a noticeable decline in cognitive function considering age-related cognitive decline but maintaining independence related to daily living activities [8,9,10]. Early detection and intervention of MCI are clinically important to decrease the conversion rate to dementia. Cholinesterase inhibitors have been suggested for treating MCI. However, a recent systematic review concluded that cholinesterase inhibitors cannot produce a constant effect for patients with MCI [11]. There is a strong need for a new strategy to manage MCI and prevent its progression.
Cognitive function decline can occur in various domains. Changes in the memory domain might occur, leading to difficulties in tracking dates and appointments. Changes in the language domain might lead to difficulties in finding appropriate words or fluency. Changes in visuospatial function may lead to difficulties in drawing or identifying familiar persons. Changes in executive function may lead to difficulties in solving complex problems [12]. Amnestic MCI (aMCI) and non-amnestic MCI (naMCI) as clinical subtypes of MCI can be distinguished according to the presence of memory impairment. MCI patients who are having impairments in multiple cognitive domains can be distinguished from patients who are having impairment in a single cognitive domain [10,13]. aMCI has been reported to have a high risk of progression to Alzheimer’s disease (AD). Memory impairment is one of the main symptoms of AD [14]. Moreover, MCI patients who are having impairments in both memory and frontal/executive function have been reported to have a high risk of dementia conversion from MCI [15].
Previous research studies [4,5] on EA for MCI have mainly performed brief cognitive tests, including the Mini-Mental State Examination (MMSE) [16] and Montreal Cognitive Assessment (MoCA) [17]. MMSE and MoCA were developed to be brief screening tools for cognitive impairment. They are not complete neuropsychological examinations [18]. Thus, they cannot generate enough information for specific cognitive functions. A few studies have used the Clock Drawing Test (CDT), picture recognition [19], and the Alzheimer’s disease assessment scale cognitive subscale (ADAS-cog) [20]. However, they could not comprehensively evaluate various cognitive functions. Therefore, there have been limited reports about which cognitive function domains are improved by EA in patients with MCI. In addition, most previous studies mainly used active controls such as donepezil and nimodipine [4,5,19]. Few studies have used a sham control [21,22]. As a result, there is limited research about the specific effect of EA on cognitive impairment. Furthermore, most studies have a treatment period of 4–8 weeks [4,5,20]. Few studies have a long-term follow-up of more than 24 weeks.
In this pilot randomized controlled study, we explored the effect of EA on cognitive function, mainly focusing on each cognitive domain using a comprehensive neuropsychological examination. As a control, a sham EA group was used in addition to the usual care group to observe specific effects of EA. We carried out a 12-week treatment of EA with a follow-up of 24 weeks. The aim of this study was to evaluate the feasibility of a larger scale of clinical research and provide basic information on the efficacy and safety of EA treatment for patients with MCI.
2. Materials and Methods
2.1. Study Design
This study was a pilot randomized controlled trial with three parallel groups: an EA group, a sham EA group, and a usual care group. Using a protocol approved by the Institutional Review Board (Approval Number: djomc-142-1 and DJDSKH-16-BM-10) of Daejeon Korean Medical Hospital of Daejeon University, this trial was conducted in two centers. This trial was registered with the clinical research information service with a registration number of KCT0002164. We submitted our trial protocol to the registry site on 28 October 2016. After the review process, it was registered on 8 December 2016. The date of the first participant recruitment was 31 October 2016, which was after the submission date of the protocol.
2.2. Participants
Sixty eligible participants in two Korean medicine university hospitals from 31 October 2016 to 24 August 2018 were recruited. Inclusion criteria were: patients aged from equal to or more than 45 years old to under 80 years old; those who met the Peterson diagnostic criteria of MCI with memory problems for at least three months; Clinical Dementia Rating (CDR) score of 0.5 and Global Deterioration Scale (GDS) score of 2–3; Hachinski ischemic score ≤4; at least six years of education; and agreed with written informed consent. Diagnostic criteria of mild cognitive impairment included self-reported cognitive complaint, objective cognitive impairment, preserved independence in functional abilities, and no dementia [10]. In our pilot trial, objective evidence of cognitive impairment was judged by a MoCA score below 23 [23]. Participants with MoCA scores <23 were included. The following participants were excluded: (1) those with dementia by the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV); (2) those with a history of cognitive impairment due to any other cause (head trauma or brain injury, etc.); (3) those with hospital anxiety and depression scale-depression (HADS-D) ≥11; (4) those with a history of cerebral hemorrhage or infarction; (5) those with brain disorders including Parkinson’s disease, Huntington’s Disease, normal pressure hydrocephalus, brain tumor, and so on; (6) those with psychiatric illnesses such as major depressive disorder, schizophrenia, delusional disorder, and bipolar disorder; (7) those with a severe medical disease (diabetic complications; cardiovascular, hepatic, or renal disorder); (8) those with anemia, hypothyroidism, vitamin deficiencies, or malignancy; (9) those with any history of drug or alcohol dependence during the past 6 months; (10) those who received any traditional Korean medical treatment for MCI during the past 4 weeks; (11) those who were illiterate; (12) those who were involved in other clinical trials within 4 weeks; (13) those with inappropriate prosthesis for electro-acupuncture (pace-maker, a heart-lung machine, an electrocardiograph, etc.) or the possibility of a hypersensitivity reaction for electro-acupuncture including epilepsy; (14) pregnant, lactating women or those suspected to be pregnant; (15) those who had difficulties to comply with treatment, visits, or questionnaires.
2.3. Randomization and Allocation Concealment
Eligible participants were randomly allocated to the EA group, the sham EA group, or the usual care group in a 1:1:1 ratio. The randomization list was generated with a block randomization method using SAS Version 9.4 (SAS institute. Inc., Cary, NC, USA) by an independent statistician. After the randomization list was created, it was coded, sealed in an opaque double envelope and numbered in order according to the randomization list, and stored in a locked cabinet. Upon participant enrollment, an individual’s envelope was opened to determine his or her group allocation. The independent investigator who was in charge of the intervention verified group allocations of participants.
