Anatomo-Functional Origins of the Cortical Silent Period: Spotlight on the Basal Ganglia
Abstract
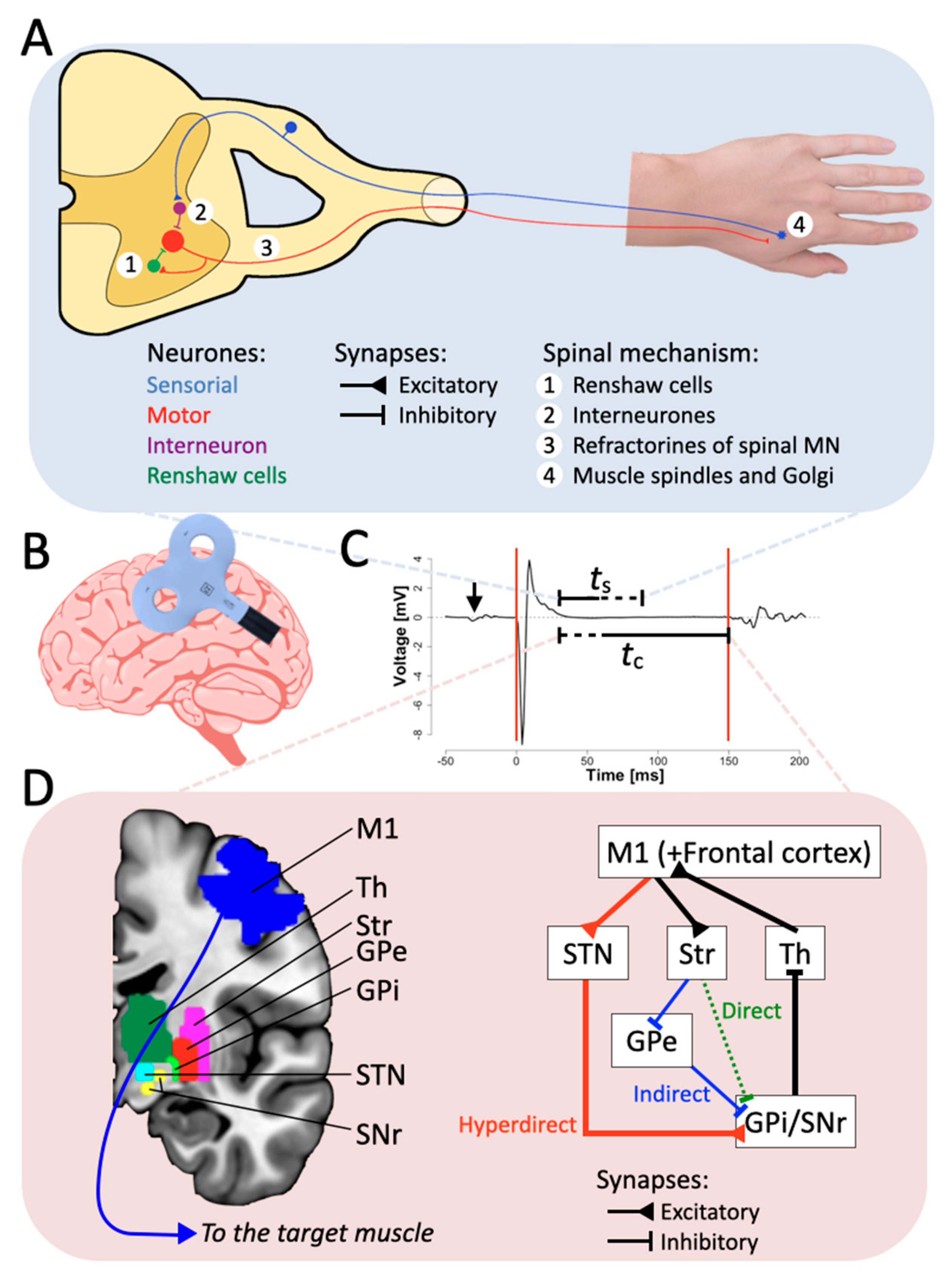
1. Introduction
2. Possible Origins of the CSP
2.1. The Spinal Origin
2.2. The Cortical Origin
2.3. Neurotransmitters
2.4. Behavior and Cognition
3. Neurological Disorders
3.1. Motor Neurological Disorders
3.2. Non-Motor Neurological Disorders
3.3. Overview
4. Neural Mechanisms of CSP
4.1. Basal Ganglia Involvement in Motor Control and CSP
4.2. Hyperdirect Pathway and Motor Neurological Disorders Influencing CSP
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSP | cortical silent period |
| TMS | transcranial magnetic stimulation |
| MEP | motor-evoked potential |
| M1 | primary motor cortex |
| GABA | gamma-aminobutyric acid |
| L-DOPA | levodopa |
| CBGTC | cortico-basal ganglia-thalamo-cortical |
References
- Hupfeld, K.E.; Swanson, C.W.; Fling, B.W.; Seidler, R.D. TMS-Induced Silent Periods: A Review of Methods and Call for Consistency. J. Neurosci. Methods 2020, 346, 108950. [Google Scholar] [CrossRef]
- Cacchio, A.; Cimini, N.; Alosi, P.; Santilli, V.; Marrelli, A. Reliability of Transcranial Magnetic Stimulation-Related Measurements of Tibialis Anterior Muscle in Healthy Subjects. Clin. Neurophysiol. 2009, 120, 414–419. [Google Scholar] [CrossRef]
- Adrian, E.D.; Moruzzi, G. Impulses in the Pyramidal Tract. J. Physiol. 1939, 97, 153–199. [Google Scholar] [CrossRef] [PubMed]
- Krnjević, K.; Randić, M.; Straughan, D.W. Cortical Inhibition. Nature 1964, 201, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Krnjević, K.; Randić, M.; Straughan, D.W. An Inhibitory Process in the Cerebral Cortex. J. Physiol. 1966, 184, 16–48. [Google Scholar] [CrossRef] [PubMed]
- Cantello, R.; Gianelli, M.; Civardi, C.; Mutani, R. Magnetic Brain Stimulation: The Silent Period after the Motor Evoked Potential. Neurology 1992, 42, 1951. [Google Scholar] [CrossRef]
- Marsden, C.D.; Merton, P.A.; Morton, H.B. Direct Electrical Stimulation of Corticospinal Pathways through the Intact Scalp in Human Subjects. Adv. Neurol. 1983, 39, 387–391. [Google Scholar] [PubMed]
- De Benedictis, A.; Sarubbo, S.; Duffau, H. Subcortical Surgical Anatomy of the Lateral Frontal Region: Human White Matter Dissection and Correlations with Functional Insights Provided by Intraoperative Direct Brain Stimulation. J. Neurosurg. 2012, 117, 1053–1069. [Google Scholar] [CrossRef]
- Montemurro, N.; Herbet, G.; Duffau, H. Right Cortical and Axonal Structures Eliciting Ocular Deviation during Electrical Stimulation Mapping in Awake Patients. Brain Topogr. 2016, 29, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Calancie, B.; Nordin, M.; Wallin, U.; Hagbarth, K.E. Motor-Unit Responses in Human Wrist Flexor and Extensor Muscles to Transcranial Cortical Stimuli. J. Neurophysiol. 1987, 58, 1168–1185. [Google Scholar] [CrossRef]
- DeLong, M.R.; Wichmann, T. Circuits and Circuit Disorders of the Basal Ganglia. Arch. Neurol. 2007, 64, 20. [Google Scholar] [CrossRef]
- Farzan, F.; Barr, M.S.; Hoppenbrouwers, S.S.; Fitzgerald, P.B.; Chen, R.; Pascual-Leone, A.; Daskalakis, Z.J. The EEG Correlates of the TMS-Induced EMG Silent Period in Humans. NeuroImage 2013, 83, 120–134. [Google Scholar] [CrossRef]
- Grimaldi, G.; Argyropoulos, G.P.; Boehringer, A.; Celnik, P.; Edwards, M.J.; Ferrucci, R.; Galea, J.M.; Groiss, S.J.; Hiraoka, K.; Kassavetis, P.; et al. Non-Invasive Cerebellar Stimulation—A Consensus Paper. Cerebellum 2014, 13, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Matsugi, A.; Douchi, S.; Suzuki, K.; Oku, K.; Mori, N.; Tanaka, H.; Nishishita, S.; Bando, K.; Kikuchi, Y.; Okada, Y. Cerebellar Transcranial Magnetic Stimulation Reduces the Silent Period on Hand Muscle Electromyography During Force Control. Brain Sci. 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-García, J.M.; da Guarda, S.N.F.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Mariën, P.; et al. Consensus Paper: Roles of the Cerebellum in Motor Control—The Diversity of Ideas on Cerebellar Involvement in Movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Dileone, M.; Pilato, F.; Capone, F.; Musumeci, G.; Ranieri, F.; Ricci, V.; Bria, P.; Di Iorio, R.; De Waure, C. Modulation of Motor Cortex Neuronal Networks by RTMS: Comparison of Local and Remote Effects of Six Different Protocols of Stimulation. J. Neurophysiol. 2011, 105, 2150–2156. [Google Scholar] [CrossRef]