2.4. Blinding
Because of the characteristic of this study, clinical investigators could not be blinded. This study was designed to blind participants and outcome assessors. Patients were informed that they would be treated with either classical EA, non-classical EA, or usual care. To obtain the blinding of participants, a Streitberger’s method [24] was used. For both the EA group and the sham EA group, a plastic ring was banded to the skin surface. Then a seemingly identical needle was inserted between the ring [25]. The Streitberger placebo needle appeared to be the same as the acupuncture needle used in the EA group. With a blunt tip, the length of the needle shaft seen outside was gradually shortened without penetration, resulting in a visual effect of penetration to the skin [26]. The new blinding index [27] and treatment credibility scale [28] were assessed at the end of the first treatment and the last treatment. Participants were asked to answer which treatment was applied, including an answer of “don’t know”. Moreover, questions to measure treatment credibility, including “how confident do you feel that this treatment can alleviate cognitive impairment?”; “how logical does this treatment seem to you?”; “how confident would you be in recommending this treatment to a friend who suffer from cognitive impairment?”; and “how successful do you think this treatment would be in alleviating other complaints?”, were asked. Patients received each intervention in an environment similar to the real clinical setting. Independent and qualified clinical psychologists performed neuropsychological assessments throughout the study period. Since they were not in charge of the intervention, blinding was fully maintained for outcome assessors.
2.5. Interventions
2.5.1. Electroacupuncture (EA)
A qualified Korean medicine doctor with clinical experience of more than two years performed EA for 24 sessions over 12 weeks (2 times a week). The skin in the treatment area was first sterilized with an isopropyl alcohol skin wipe. Sterile stainless steel acupuncture needles (0.25 mm in diameter and 25 mm in length, Asiamed, Suhl, Germany) were then inserted 5–25 mm into 14 classical acupoints, including GV20, GV24, EX-HN1, bilateral ST36, and bilateral KI3 (Figure 1a). Electric stimulation was applied at bilateral PC6 and HT7 [29] using an electroacupuncture device (ES-160, ITO Co. Ltd.) at a frequency of 3 Hz for 30 min. The intensity of the stimulation was adjusted to 80% of the intensity at which participants perceived electric stimulation. EA treatment was established using guidelines of the revised Standards for Reporting Interventions in Clinical Trial of Acupuncture (STRICTA) [30].
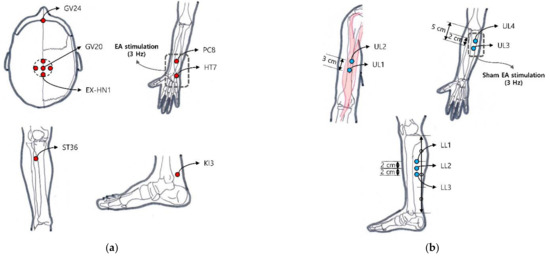
Figure 1.
Locations of classical acupoint and non-classical acupoint: (a) GV24, GV20, EX-HN1, bilateral ST36, PC8, HT7, KI3 were selected as classical acupoints in the EA group; (b) On the other hand, four bilateral points located in upper limbs and three bilateral points located in lower limbs were selected for the sham electro-acupuncture group. Sham EA stimulation was applied with light and sound stimulation at a frequency of 3Hz without electric stimulation.
2.5.2. Sham Electroacupuncture (Sham EA)
Sham EA was performed using the Streitberger placebo needle (Asiamed, Suhl, Germany) with a blunt tip that would not penetrate the skin [24]. The overall treatment process was applied the same as EA by a qualified Korean medicine doctor. Fourteen non-classical acupoints located in the upper limbs and lower limbs were selected to avoid the location-specific effect of EA. We chose sham acupoint locations not sharing the dermatomes with acupoints used in the EA group [31]. UL1 is on the anterolateral aspect of the arm at the prominence of the biceps brachii muscle belly. UL2 is located on the upper limb 2 cm superior to UL1. UL3 is on the anterior aspect of the elbow 5 cm inferior to the cubital crease and 1 cm lateral from the midline. UL4 is located on the upper limb 2 cm superior to UL3. LL1 is on the superior part of the 1/3 medial aspect of the tibia. LL2 is located on the lower limb 2 cm inferior to LL1. LL3 is located on the lower limb 2 cm inferior to LL2 (Figure 1b). Sham electric stimulation was applied with light and sound stimulation at a frequency of 3 Hz without a current flow.
2.5.3. Usual Care for MCI Patients
All participants received education to enhance their cognitive function at every visit with a brochure. At the first visit, a qualified Korean medicine doctor conducted an education program for 10 to 15 min individually based on the brochure. At every visit, the progress of cognitive problems was checked for about 5 to 10 min. The education program was composed of basic information on MCI, lifestyle management to prevent cognitive disorders, and exercise guidelines with detailed pictures and descriptions to stimulate the facial/cervical region and to enhance whole body circulation. The education program and the brochure were developed based on previous clinical guidelines for dementia in Korea and the report by the international working group on mild cognitive impairment [32].
2.6. Outcomes
This pilot trial mainly focused on exploring potential effects of EA on various cognitive function domains. A detailed neuropsychological assessment battery of the Seoul neuropsychological screening battery II (SNSB-II) was examined at baseline, after a 12-week treatment (post-treatment), and at the 24-week time point of the follow-up assessment. It was composed of various validated cognitive tests to provide T scores for five cognitive function domains (attention, language and related functions, visuospatial functions, memory, and frontal/executive functions) [33,34] adjusted by age and education level. The T score of each cognitive function domain was determined based on various tests included in the battery. The battery consisted of the Digit Span Test (DST) forward and backward score [35] for the attention domain; Comprehension and Repetition score and Korean-Boston Naming Test (K-BNT) [36] for the language domain; Rey Complex Figure Test (RCFT) Copy score [37] and Clock drawing test (CDT) for the visuospatial domain; Rey Complex Figure Test (RCFT) [37] and Seoul Verbal Learning Test (SVLT) [38] for the memory domain; and Go-No Go, Controlled Oral Word Association Test (K-COWAT) [39], Korean-Color Word Stroop Test (K-CWST) [40], Digit Symbol Coding (DSC), and Korean-Trail Making Test-Elderly’s version (K-TMT) for the frontal/executive function domain.
The Alzheimer’s disease assessment scale cognitive subscale-11 items (ADAS-Cog-11) [41,42] and MoCA-K [17,23] were examined at baseline, every four weeks during the treatment period at 4-, 8-, and 12-week time points, and at the 24-week time point of the follow-up assessment. The Hospital Anxiety and Depression Scale (HADS) [43,44] and short version of geriatric depression scale (SGDepS) [45] were measured at baseline, post-treatment, and at the follow-up assessment. Additionally, the Patient Global Impression of Change (PGIC) was examined at the post-treatment and follow-up assessment. The following question was asked to the participants: “How much did cognitive impairment improve compared to before participation in the study?”. Participants were asked to choose answers among “very much improved”, “much improved”, “minimally improved”, “no change”, “minimally worse”, “much worse”, and “very much worse”. Adverse events were carefully recorded during the study. Symptoms, severity, and causality of adverse events were documented. Laboratory tests were done at the baseline and post-treatment (12 weeks).
2.7. Statistical Analysis
This was a pilot clinical study to explore the effectiveness, safety, and feasibility of electro-acupuncture for mild cognitive impairment. For the purpose of this study, the sample size was determined to be 16 for each group considering that the standardized effect size of EA for MCI would be medium [46]. A total of 60 participants were planned to be recruited, accounting for an expected dropout rate of 20%. Adjustment for multiple comparisons of five cognitive domains was not considered in the sample size calculation, which was one limitation of this study.
Continuous variables are presented as the mean (standard deviation), and categorical variables are presented as a frequency (%). Statistical analyses for clinical outcomes were performed using the full analysis set (FAS) of the population, which included a population as similar to intent-to-treat (ITT) as possible. Participants who did not meet inclusion and exclusion criteria were excluded from the FAS. Per protocol (PP) analysis of clinical outcomes was carried out supplementarily. Safety analysis was performed for participants who received the intervention and safety evaluation more than once. All analyses were performed using SAS Version 9.4 with a significance level of 5% and two-sided tests. This pilot trial was designed as a three-arm study. There were two null hypotheses: (1) there was no difference in T scores on the SNSB cognitive function domain between the EA group and the usual care group at the post-treatment; and (2) there was no difference in T scores on the SNSB cognitive function domain between the EA group and the sham EA group at the post-treatment. A fixed sequence procedure was applied. If the previous null hypothesis was rejected, a subsequent hypothesis was then tested [47]. The least square mean difference between two groups was calculated by the analysis of covariance (ANCOVA) with the baseline score as the covariate and group as a fixed factor. Missing data were replaced by multiple imputations. No adjustment was made for multiple comparisons. Therefore, outcomes should be interpreted as exploratory.
3. Results
3.1. Participant Flow
Of 166 participants screened for eligibility, 106 were excluded due to not meeting the inclusion and exclusion criteria (n = 98) or declining to participate (n = 8). Most participants were excluded because of not meeting the criteria of MCI (n = 64) and high depression subscale in HADS (n = 35). Sixty participants were included and randomly allocated to each group in this trial (Figure 2). Twenty patients were assigned to each group (the EA group, the sham EA group, and the usual care group). During the treatment period, 11 participants declined to participate and discontinued the study, and two serious adverse events of admission not related to the intervention occurred. Two participants were found not to meet the inclusion criteria whose MoCA-K score was 23 after the randomization. Thus, they were excluded from the study. There were no notable differences in demographic characteristics at baseline among the three groups (Table 1). The subtype of MCI was judged based on the cognitive domain T score on the SNSB. Patients with a T score <40 for a domain were judged as having impairment in that domain. There were 31 (53.4%) patients with amnestic MCI and 19 (32.8%) patients with non-amnestic MCI. Eight (13.8%) patients had unspecified MCI with the above method. Their MoCA scores were below 23. They were included in this study, although they did not show impairment based on the T score on the SNSB-II.
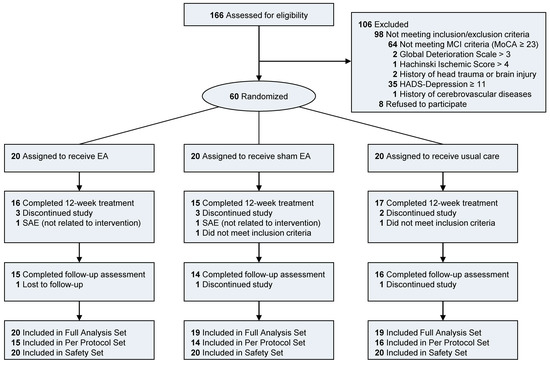
Figure 2.
Flow diagram of participants in the pilot trial of EA for mild cognitive impairment. Number of participants not meeting each inclusion/exclusion criteria were counted individually. There were six participants who did not meet the MCI criteria with higher HADS-D than 11. One participant did meet the MCI criteria with GDS higher than 3. Two serious adverse events (SAE) occurred. Both were definitely not related to interventions. Two participants did not meet the inclusion criteria after the randomization. Thus, they were excluded from the Full Analysis Set (FAS).

Table 1.
Baseline Characteristics of Participants.
From November to December 2016, 7 participants were recruited from one hospital. From December 2016 to August 2018, 53 participants were recruited from another hospital. Recruitment rates were 3.5 participants/month and 2.5 participants/month, respectively. Of 17 and 149 screened participants, 7 (41.2%) and 53 (35.6%) participants were enrolled from the two centers, respectively.
3.2. Effect of EA on Five Cognitive Function Domains
Results for the five cognitive function domains after the treatment are presented in Figure 3 and Table 2. Among the five cognitive domains of the SNSB, the visuospatial function domain score showed a difference between the EA group and the usual care group post-treatment. The visuospatial function T score in the EA group was increased from 36.40 ± 13.43 at baseline to 45.78 ± 13.20 (p = 0.0224) at the post-treatment assessment. In the usual care group, it changed from 35.72 ± 14.52 to 35.62 ± 15.15 (p = 0.8802). The LS mean difference of visuospatial function T score between the EA and the usual care group at post-treatment assessment was 10.16 (95% CI: 1.14, 19.18) (p = 0.0283). The effect size of EA compared to usual care for the visuospatial function T score calculated as the Cohen’s d was 0.72 (95% CI: 0.08, 1.35). The frontal/executive function T score was increased from 41.35 ± 10.93 to 49.29 ± 9.71 after 12 weeks of the treatment period in the EA group (p = 0.0100). In the usual care group, it changed from 42.65 ± 11.03 to 44.45 ± 9.32 (p = 0.4917). The LS mean difference of frontal/executive function T score between the EA and the usual care group at post-treatment assessment was 4.83 (95% CI: −1.30, 10.97) (p = 0.1190) with a Cohen’s d value of 0.51 (95% CI: −0.14, 1.15). For other cognitive domains, there was no noticeable difference between the EA group and the usual care group.
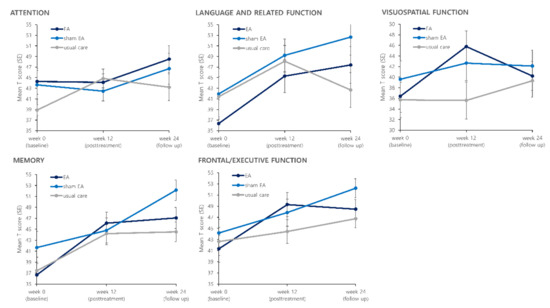
Figure 3.