- Shibasaki, H. Cortical Activities Associated with Voluntary Movements and Involuntary Movements. Clin. Neurophysiol. 2012, 123, 229–243. [Google Scholar] [CrossRef]
- Merton, P.A. The Silent Period in a Muscle of the Human Hand. J. Physiol. 1951, 114, 183–198. [Google Scholar] [CrossRef]
- Ryall, R.; Piercey, M.; Polosa, C.; Goldfarb, J. Excitation of Renshaw Cells in Relation to Orthodromic and Antidromic Excitation of Motoneurons. J. Neurophysiol. 1972, 35, 137–148. [Google Scholar] [CrossRef]
- Ziemann, U.; Netz, J.; Szelényi, A.; Hömberg, V. Spinal and Supraspinal Mechanisms Contribute to the Silent Period in the Contracting Soleus Muscle after Transcranial Magnetic Stimulation of Human Motor Cortex. Neurosci. Lett. 1993, 156, 167–171. [Google Scholar] [CrossRef]
- Ryall, R. Renshaw Cell Mediated Inhibition of Renshaw Cells: Patterns of Excitation and Inhibition from Impulses in Motor Axon Collaterals. J. Neurophysiol. 1970, 33, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Person, R.S.; Kozhina, G.V. Investigation of the Silent Period by a Poststimulus Histogram Method. Neurophysiology 1978, 10, 123–129. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, E.; Bussel, B.; Held, J.P.; Katz, R. Excitability of Human Motoneurones after Discharge in a Conditioning Reflex. Electroencephalogr. Clin. Neurophysiol. 1976, 40, 279–287. [Google Scholar] [CrossRef]
- Hoffmann, P. Demonstration Eines Hemmungsreflexes Ira Mensehlichen Rtiekeumark. Z. Biol. 1920, 515, 70. [Google Scholar]
- Inghilleri, M.; Berardelli, A.; Marchetti, P.; Manfredi, M. Effects of Diazepam, Baclofen and Thiopental on the Silent Period Evoked by Transcranial Magnetic Stimulation in Humans. Exp. Brain Res. 1996, 109, 467–472. [Google Scholar] [CrossRef]
- Fuhr, P.; Agostino, R.; Hallett, M. Spinal Motor Neuron Excitability during the Silent Period after Cortical Stimulation. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1991, 81, 257–262. [Google Scholar] [CrossRef]
- Classen, J.; Benecke, R. Inhibitory Phenomena in Individual Motor Units Induced by Transcranial Magnetic Stimulation. Electroencephalogr. Clin. Neurophysiol. 1995, 11, 264–274. [Google Scholar]
- Škarabot, J.; Mesquita, R.N.O.; Brownstein, C.G.; Ansdell, P. Myths and Methodologies: How Loud Is the Story Told by the Transcranial Magnetic Stimulation-evoked Silent Period? Exp. Physiol. 2019, 104, 635–642. [Google Scholar] [CrossRef]
- Butler, J.E.; Petersen, N.C.; Herbert, R.D.; Gandevia, S.C.; Taylor, J.L. Origin of the Low-Level EMG during the Silent Period Following Transcranial Magnetic Stimulation. Clin. Neurophysiol. 2012, 123, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.C.; Stinear, J.W.; Rich, T.; Chen, C.-Y.; Feyma, T.; Meekins, G.D.; Gillick, B.T. EMG Breakthrough during Cortical Silent Period in Congenital Hemiparesis: A Descriptive Case Series. Braz. J. Phys. Ther. 2020, 24, 20–29. [Google Scholar] [CrossRef]
- Vry, J.; Linder-Lucht, M.; Berweck, S.; Bonati, U.; Hodapp, M.; Uhl, M.; Faist, M.; Mall, V. Altered Cortical Inhibitory Function in Children with Spastic Diplegia: A TMS Study. Exp. Brain Res. 2008, 186, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Roick, H.; von Giesen, H.J.; Benecke, R. On the Origin of the Postexcitatory Inhibition Seen after Transcranial Magnetic Brain Stimulation in Awake Human Subjects. Exp. Brain Res. 1993, 94, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, A.; Benecke, R. The Silent Period after Transcranial Magnetic Stimulation Is of Exclusive Cortical Origin: Evidence from Isolated Cortical Ischemic Lesions in Man. Neurosci. Lett. 1994, 180, 41–45. [Google Scholar] [CrossRef]
- Yacyshyn, A.F.; Woo, E.J.; Price, M.C.; McNeil, C.J. Motoneuron Responsiveness to Corticospinal Tract Stimulation during the Silent Period Induced by Transcranial Magnetic Stimulation. Exp. Brain Res. 2016, 234, 3457–3463. [Google Scholar] [CrossRef]
- Chen, R.; Lozano, A.M.; Ashby, P. Mechanism of the Silent Period Following Transcranial Magnetic Stimulation. Exp. Brain Res. 1999, 128, 539–542. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial Magnetic Stimulation. Negative Effects. Adv. Neurol. 1995, 67, 107–113. [Google Scholar]