Effects of treatment on five cognitive domains of Seoul Neuropsychological Screening Battery II (SNSB-II).

Table 2.
Effects of 12-week EA Treatment on T scores of Five Cognitive Function Domains Calculated with Cognitive Tests Included in Seoul Neuropsychological Screening Battery II (SNSB-II).
3.3. Effects of EA on ADAS-cog-11 and MoCA
Table 3 and Table S1 present results of the ADAS-cog-11 and MoCA after treatment. The ADAS-cog score was gradually decreased during the treatment period. It then slightly increased at the follow-up assessment in all three groups. There was no significant difference in the ADAS-cog between the EA group and the usual care group at the post-treatment assessment. The MoCA score was increased from the baseline after treatment in all three groups. There was no significant difference in its change among the three groups. For the subdomain of the MoCA, the visuospatial/executive score at week 8 was higher in the EA group than that in the usual care group (LS mean difference: 0.92 (95% CI: 0.17, 1.66), Cohen’s d = 0.81, p = 0.0170). It also tended to be higher than that in the sham EA group at week 8 (LS mean difference 0.66 (95% CI: −0.10, 1.41), Cohen’s d = 0.58, p = 0.0853).

Table 3.
Effects of 12-week EA Treatment on MoCA and ADAS-cog-11.
3.4. Effects of EA on HADS and SGDepS
Table 4 presents results of the HADS and SGDepS after treatment. They showed no difference between the EA group and the usual care group at the post-treatment assessment. In the EA group, the anxiety score of the HADS gradually decreased from 6.80 ± 3.87 to 4.14 ± 3.59 at follow-up (p = 0.0016). In the sham EA group and the usual care group, it changed from 7.89 ± 3.11 to 6.55 ± 3.88 (p = 0.2797) and from 8.26 ± 5.01 to 6.57 ± 3.68 (p = 0.1954), respectively. The depression score of the HADS and SGDepS seemed to be decreased after treatment in the EA group. At the follow-up, the SGDepS score was lower in the EA group than in the usual care group (LS mean difference: −2.50 (95% CI: −4.79, −0.21), Cohen’s d = 0.67, p = 0.0334).

Table 4.
Effects of 12-week EA Treatment on HADS and SGDepS.
3.5. Effect of EA on PGIC
Figure 4 presents results of the PGIC after treatment. At the post-treatment assessment, 37.5%, 28.57%, and 29.41% of participants in the EA group, the sham EA group, and the usual care group reported that their symptoms were much/very much improved, respectively. At the follow-up assessment, 60%, 15.38%, and 25% of participants in the EA group, the sham EA group, and the usual care group reported that their symptoms were much/very much improved, respectively, showing no significant difference among the three groups (p = 0.687 at week 12 and p = 0.101 at week 24).
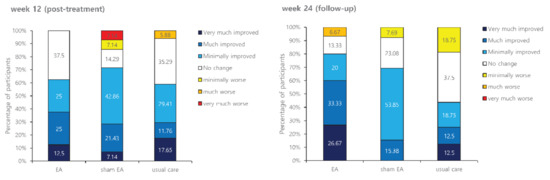
Figure 4.
Effects of treatment on Patient Global Impression of Change (PGIC).
3.6. Adverse Events
All adverse events were carefully reported during the study. The most frequent adverse event was an upper respiratory infection, which was unlikely to be related to interventions. In the EA group, 7 (1.7%) cases of adverse events were possibly or probably related to the intervention, including headache, bruising, and pruritus in a total of 407 times of the EA intervention. In the sham EA group, 1 (0.3%) case of an adverse event was possibly or probably related to the intervention in a total of 371 times of the sham EA intervention. All adverse events that were probably and possibly related to EA and sham EA were mild and recovered naturally. Two severe adverse events occurred in the EA group and the sham EA group. They were definitely not related to the intervention. One was due to a traffic accident in the EA group, and the other was due to an accidental right knee bone fracture in the sham EA group (Table 5).

Table 5.
Adverse events (all causalities) reported during the study.
3.7. Blinding Test
A blinding test was done twice for the EA group and the sham EA group after the first treatment period (week 0) and at the end of the treatment period (week 12). At the first blinding test, the new blinding index was 0.7 (0.499, 0.901) for the EA group and −0.55 (−0.808, −0.292) for the sham EA group. At the second blinding test, the blinding index was 0.875 (0.713, 1.037) for the EA group and −0.667 (−1.021, −0.313) for the sham EA group. Participants in both groups thought that they received classical electro-acupuncture, meaning that the blinding was successful. The blinding was successful throughout the study. It was maintained from the first treatment to the last treatment (Table 6). The credibility of treatment was also measured at the same time, showing no significant difference between the EA group and the sham EA group, meaning that the blinding was successful (Table S4).

Table 6.
Blinding test.
4. Discussion
The aim of this pilot study was to explore which cognitive domain could be improved by the EA. Due to the small sample size, it was not enough to conclude the effect of EA on various cognitive functions. According to the results of our pilot study, the estimated effect size (Cohen’s d) of EA on visuospatial function compared to usual care was 0.72, indicating a medium effect [48]. Moreover, the effect size of EA on the frontal/executive domain compared to usual care was 0.51, also indicating a medium effect. A noticeable difference between the EA group and the sham EA group was not observed in this study.
Visuospatial dysfunction that relies on the parietal lobe is one of the prominent early features of Alzheimer’s disease [49]. Executive dysfunction may also contribute to the impairment of instrumental activities of daily living, which is important for patients’ and caregivers’ quality of life [50]. According to a large longitudinal aMCI cohort in Korea, frontal/executive dysfunction can increase the conversion rate from aMCI to dementia [15]. A previous study on healthy older adults showed that EA treatment on GV20, EX-HN1, GV24, LI4, HT7, PC6, ST36, LR3, SP6, KI3 can enhance the visuospatial/executive function score of MoCA compared to the control [51]. Another study on patients with vascular cognitive impairment reported that EA can improve the visuospatial/executive function score of MoCA compared to the control [52]. A randomized controlled trial in China showed that MCI patients treated with EA have a better improvement in the CDT score, a representative test for visuospatial function, than MCI patients treated with nimodipine [53]. Thus, improvements in visuospatial/executive function after the EA treatment could be expected [22].
There have been a few pilot studies on acupuncture for attention, language, and memory domains. In a previous pilot study of acupuncture for MCI patients, acupuncture at EX-HN1, EX-HN3, PC6, KI3, ST40, and LR3 improved the digit span test score, a representative test for attention [22]. In another study, stroke patients with impaired cognition who applied EA at PC6 and HT7 showed a tendency of higher memory function compared to the control group, which was not statistically significant [29]. Moreover, a systematic review summarized that acupuncture is effective in improving language function in patients with post-stroke aphasia [54]. In our pilot study, the estimated effect sizes of EA for the attention, language, and memory domains were relatively small. However, this pilot study was conducted with a limited sample size, and the 95% CI ranges of the estimated effect size were relatively wide. Therefore, in the future, with confirmative research with a sufficient sample size, there is a possibility that results will differ from those of this pilot study. Based on the results of this study, it will be possible to plan a confirmative trial with an appropriate sample size to find which cognitive function domains are specifically improved by EA.
There have been limited studies about the effects of EA and sham EA on cognitive impairment. In most previous randomized controlled trials of EA for cognitive disorder, active controls (e.g., nimodipine and donepezil) were selected for comparison while a sham control was rarely used [5,55]. In our pilot study, we used the Streitberger placebo needle [24] that would not penetrate the skin at non-classical acupoints without applying an electric stimulation. Blinding was assessed as successful throughout the study, considering that most participants in both groups thought that they received classical EA. The credibility of treatment between the two groups was not statistically different. EA has three active components [56]: acupuncture needle location, depth of acupuncture needle insertion, and electric stimulation. First, the acupoint location used in this study—PC6 and HT7—might not have a specific effect on EA stimulation in MCI patients. Another previous study reported that KI3 has a different effect on intrinsic brain activity compared to the sham acupoint in patients with mild cognitive impairment [21]. Moreover, GV20, which has been commonly used in other trials of MCI, may have a potential specific effect. Limited studies have compared GV20 with sham acupoints for MCI patients. One trial using GV20 for depressed patients reported a location-specific effect of GV20 [57]. Second, the Streitberger placebo needle that could touch the skin without penetrating the skin was used in this study. For pain management, there have been reports that EA with skin penetration is superior to sham EA without skin penetration [58]. Meanwhile, a previous study on MCI patients reported that deep needle insertion on KI3 shows a different effect on the brain compared to superficial insertion [59], which required further research about the specific effect of the depth of acupuncture insertion for MCI. Third, 3 Hz of electric stimulation was used in this study. Different methods of EA stimulation may be required. An in vivo study showed that high-frequency electric stimulation (50 Hz) was superior to ameliorate cognitive impairments in a rat model compared to low (2 Hz) or medium (30 Hz) frequency electric stimulation [60].
In our pilot trial, the SGDepS score, which measures geriatric depression symptoms, seemed to show improvement after the EA treatment. We excluded patients who were having cognitive impairment due to depression. The baseline mean HADS-D score was 5.90, which was under the 8-point of cut-off value for screening depression [44]. However, the co-morbid prevalence of MCI and depression has been reported to be high, ranging from 25% to 50%. Managing geriatric depressive symptoms in MCI patients is also important [61]. EA has been reported to have a potential for relieving depression [62,63,64]. Our pilot study results suggest that EA may improve depressive symptoms in MCI patients. Further studies are needed to explore effects of EA on both depressive symptoms and cognitive decline among elderly people.
Mild adverse events that were reported mainly included bruising after EA treatment. In a previous study, hematoma and bleeding occurred in 3.19% and 1.38% of patients who received acupuncture treatment, respectively [65]. In another prospective observational study, 6.14% of acupuncture-treated patients experienced bleeding/hematoma, and 81.4% of those adverse events did not need treatment [66]. In our study, bruising occurred four times (0.98%) in a total of 407 times of EA treatment. Electric stimulation was applied at PC6 and HT7 located in the ventral forearm [67] and acupuncture points on the cranial skin and superficial fascia known to have abundant blood circulation [68]. This might be a reason for the high frequency of bruising in the EA group. Based on our inclusion criteria, elderly participants with an age over 40 years but under 80 years were included. The mean age of participants was over 60 years. It has been reported that the older the patient, the higher the risk of side effects [68]. When EA treatment is applied to elderly MCI patients, the common risk of minor bleeding and bruising may be considered. In every case of our pilot trial, those adverse events were minor. They did not need additional treatment other than close observation.
Various acupuncture points were used for cognitive disorders. In our trial, bilateral PC6 and HT7 were used for electric stimulation based on a previous study about the effect of EA on the cognitive function of stroke patients [29]. In other clinical trials of EA for MCI that excluded vascular MCI (vMCI), EA stimulation was mainly applied on the head and neck region, including EX-HN1, GV20, GV24, and GB20 [4]. Frequently used acupoints for MCI, including GV24, GV20, EX-HN1, and KI3, were also used in our study. However, electric stimulation was not applied at these acupoints. In animal studies, EA stimulation was applied on the head and neck region or KI3 on the ankle region [69,70,71,72]. Further studies are required to test the potential effect of electric stimulation on acupoints located in the head and neck region for improving cognitive decline.
This study has several limitations. First, considering the Bonferroni correction for multiple comparisons of the five cognitive function domains, the sample size should be larger. After the Bonferonni correction, a p-value less than 0.01 indicated statistical significance. Results of our study were insufficient to conclude the effect of EA on cognitive functions. A large-scale clinical study is needed. Second, EA treatment twice a week might not have been sufficient enough to be effective in improving cognitive functions. In a previous study, EA treatment was performed three times a week [19]. Third, the drop-out ratio was relatively high because the treatment period and follow-up period were long. PP analysis was also conducted. Results are presented in Table S2, showing a similar tendency to results of the FAS analysis. Another analysis adjusting the age, sex, education year, and site also showed similar results (Table S3).
This pilot study also aimed to evaluate the feasibility of a massive clinical trial. Sixty participants were recruited from two Korean medical university hospitals during about two years. Approximately 2–3 participants were recruited per month. The screening failure rate was 66.85% mainly because of not meeting the inclusion criteria (MoCA-K score over 22 or HADS score over 11). The drop-out rate was 25.00% at the follow-up period. The assessment for each participant took more than two hours. Modifying the assessment to be simpler and briefer would lower the drop-out rate. If the visuospatial function T score of the SNSB-II is used as the primary outcome in further research, the required sample size is estimated to be 31 per group based on our pilot study result. The ADAS-cog-11 did not appear to be appropriate to measure the effect of EA for patients with MCI. The ADAS-cog-13 with additional tests for MCI [41,73,74] should be considered. Regarding sham control, it would be better to find ways to increase the specific effect of EA by improving the electric stimulation method and acupoint selection for cognitive impairment.