- Kobayashi, M.; Pascual-Leone, A. Transcranial Magnetic Stimulation in Neurology. Lancet Neurol. 2003, 2, 145–156. [Google Scholar] [CrossRef]
- Tazoe, T.; Endoh, T.; Nakajima, T.; Sakamoto, M.; Komiyama, T. Disinhibition of Upper Limb Motor Area by Voluntary Contraction of the Lower Limb Muscle. Exp. Brain Res. 2007, 177, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Kimberley, T.J.; Borich, M.R.; Arora, S.; Siebner, H.R. Multiple Sessions of Low-Frequency Repetitive Transcranial Magnetic Stimulation in Focal Hand Dystonia: Clinical and Physiological Effects. Restor. Neurol. Neurosci. 2013, 31, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Stinear, C.M.; Coxon, J.P.; Byblow, W.D. Primary Motor Cortex and Movement Prevention: Where Stop Meets Go. Neurosci. Biobehav. Rev. 2009, 33, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Davranche, K.; Tandonnet, C.; Burle, B.; Meynier, C.; Vidal, F.; Hasbroucq, T. The Dual Nature of Time Preparation: Neural Activation and Suppression Revealed by Transcranial Magnetic Stimulation of the Motor Cortex. Eur. J. Neurosci. 2007, 25, 3766–3774. [Google Scholar] [CrossRef] [PubMed]
- Desiato, M.T.; Caramia, M.D. Towards a Neurophysiological Marker of Amyotrophic Lateral Sclerosis as Revealed by Changes in Cortical Excitability. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1997, 105, 1–7. [Google Scholar] [CrossRef]
- Orth, M.; Rothwell, J.C. The Cortical Silent Period: Intrinsic Variability and Relation to the Waveform of the Transcranial Magnetic Stimulation Pulse. Clin. Neurophysiol. 2004, 115, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Säisänen, L.; Pirinen, E.; Teitti, S.; Könönen, M.; Julkunen, P.; Määttä, S.; Karhu, J. Factors Influencing Cortical Silent Period: Optimized Stimulus Location, Intensity and Muscle Contraction. J. Neurosci. Methods 2008, 169, 231–238. [Google Scholar] [CrossRef]
- Krause, P.; Straube, A. Peripheral Repetitive Magnetic Stimulation Induces Intracortical Inhibition in Healthy Subjects. Neurol. Res. 2008, 30, 690–694. [Google Scholar] [CrossRef]
- Trompetto, C.; Buccolieri, A.; Abbruzzese, G. Intracortical Inhibitory Circuits and Sensory Input: A Study with Transcranial Magnetic Stimulation in Humans. Neurosci. Lett. 2001, 297, 17–20. [Google Scholar] [CrossRef]
- Wassermann, E.M.; Fuhr, P.; Cohen, L.G.; Hallett, M. Effects of Transcranial Magnetic Stimulation on Ipsilateral Muscles. Neurology 1991, 41, 1795. [Google Scholar] [CrossRef]
- Meyer, B.-U.; Röricht, S.; von Einsiedel, H.G.; Kruggel, F.; Weindl, A. Inhibitory and Excitatory Interhemispheric Transfers between Motor Cortical Areas in Normal Humans and Patients with Abnormalities of the Corpus Callosum. Brain 1995, 118, 429–440. [Google Scholar] [CrossRef]
- Meyer, B.; Röricht, S.; Woiciechowsky, C. Topography of Fibers in the Human Corpus Callosum Mediating Interhemispheric Inhibition between the Motor Cortices. Ann. Neurol. 1998, 43, 360–369. [Google Scholar] [CrossRef]
- Li, J.-Y.; Lai, P.-H.; Chen, R. Transcallosal Inhibition in Patients with Callosal Infarction. J. Neurophysiol. 2013, 109, 659–665. [Google Scholar] [CrossRef]
- Rothwell, J.; Thompson, P.; Day, B.; Boyd, S.; Marsden, C. Stimulation of the Human Motor Cortex through the Scalp. Exp. Physiol. 1991, 76, 159–200. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Williamson, A. Convergence and Divergence of Neurotransmitter Action in Human Cerebral Cortex. Proc. Natl. Acad. Sci. USA 1989, 86, 8098–8102. [Google Scholar] [CrossRef]
- Lang, N.; Sueske, E.; Hasan, A.; Paulus, W.; Tergau, F. Pregabalin Exerts Oppositional Effects on Different Inhibitory Circuits in Human Motor Cortex: A Double-blind, Placebo-controlled Transcranial Magnetic Stimulation Study. Epilepsia 2006, 47, 813–819. [Google Scholar] [CrossRef]
- McCormick, D.A. GABA as an Inhibitory Neurotransmitter in Human Cerebral Cortex. J. Neurophysiol. 1989, 62, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, S.; Beaulé, V.; Proulx, S.; de Beaumont, L.; Marjańska, M.; Doyon, J.; Pascual-Leone, A.; Lassonde, M.; Théoret, H. Relationship between Transcranial Magnetic Stimulation Measures of Intracortical Inhibition and Spectroscopy Measures of GABA and Glutamate+glutamine. J. Neurophysiol. 2013, 109, 1343–1349. [Google Scholar] [CrossRef]