5. Conclusions
In this pilot trial, we could see a possible but not statistically significant effect of EA on visuospatial function in patients with MCI. From our results, the effect size of EA on visuospatial function was estimated to be medium. It was not enough to conclude the effect of EA on various cognitive functions based on our study. Large-scale clinical trials are needed to confirm effects of EA on cognitive functions. Results of this pilot trial may provide useful information for further clinical trials of EA for MCI patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/brainsci11060756/s1, Table S1: Effects of treatment on cognitive tests included in SNSB, subdomain of MoCA, and subdomain of ADAS-cog-11 (FAS analysis); Table S2: Effects of treatment on SNSB five cognitive domains, cognitive tests included in SNSB, HADS, MoCA, and ADAS-cog-11 (PP analysis); Table S3: Effects of treatment on SNSB five cognitive domains, cognitive tests included in SNSB, HADS, MoCA, and ADAS-cog-11 (FAS analysis, adjusted by the age, sex, education year, and site); Table S4: Results of credibility of treatment assessment.
Author Contributions
Conceptualization, J.-H.K.; formal analysis, Y.C. and O.K.; investigation, I.-C.J., A.-R.K., and H.-J.P.; data curation, O.K.; writing—original draft preparation, Y.C.; writing—review and editing, I.-C.J., A.-R.K., H.-J.P., O.K., J.-H.L., and J.-H.K.; supervision, J.-H.K.; funding acquisition, J.-H.L. and J.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Korea Institute of Oriental Medicine, grant number K16122 and KSN1522120.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Daejeon Korean Medical Hospital of Daejeon University (protocol no: KCR1604, date of approval: 2016-10-22).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank all the participants and the research staff for their contributions to collecting data.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mayor, D. An exploratory review of the electroacupuncture literature: Clinical applications and endorphin mechanisms. Acupunct. Med. 2013, 31, 409–415. [Google Scholar] [CrossRef]
- Zhang, R.; Lao, L.; Ren, K.; Berman, B.M. Mechanisms of acupuncture–electroacupuncture on persistent pain. Anesthesiol. J. Am. Soc. Anesthesiol. 2014, 120, 482–503. [Google Scholar] [CrossRef]
- Song, H.-J.; Cho, M.-R. The Effects of Acupuncture at Sobu (HT8) and Haenggan (LR2) on Scopolamine-induced Cognitive Impairment in Rat Model. J. Neuroimmune Pharmacol. 2018, 35, 28–36. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.K.; Kim, S.Y.; Kim, Y.I.; Yoo, H.R.; Jung, I.C. Cognitive improvement effects of electro-acupuncture for the treatment of MCI compared with Western medications: A systematic review and Meta-analysis. BMC Complementary Altern. Med. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Deng, M.; Wang, X.F. Acupuncture for amnestic mild cognitive impairment: A meta-analysis of randomised controlled trials. Acupunct. Med. 2016, 34, 342–348. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Yu, S.-F.; Xue, H.-Y.; Li, Y.; Zhao, C.; Jin, Y.-H. Effectiveness and Safety of Acupuncture for the Treatment of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 98. [Google Scholar] [CrossRef]
- Kim, M.W.; Yoo, J.H.; Go, H.J.; Kim, S.W.; Jang, S.W.; Jeong, H.J.; Kim, J.H. Systematic Review of Acupuncture Treatment for Mild Cognitive Impairment. Journal of Acupuncture Research 2019, 36, 72–79. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Sachs-Ericsson, N.; Blazer, D.G. The new DSM-5 diagnosis of mild neurocognitive disorder and its relation to research in mild cognitive impairment. Aging Ment. Health 2015, 19, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.; Li, R.; Lyketsos, C.; Livingston, G. Treatment for mild cognitive impairment: Systematic review. Br. J. Psychiatry 2013, 203, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Langa, K.M.; Levine, D.A. The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 2014, 312, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, K.; Hermeneit, S. High rate of conversion to Alzheimer’s disease in a cohort of amnestic MCI patients. Int. Psychogeriatr. 2008, 20, 96–108. [Google Scholar] [CrossRef]
- Jung, Y.H.; Park, S.; Jang, H.; Cho, S.H.; Kim, S.J.; Kim, J.P.; Kim, S.T.; Na, D.L.; Seo, S.W.; Kim, H.J. Frontal-executive dysfunction affects dementia conversion in patients with amnestic mild cognitive impairment. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Cockrell, J.R.; Folstein, M.F. Mini-Mental State Examination in Principles and Practice of Geriatric Psychiatry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2002; pp. 140–141. [Google Scholar]
- Zhang, H.; Zhao, L.; Yang, S.; Chen, Z.; Li, Y.; Peng, X.; Yang, Y.; Zhu, M. Clinical observation on effect of scalp electroacupuncture for mild cognitive impairment. J. Tradit. Chin. Med. 2013, 33, 46–50. [Google Scholar] [CrossRef]
- Kim, J.-H.; Han, J.-Y.; Park, G.-C.; Lee, J.-S. Cognitive Improvement Effects of Electroacupuncture Combined with Computer-Based Cognitive Rehabilitation in Patients with Mild Cognitive Impairment: A Randomized Controlled Trial. Brain Sci. 2020, 10, 984. [Google Scholar] [CrossRef]
- Jia, B.; Liu, Z.; Min, B.; Wang, Z.; Zhou, A.; Li, Y.; Qiao, H.; Jia, J. The effects of acupuncture at real or sham acupoints on the intrinsic brain activity in mild cognitive impairment patients. Evid. Based Complementary Altern. Med. 2015, 2015, 529675. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.-t.; Wang, D.; Huang, J.-k.; Zhou, X.-m.; Yuan, X.; Liang, J.-p.; Yin, L.; Xie, H.-l.; Jia, X.-y.; Shi, J. Modulatory effects of acupuncture on brain networks in mild cognitive impairment patients. Neural Regen. Res. 2017, 12, 250. [Google Scholar]
- Lee, J.-Y.; Lee, D.W.; Cho, S.-J.; Na, D.L.; Jeon, H.J.; Kim, S.-K.; Lee, Y.R.; Youn, J.-H.; Kwon, M.; Lee, J.-H. Brief screening for mild cognitive impairment in elderly outpatient clinic: Validation of the Korean version of the Montreal Cognitive Assessment. J. Geriatr. Psychiatry Neurol. 2008, 21, 104–110. [Google Scholar] [CrossRef]
- Streitberger, K.; Kleinhenz, J. Introducing a placebo needle into acupuncture research. The Lancet 1998, 352, 364–365. [Google Scholar] [CrossRef]
- McManus, C.A.; Schnyer, R.N.; Kong, J.; Nguyen, L.T.; Hyun Nam, B.; Goldman, R.; Stason, W.B.; Kaptchuk, T.J. Sham acupuncture devices--practical advice for researchers. Acupunct. Med. 2007, 25, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Gao, Y.; Chang, J.; Kong, J. Placebo acupuncture devices: Considerations for acupuncture research. Evid. Based Complementary Altern. Med. 2013, 2013, 628907. [Google Scholar] [CrossRef]
- Bang, H.; Ni, L.; Davis, C.E. Assessment of blinding in clinical trials. Control Clin. Trials 2004, 25, 143–156. [Google Scholar] [CrossRef]
- Vincent, C. Credibility assessment in trials of acupuncture. Complementary Med. Res. 1990, 4, 8–11. [Google Scholar]
- Chou, P.; Chu, H.; Lin, J.-G. Effects of electroacupuncture treatment on impaired cognition and quality of life in Taiwanese stroke patients. J. Altern. Complementary Med. 2009, 15, 1067–1073. [Google Scholar]
- MacPherson, H.; Altman, D.G.; Hammerschlag, R.; Youping, L.; Taixiang, W.; White, A.; Moher, D. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT statement. J. Altern. Complement. Med. 2010, 16, St1-14. [Google Scholar] [CrossRef]
- Ots, T.; Kandirian, A.; Szilagyi, I.; DiGiacomo, S.M.; Sandner-Kiesling, A. The selection of dermatomes for sham (placebo) acupuncture points is relevant for the outcome of acupuncture studies: A systematic review of sham (placebo)-controlled randomized acupuncture trials. Acupunct. Med. 2020, 38, 211–226. [Google Scholar] [CrossRef]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef]
- Kang, Y.; Jang, S.; Na, D. Seoul neuropsychological screening battery (SNSB-II;); Human Brain Research & Consulting Co.: Seoul, Korea, 2012. [Google Scholar]
- Ahn, H.-J.; Chin, J.; Park, A.; Lee, B.H.; Suh, M.K.; Seo, S.W.; Na, D.L. Seoul Neuropsychological Screening Battery-dementia version (SNSB-D): A useful tool for assessing and monitoring cognitive impairments in dementia patients. J. Korean Med. Sci. 2010, 25, 1071–1076. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, D.Y.; Seo, E.H.; Jo, M.K.; Sohn, B.K.; Choe, Y.M.; Byun, M.S.; Kim, J.W.; Kim, S.G.; Yoon, J.C.; et al. A normative study of the digit span in an educationally diverse elderly population. Psychiatry Investig. 2014, 11, 39–43. [Google Scholar] [CrossRef]
- Kim, H.; Na, D.L. Normative data on the Korean version of the Boston naming test. J. Clin. Exp. Neuropsychol. 1999, 21, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.E.; Meyers, K.R. Rey Complex Figure Test and recognition trial professional manual; Psychological Assessment Resources: Odessa, FL, USA, 1995. [Google Scholar]
- Baek, M.J.; Kim, H.J.; Kim, S. Comparison between the story recall test and the word-list learning test in Korean patients with mild cognitive impairment and early stage of Alzheimer’s disease. J. Clin. Exp. Neuropsychol 2012, 34, 396–404. [Google Scholar] [CrossRef]
- Kang, Y.; Chin, J.-H.; Na, D.; Lee, J.; Park, J. A normative study of the Korean version of Controlled Oral Word Association Test (COWAT) in the elderly. Korean journal of clinical psychology 2000, 19, 385–392. [Google Scholar]
- Lee, J.; Kang, Y.; Na, D. Efficiencies of stroop interference indexes in healthy older adults and dementia patients. Korean J. Clin. Psychol. 2000, 19, 807–818. [Google Scholar]
- Mohs, R.C.; Knopman, D.; Petersen, R.C.; Ferris, S.H.; Ernesto, C.; Grundman, M.; Sano, M.; Bieliauskas, L.; Geldmacher, D.; Clark, C. Development of cognitive instruments for use in clinical trials of antidementia drugs: Additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. Alzheimer Dis. Assoc. Disord. 1997, 11, S13–S21. [Google Scholar] [CrossRef]
- Youn, J.; Lee, D.; Kim, K.; Lee, J.; Jhoo, J.; Lee, K. Development of the Korean version of Alzheimer’s Disease Assess ment Scale (ADAS-K). Int. J. Geriatr. Psychiatry 2002, 17, 797–803. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Oh, S.M.; Min, K.J.; Park, D.B. A study on the standardization of the hospital anxiety and depression scale for Koreans: A comparison of normal, depressed and anxious groups. J. Korean Neuropsychiatr. Assoc. 1999, 38, 289–296. [Google Scholar]
- Burke, W.J.; Roccaforte, W.H.; Wengel, S.P. The short form of the Geriatric Depression Scale: A comparison with the 30-item form. Top. Geriatr. 1991, 4, 173–178. [Google Scholar] [CrossRef]
- Whitehead, A.L.; Julious, S.A.; Cooper, C.L.; Campbell, M.J. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat. Methods Med. Res. 2015, 25, 1057–1073. [Google Scholar] [CrossRef]
- Wiens, B.L. A fixed sequence Bonferroni procedure for testing multiple endpoints. Pharm. Stat. J. Appl. Stat. Pharm. Ind. 2003, 2, 211–215. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Mandal, P.K.; Joshi, J.; Saharan, S. Visuospatial perception: An emerging biomarker for Alzheimer’s disease. J. Alzheimers Dis. 2012, 31, S117–S135. [Google Scholar] [CrossRef]
- Marshall, G.A.; Rentz, D.M.; Frey, M.T.; Locascio, J.J.; Johnson, K.A.; Sperling, R.A. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2011, 7, 300–308. [Google Scholar] [CrossRef]