- Siebner, H.R.; Dressnandt, J.; Auer, C.; Conrad, B. Continuous Intrathecal Baclofen Infusions Induced a Marked Increase of the Transcranially Evoked Silent Period in a Patient with Generalized Dystonia. Muscle Nerve 1998, 4, 1209–1212. [Google Scholar] [CrossRef]
- Stetkarova, I.; Kofler, M. Differential Effect of Baclofen on Cortical and Spinal Inhibitory Circuits. Clin. Neurophysiol. 2013, 124, 339–345. [Google Scholar] [CrossRef]
- Werhahn, K.J.; Kunesch, E.; Noachtar, S.; Benecke, R.; Classen, J. Differential Effects on Motorcortical Inhibition Induced by Blockade of GABA Uptake in Humans. J. Physiol. 1999, 517, 591–597. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Orekhov, Y.; Ziemann, U. The Role of GABAB Receptors in Intracortical Inhibition in the Human Motor Cortex. Exp. Brain Res. 2006, 173, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, U.; Bruns, D.; Paulus, W. Enhancement of Human Motor Cortex Inhibition by the Dopamine Receptor Agonist Pergolide: Evidence from Transcranial Magnetic Stimulation. Neurosci. Lett. 1996, 208, 187–190. [Google Scholar] [CrossRef]
- Bäumer, T.; Hidding, U.; Hamel, W.; Buhmann, C.; Moll, C.K.E.; Gerloff, C.; Orth, M.; Siebner, H.R.; Münchau, A. Effects of DBS, Premotor RTMS, and Levodopa on Motor Function and Silent Period in Advanced Parkinson’s Disease. Mov. Disord. 2009, 24, 672–676. [Google Scholar] [CrossRef]
- Thorstensen, J.R.; Taylor, J.L.; Tucker, M.G.; Kavanagh, J.J. Enhanced Serotonin Availability Amplifies Fatigue Perception and Modulates the TMS-induced Silent Period during Sustained Low-intensity Elbow Flexions. J. Physiol. 2020, 598, 2685–2701. [Google Scholar] [CrossRef]
- Gandevia, S.C.; Allen, G.M.; Butler, J.E.; Taylor, J.L. Supraspinal Factors in Human Muscle Fatigue: Evidence for Suboptimal Output from the Motor Cortex. J. Physiol. 1996, 490, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R. Mechanisms of Muscular Fatigue. Med. Sport Sci. 2004, 46, 279–300. [Google Scholar]
- Allen, D.G.; Kabbara, A.A.; Westerblad, H. Muscle Fatigue: The Role of Intracellular Calcium Stores. Can. J. Appl. Physiol. 2002, 27, 83–96. [Google Scholar] [CrossRef]
- Taylor, J.L.; Butler, J.E.; Allen, G.M.; Gandevia, S.C. Changes in Motor Cortical Excitability during Human Muscle Fatigue. J. Physiol. 1996, 490, 519–528. [Google Scholar] [CrossRef]
- Zghal, F.; Cottin, F.; Kenoun, I.; Rebaï, H.; Moalla, W.; Dogui, M.; Tabka, Z.; Martin, V. Improved Tolerance of Peripheral Fatigue by the Central Nervous System after Endurance Training. Eur. J. Appl. Physiol. 2015, 115, 1401–1415. [Google Scholar] [CrossRef]
- Levin, O.; Netz, Y.; Ziv, G. Behavioral and Neurophysiological Aspects of Inhibition—The Effects of Acute Cardiovascular Exercise. J. Clin. Med. 2021, 10, 282. [Google Scholar] [CrossRef]
- Maruyama, A.; Matsunaga, K.; Tanaka, N.; Rothwell, J.C. Muscle Fatigue Decreases Short-Interval Intracortical Inhibition after Exhaustive Intermittent Tasks. Clin. Neurophysiol. 2006, 117, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Arias, P.; Robles-García, V.; Corral-Bergantiños, Y.; Madrid, A.; Espinosa, N.; Valls-Solé, J.; Grieve, K.L.; Oliviero, A.; Cudeiro, J. Central Fatigue Induced by Short-Lasting Finger Tapping and Isometric Tasks: A Study of Silent Periods Evoked at Spinal and Supraspinal Levels. Neuroscience 2015, 305, 316–327. [Google Scholar] [CrossRef]
- Gruet, M.; Temesi, J.; Rupp, T.; Levy, P.; Verges, S.; Millet, G.Y. Dynamics of Corticospinal Changes during and after High-Intensity Quadriceps Exercise: Corticospinal Responses to Quadriceps Fatigue. Exp. Physiol. 2014, 99, 1053–1064. [Google Scholar] [CrossRef]
- Liepert, J.; Kotterba, S.; Tegenthoff, M.; Malin, J. Central Fatigue Assessed by Transcranial Magnetic Stimulation. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. Med. 1996, 19, 1429–1434. [Google Scholar] [CrossRef]
- Taylor, J.L.; Gandevia, S.C. A Comparison of Central Aspects of Fatigue in Submaximal and Maximal Voluntary Contractions. J. Appl. Physiol. 2008, 104, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Benwell, N.M.; Mastaglia, F.L.; Thickbroom, G.W. Differential Changes in Long-Interval Intracortical Inhibition and Silent Period Duration during Fatiguing Hand Exercise. Exp. Brain Res. 2007, 179, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mathis, J.; de Quervain, D.; Hess, C.W. Dependence of the Transcranially Induced Silent Period on the ‘instruction Set’ and the Individual Reaction Time. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1998, 109, 426–435. [Google Scholar] [CrossRef]
- Bonnard, M.; Spieser, L.; Meziane, H.B.; de Graaf, J.B.; Pailhous, J. Prior Intention Can Locally Tune Inhibitory Processes in the Primary Motor Cortex: Direct Evidence from Combined TMS-EEG. Eur. J. Neurosci. 2009, 30, 913–923. [Google Scholar] [CrossRef]
- Hess, A.; Kunesch, E.; Classen, J.; Hoeppner, J.; Stefan, K.; Benecke, R. Task-Dependent Modulation of Inhibitory Actions within the Primary Motor Cortex. Exp. Brain Res. 1999, 124, 321–330. [Google Scholar] [CrossRef]
- Pearce, A.J.; Kidgell, D.J. Comparison of Corticomotor Excitability during Visuomotor Dynamic and Static Tasks. J. Sci. Med. Sport 2010, 13, 167–171. [Google Scholar] [CrossRef]
- Tinazzi, M.; Farina, S.; Tamburin, S.; Facchini, S.; Fiaschi, A.; Restivo, D.; Berardelli, A. Task-Dependent Modulation of Excitatory and Inhibitory Functions within the Human Primary Motor Cortex. Exp. Brain Res. 2003, 150, 222–229. [Google Scholar] [CrossRef]
- Conte, A.; Gilio, F.; Iezzi, E.; Frasca, V.; Inghilleri, M.; Berardelli, A. Attention Influences the Excitability of Cortical Motor Areas in Healthy Humans. Exp. Brain Res. 2007, 182, 109–117. [Google Scholar] [CrossRef]
- Conte, A.; Belvisi, D.; Iezzi, E.; Mari, F.; Inghilleri, M.; Berardelli, A. Effects of Attention on Inhibitory and Facilitatory Phenomena Elicited by Paired-Pulse Transcranial Magnetic Stimulation in Healthy Subjects. Exp. Brain Res. 2008, 186, 393–399. [Google Scholar] [CrossRef]
- Holste, K.G.; Yasen, A.L.; Hill, M.J.; Christie, A.D. Motor Cortex Inhibition Is Increased during a Secondary Cognitive Task. Mot. Control 2016, 20, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Fiorio, M.; Emadi Andani, M.; Marotta, A.; Classen, J.; Tinazzi, M. Placebo-Induced Changes in Excitatory and Inhibitory Corticospinal Circuits during Motor Performance. J. Neurosci. 2014, 34, 3993–4005. [Google Scholar] [CrossRef] [PubMed]
- Andani, M.E.; Tinazzi, M.; Corsi, N.; Fiorio, M. Modulation of Inhibitory Corticospinal Circuits Induced by a Nocebo Procedure in Motor Performance. PLoS ONE 2015, 10, e0125223. [Google Scholar] [CrossRef]
- Entezari-Taher, M.; Dean, A.C. Alteration of Motor Cortex Excitability in Response to Intermittent Photic Stimulation. Clin. Neurophysiol. 2000, 111, 1809–1812. [Google Scholar] [CrossRef]
- Cantello, R.; Civardi, C.; Cavalli, A.; Varrasi, C.; Vicentini, R. Effects of a Photic Input on the Human Cortico-Motoneuron Connection. Clin. Neurophysiol. 2000, 111, 1981–1989. [Google Scholar] [CrossRef]
- Nakahara, H.; Doya, K.; Hikosaka, O. Parallel Cortico-Basal Ganglia Mechanisms for Acquisition and Execution of Visuomotor Sequences—A Computational Approach. J. Cogn. Neurosci. 2001, 13, 626–647. [Google Scholar] [CrossRef]
- Säisänen, L.; Julkunen, P.; Lakka, T.; Lindi, V.; Könönen, M.; Määttä, S. Development of Corticospinal Motor Excitability and Cortical Silent Period from Mid-Childhood to Adulthood—A Navigated TMS Study. Neurophysiol. Clin. 2018, 48, 65–75. [Google Scholar] [CrossRef]
- Berardelli, A.; Rona, S.; Inghilleri, M.; Manfredi, M. Cortical Inhibition in Parkinson’s Disease: A Study with Paired Magnetic Stimulation. Brain 1996, 119, 71–77. [Google Scholar] [CrossRef]
- Li, J.-Y.; Espay, A.J.; Gunraj, C.A.; Pal, P.K.; Cunic, D.I.; Lang, A.E.; Chen, R. Interhemispheric and Ipsilateral Connections in Parkinson’s Disease: Relation to Mirror Movements. Mov. Disord. 2007, 22, 813–821. [Google Scholar] [CrossRef]
- Nakashima, K.; Wang, Y.; Shimoda, M.; Sakuma, K.; Takahashi, K. Shortened Silent Period Produced by Magnetic Cortical Stimulation in Patients with Parkinson’s Disease. J. Neurol. Sci. 1995, 130, 209–214. [Google Scholar] [CrossRef]