- Fan, D.Q.; Zhao, H.C.; Sheng, J.; Liu, Y.R.; Yu, J. Electroacupuncture Modulates Resting-State Functional Connectivity in the Default Mode Network for Healthy Older Adults. J. Geriatr. Psychiatry Neurol. 2020, 33, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Su, W.; Hu, H.; Zhou, L.; Jin, X. The effect of medicated thread moxibustion combined with electro acupuncture treatment on Montreal Cognitive Assessment (MoCA) of patients with vascular cognitive impairment of none dementia. Biomed. Res. 2017, 28, 1871–1877. [Google Scholar]
- Xu, J.-g.; Peng, C.-b. The clinical study of the electroacupuncture for treatment of amnestic mild cognitive impairment. Chin. J. Gen. Pract. 2017, 15, 393–396. [Google Scholar]
- Zhang, B.; Han, Y.; Huang, X.; Liu, Z.; Li, S.; Chang, J.; Gao, Y. Acupuncture is effective in improving functional communication in post-stroke aphasia. Wiener klinische Wochenschrift 2019, 131, 221–232. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, W.; Xu, M.; Li, W.; Liu, Z. The effectiveness and safety of acupuncture for patients with Alzheimer disease: A systematic review and meta-analysis of randomized controlled trials. Medicine 2015, 94, e933. [Google Scholar] [CrossRef]
- Chen, Z.-x.; Li, Y.; Zhang, X.-g.; Chen, S.; Yang, W.-t.; Zheng, X.-w.; Zheng, G.-q. Sham electroacupuncture methods in randomized controlled trials. Sci. Rep. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Song, C.; Halbreich, U.; Han, C.; Leonard, B.; Luo, H. Imbalance between pro-and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: The effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry 2009, 42, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ning, Z.; Lam, W.L.; Lam, W.-Y.; Zhao, Y.K.; Yeung, J.W.F.; Ng, B.F.-L.; Ziea, E.T.-C.; Lao, L. Types of control in acupuncture clinical trials might affect the conclusion of the trials: A review of acupuncture on pain management. J. Acupunct. Meridian Stud. 2016, 9, 227–233. [Google Scholar] [CrossRef]
- Feng, Y.; Bai, L.; Ren, Y.; Chen, S.; Wang, H.; Zhang, W.; Tian, J. FMRI connectivity analysis of acupuncture effects on the whole brain network in mild cognitive impairment patients. Magn. Reson. Imaging 2012, 30, 672–682. [Google Scholar] [CrossRef]
- Yu, C.-C.; Wang, Y.; Shen, F.; Kong, L.-H.; Wang, Y.-W.; Zhou, H.; Tang, L. High-frequency (50 Hz) electroacupuncture ameliorates cognitive impairment in rats with amyloid beta 1–42-induced Alzheimer’s disease. Neural Regen. Res 2018, 13, 1833. [Google Scholar] [PubMed]
- Steffens, D.C.; Potter, G. Geriatric depression and cognitive impairment. Psychiatry Clin. Neurosci. 2008, 38, 163–175. [Google Scholar] [CrossRef]
- Luo, H.; MENG, F.; JIA, Y.; ZHAO, X. Clinical research on the therapeutic effect of the electro-acupuncture treatment in patients with depression. Psychiatry Clin. Neurosci. 1998, 52, S338–S340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-j.; Shi, Z.-y.; Liu, S.; Gong, S.-h.; Liu, J.-q.; Liu, J.-s. Clinical observation on treatment of depression by electro-acupuncture combined with Paroxetine. Chinese journal of integrative medicinChin. J. Integr. Med. 2007, 13, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Armour, M.; Lee, M.S.; Wang, L.Q.; Hay, P.J. Acupuncture for depression. Cochrane Database Syst. Rev. 2018, 3, Cd004046. [Google Scholar] [CrossRef]
- Melchart, D.; Weidenhammer, W.; Streng, A.; Reitmayr, S.; Hoppe, A.; Ernst, E.; Linde, K. Prospective investigation of adverse effects of acupuncture in 97 733 patients. Arch. Intern. Med. 2004, 164, 104–105. [Google Scholar] [CrossRef]
- Witt, C.M.; Pach, D.; Brinkhaus, B.; Wruck, K.; Tag, B.; Mank, S.; Willich, S.N. Safety of acupuncture: Results of a prospective observational study with 229,230 patients and introduction of a medical information and consent form. Complementary Med. Res. 2009, 16, 91–97. [Google Scholar] [CrossRef]
- Enblom, A.; Johnsson, A. Type and frequency of side effects during PC6 acupuncture: Observations from therapists and patients participating in clinical efficacy trials of acupuncture. Acupunct. Med. 2017, 35, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, F.-w.; Li, Y.; Wu, X.; Zheng, H.; Cheng, L.-h.; Liang, F.-r. Adverse events associated with acupuncture: Three multicentre randomized controlled trials of 1968 cases in China. Trials 2011, 12, 87. [Google Scholar] [CrossRef]
- Cai, M.; Lee, J.H.; Yang, E.J. Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model. J. Neuroinflammation 2019, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xv, G.-H.; Wang, W.-X.; Meng, D.-J.; Ji, Y. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer’s disease. Acupunct. Med. 2017, 35, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Tang, Y.; Li, Y.; Gao, K.; Shi, X.; Li, Z. Behavioral changes and hippocampus glucose metabolism in APP/PS1 transgenic mice via electro-acupuncture at governor vessel acupoints. Front. Aging Neurosci. 2017, 9, 5. [Google Scholar] [CrossRef]
- Jiang, J.; Gao, K.; Zhou, Y.; Xu, A.; Shi, S.; Liu, G.; Li, Z. Electroacupuncture treatment improves learning-memory ability and brain glucose metabolism in a mouse model of Alzheimer’s disease: Using morris water maze and micro-PET. Evid. Based Complement. Alternat. Med. 2015, 2015, 142129. [Google Scholar] [CrossRef]
- Raghavan, N.; Samtani, M.N.; Farnum, M.; Yang, E.; Novak, G.; Grundman, M.; Narayan, V.; DiBernardo, A.; Initiative, A.s.D.N. The ADAS-Cog revisited: Novel composite scales based on ADAS-Cog to improve efficiency in MCI and early AD trials. Alzheimer Dement. 2013, 9, S21–S31. [Google Scholar] [CrossRef]
- Podhorna, J.; Krahnke, T.; Shear, M.; Harrison, J.E. Alzheimer’s Disease Assessment Scale–Cognitive subscale variants in mild cognitive impairment and mild Alzheimer’s disease: Change over time and the effect of enrichment strategies. Alzheimer Res. Ther. 2016, 8, 1–13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).