- Priori, A.; Berardelli, A.; Inghilleri, M.; Accornero, N.; Manfredi, M. Motor Cortical Inhibition and the Dopaminergic System: Pharmacological Changes in the Silent Period after Transcranial Brain Stimulation in Normal Subjects, Patients with Parkinson’s Disease and Drug-Induced Parkinsonism. Brain 1994, 117, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Ridding, M.C.; Rothwell, J.C.; Inzelberg, R. Changes in Excitability of Motor Cortical Circuitry in Patients with Parkinson’s Disease. Ann. Neurol. 1995, 37, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Siebner, H.R.; Mentschel, C.; Auer, C.; Lehner, C.; Conrad, B. Repetitive Transcranial Magnetic Stimulation Causes a Short-Term Increase in the Duration of the Cortical Silent Period in Patients with Parkinson’s Disease. Neurosci. Lett. 2000, 284, 147–150. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s Disease: Clinical Features and Diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional Changes of the Basal Ganglia Circuitry in Parkinson’s Disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef]
- Obeso, J.A.; Rodriguez-Oroz, M.C.; Rodriguez, M.; Lanciego, J.L.; Artieda, J.; Gonzalo, N.; Olanow, C.W. Pathophysiology of the Basal Ganglia in Parkinson’s Disease. Trends Neurosci. 2000, 23, S8–S19. [Google Scholar] [CrossRef]
- Parent, A.; Hazrati, L.-N. Functional Anatomy of the Basal Ganglia. I. The Cortico-Basal Ganglia-Thalamo-Cortical Loop. Brain Res. Rev. 1995, 20, 91–127. [Google Scholar] [CrossRef]
- Cotzias, G.C.; Papavasiliou, P.S.; Gellene, R. Modification of Parkinsonism—Chronic Treatment with L-Dopa. N. Engl. J. Med. 1969, 280, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, J.-P. Motor Cortex Dysfunction Revealed by Cortical Excitability Studies in Parkinson’s Disease: Influence of Antiparkinsonian Treatment and Cortical Stimulation. Clin. Neurophysiol. 2005, 116, 244–253. [Google Scholar] [CrossRef]
- Jensen, R.N.; Bolwig, T.; Sørensen, S.A. Psychiatric Symptoms in Patients with Huntington’s Disease. Ugeskr. Laeger 2018, 180, 13. [Google Scholar]
- Walker, F.O. Huntington’s Disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Mehrabi, N.F.; Singh-Bains, M.K.; Faull, R.L. Cortico-Basal Ganglia Interactions in Huntington’s Disease. Ann. Neurodegener. Disord. 2016, 6, 1–6. [Google Scholar]
- Modugno, N.; Currà, A.; Giovannelli, M.; Priori, A.; Squitieri, F.; Ruggieri, S.; Manfredi, M.; Berardelli, A. The Prolonged Cortical Silent Period in Patients with Huntington’s Disease. Clin. Neurophysiol. 2001, 112, 1470–1474. [Google Scholar] [CrossRef]
- Nardone, R.; Lochner, P.; Marth, R.; Ausserer, H.; Bratti, A.; Tezzon, F. Abnormal Intracortical Facilitation in Early-Stage Huntington’s Disease. Clin. Neurophysiol. 2007, 118, 1149–1154. [Google Scholar] [CrossRef]
- Schippling, S.; Schneider, S.A.; Bhatia, K.P.; Münchau, A.; Rothwell, J.C.; Tabrizi, S.J.; Orth, M. Abnormal Motor Cortex Excitability in Preclinical and Very Early Huntington’s Disease. Biol. Psychiatry 2009, 65, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Tarsy, D.; Simon, D.K. Dystonia. N. Engl. J. Med. 2006, 355, 818–829. [Google Scholar] [CrossRef]
- Filipović, S.R.; Ljubisavljević, M.; Svetel, M.; Milanović, S.; Kačar, A.; Kostić, V.S. Impairment of Cortical Inhibition in Writer’s Cramp as Revealed by Changes in Electromyographic Silent Period after Transcranial Magnetic Stimulation. Neurosci. Lett. 1997, 222, 167–170. [Google Scholar] [CrossRef]
- Siebner, H.R. Patients with Focal Arm Dystonia Have Increased Sensitivity to Slow-Frequency Repetitive TMS of the Dorsal Premotor Cortex. Brain 2003, 126, 2710–2725. [Google Scholar] [CrossRef]
- Trompetto, C.; Avanzino, L.; Marinelli, L.; Mori, L.; Pelosin, E.; Roccatagliata, L.; Abbruzzese, G. Corticospinal Excitability in Patients with Secondary Dystonia Due to Focal Lesions of the Basal Ganglia and Thalamus. Clin. Neurophysiol. 2012, 123, 808–814. [Google Scholar] [CrossRef]
- Kukowski, B.; Haug, B. Quantitative Evaluation of the Silent Period, Evoked by Transcranial Magnetic Stimulation during Sustained Muscle Contraction, in Normal Man and in Patients with Stroke. Electromyogr. Clin. Neurophysiol. 1992, 32, 373–378. [Google Scholar]
- Ahonen, J.-P.; Jehkonen, M.; Dastidar, P.; Molnar, G.; Häkkinen, V. Cortical Silent Period Evoked by Transcranial Magnetic Stimulation in Ischemic Stroke. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1998, 109, 224–229. [Google Scholar] [CrossRef]
- Braune, H.J.; Fritz, C. Transcranial Magnetic Stimulation–Evoked Inhibition of Voluntary Muscle Activity (Silent Period) Is Impaired in Patients with Ischemic Hemispheric Lesion. Stroke 1995, 26, 550–553. [Google Scholar] [CrossRef]
- Catano, A.; Houa, M.; Noël, P. Magnetic Transcranial Stimulation: Clinical Interest of the Silent Period in Acute and Chronic Stages of Stroke. Electroencephalogr. Clin. Neurophysiol. Mot. Control 1997, 105, 290–296. [Google Scholar] [CrossRef]
- Classen, J.; Schnitzler, A.; Binkofski, F.; Werhahn, K.J.; Kim, Y.-S.; Kessler, K.R.; Benecke, R. The Motor Syndrome Associated with Exaggerated Inhibition within the Primary Motor Cortex of Patients with Hemiparetic Stroke. Brain 1997, 120, 605–1915. [Google Scholar] [CrossRef]
- van Kuijk, A.A.; Pasman, J.W.; Geurts, A.C.; Hendricks, H.T. How Salient Is the Silent Period? The Role of the Silent Period in the Prognosis of Upper Extremity Motor Recovery after Severe Stroke. J. Clin. Neurophysiol. 2005, 22, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Liepert, J.; Storch, P.; Fritsch, A.; Weiller, C. Motor Cortex Disinhibition in Acute Stroke. Clin. Neurophysiol. 2000, 111, 671–676. [Google Scholar] [CrossRef]
- Cincotta, M.; Giovannelli, F.; Borgheresi, A.; Tramacere, L.; Viggiano, M.P.; Zaccara, G. A Meta-Analysis of the Cortical Silent Period in Epilepsies. Brain Stimulat. 2015, 8, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, M.; Borgheresi, A.; Lori, S.; Fabbri, M.; Zaccara, G. Interictal Inhibitory Mechanisms in Patients with Cryptogenic Motor Cortex Epilepsy: A Study of the Silent Period Following Transcranial Magnetic Stimulation. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 1–7. [Google Scholar] [CrossRef]
- Macdonell, R.A.L.; King, M.A.; Newton, M.R.; Curatolo, J.M.; Reutens, D.C.; Berkovic, S.F. Prolonged Cortical Silent Period after Transcranial Magnetic Stimulation in Generalized Epilepsy. Neurology 2001, 57, 706–708. [Google Scholar] [CrossRef]
- Ertas, N.K.; Gül, G.; Altunhalka, A.; Kirbas, D. Cortical Silent Period Following Transcranial Magnetic Stimulation in Epileptic Patients. Epileptic. Disord. 2000, 2, 137–140. [Google Scholar] [PubMed]
- Tataroglu, C.; Ozkiziltan, S.; Baklan, B. Motor Cortical Thresholds and Cortical Silent Periods in Epilepsy. Seizure 2004, 13, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Joo, E.Y.; Kim, S.H.; Seo, D.W.; Hong, S.B. Zonisamide Decreases Cortical Excitability in Patients with Idiopathic Generalized Epilepsy. Clin. Neurophysiol. 2008, 119, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Sajan, P.; Chowdhury, V.; Chaudhry, N. Cortical Excitability in Drug Naive Juvenile Myoclonic Epilepsy. Seizure 2013, 22, 662–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varrasi, C.; Civardi, C.; Boccagni, C.; Cecchin, M.; Vicentini, R.; Monaco, F.; Cantello, R. Cortical Excitability in Drug-Naive Patients with Partial Epilepsy: A Cross-Sectional Study. Neurology 2004, 63, 2051–2055. [Google Scholar] [CrossRef]
- Klimpe, S.; Behrang-Nia, M.; Bott, M.C.; Werhahn, K.J. Recruitment of Motor Cortex Inhibition Differentiates between Generalized and Focal Epilepsy. Epilepsy Res. 2009, 84, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Manganotti, P.; Bongiovanni, L.G.; Zanette, G.; Fiaschi, A. Early and Late Intracortical Inhibition in Juvenile Myoclonic Epilepsy. Epilepsia 2000, 41, 1129–1138. [Google Scholar] [CrossRef]
- Werhahn, K.; Lieber, J.; Classen, J.; Noachtar, S. Motor Cortex Excitability in Patients with Focal Epilepsy. Epilepsy Res. 2000, 41, 179–189. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, H.W.; Cohen, L.G.; Park, K.; Choi, K. Motor Cortical Excitability in Patients with Poststroke Epilepsy. Epilepsia 2008, 49, 117–124. [Google Scholar] [CrossRef]
- Richter, M.A.; de Jesus, D.R.; Hoppenbrouwers, S.; Daigle, M.; Deluce, J.; Ravindran, L.N.; Fitzgerald, P.B.; Daskalakis, Z.J. Evidence for Cortical Inhibitory and Excitatory Dysfunction in Obsessive Compulsive Disorder. Neuropsychopharmacology 2012, 37, 1144–1151. [Google Scholar] [CrossRef]
- Russo, M.; Naro, A.; Mastroeni, C.; Morgante, F.; Terranova, C.; Muscatello, M.R.; Zoccali, R.; Calabrò, R.S.; Quartarone, A. Obsessive-Compulsive Disorder: A “Sensory-Motor” Problem? Int. J. Psychophysiol. 2014, 92, 74–78. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D. Functional Architecture of Basal Ganglia Circuits: Neural Substrates of Parallel Processing. Trends Neurosci. 1990, 13, 266–271. [Google Scholar] [CrossRef]
- DeLong, M.R.; Georgopoulos, A.P. Motor Functions of the Basal Ganglia. Compr. Physiol. 2011, 1017–1061. [Google Scholar]
- Smith, Y.; Bevan, M.; Shink, E.; Bolam, J.P. Microcircuitry of the Direct and Indirect Pathways of the Basal Ganglia. Neuroscience 1998, 86, 353–387. [Google Scholar] [PubMed]
- Waldvogel, H.J.; Billinton, A.; White, J.H.; Emson, P.C.; Faull, R.L.M. Comparative Cellular Distribution of GABAA and GABAB Receptors in the Human Basal Ganglia: Immunohistochemical Colocalization of the ?1 Subunit of the GABAA Receptor, and the GABABR1 and GABABR2 Receptor Subunits. J. Comp. Neurol. 2004, 470, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Kita, H.; Kita, S.T. The Morphology of Globus Pallidus Projection Neurons in the Rat: An Intracellular Staining Study. Brain Res. 1994, 636, 308–319. [Google Scholar] [CrossRef]
- Mink, J.W.; Thach, W.T. Basal Ganglia Motor Control. III. Pallidal Ablation: Normal Reaction Time, Muscle Cocontraction, and Slow Movement. J. Neurophysiol. 1991, 65, 330–351. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Tokuno, H.; Takada, M. Functional Significance of the CorticoÁ/SubthalamoÁ/Pallidal ‘Hyperdirect’ Pathway. Neurosci. Res. 2002, 7. [Google Scholar]
- Braak, H.; Del Tredici, K. Cortico-Basal Ganglia-Cortical Circuitry in Parkinson’s Disease Reconsidered. Exp. Neurol. 2008, 212, 226–229. [Google Scholar] [CrossRef]
- Cai, W.; Duberg, K.; Padmanabhan, A.; Rehert, R.; Bradley, T.; Carrion, V.; Menon, V. Hyperdirect Insula-Basal-Ganglia Pathway and Adult-like Maturity of Global Brain Responses Predict Inhibitory Control in Children. Nat. Commun. 2019, 10, 4798. [Google Scholar] [CrossRef]
- Wang, H.; Fan, L.; Song, M.; Liu, B.; Wu, D.; Jiang, R.; Li, J.; Li, A.; Banaschewski, T.; Bokde, A.L.W.; et al. Functional Connectivity Predicts Individual Development of Inhibitory Control during Adolescence. Cereb. Cortex 2020, bhaa383. [Google Scholar] [CrossRef]
- Greenhouse, I.; Oldenkamp, C.L.; Aron, A.R. Stopping a Response Has Global or Nonglobal Effects on the Motor System Depending on Preparation. J. Neurophysiol. 2012, 107, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. J. Neurosci. 2006, 26, 2424–2433. [Google Scholar] [CrossRef]
- Aron, A.R.; Durston, S.; Eagle, D.M.; Logan, G.D.; Stinear, C.M.; Stuphorn, V. Converging Evidence for a Fronto-Basal-Ganglia Network for Inhibitory Control of Action and Cognition. J. Neurosci. 2007, 27, 11860–11864. [Google Scholar] [CrossRef] [PubMed]
- Badry, R.; Mima, T.; Aso, T.; Nakatsuka, M.; Abe, M.; Fathi, D.; Foly, N.; Nagiub, H.; Nagamine, T.; Fukuyama, H. Suppression of Human Cortico-Motoneuronal Excitability during the Stop-Signal Task. Clin. Neurophysiol. 2009, 120, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Jahfari, S.; Waldorp, L.; van den Wildenberg, W.P.M.; Scholte, H.S.; Ridderinkhof, K.R.; Forstmann, B.U. Effective Connectivity Reveals Important Roles for Both the Hyperdirect (Fronto-Subthalamic) and the Indirect (Fronto-Striatal-Pallidal) Fronto-Basal Ganglia Pathways during Response Inhibition. J. Neurosci. 2011, 31, 6891–6899. [Google Scholar] [CrossRef]
- Fecteau, S.; Lassonde, M.; Théoret, H. Intrahemispheric Dysfunction in Primary Motor Cortex without Corpus Callosum: A Transcranial Magnetic Stimulation Study. BMC Neurol. 2006, 6, 21. [Google Scholar] [CrossRef][Green Version]
- Kühn, A.A.; Brandt, S.A.; Kupsch, A.; Trottenberg, T.; Brocke, J.; Irlbacher, K.; Schneider, G.H.; Meyer, B.-U. Comparison of Motor Effects Following Subcortical Electrical Stimulation through Electrodes in the Globus Pallidus Internus and Cortical Transcranial Magnetic Stimulation. Exp. Brain Res. 2004, 155, 48–55. [Google Scholar] [CrossRef]
- Chu, H.-Y.; McIver, E.L.; Kovaleski, R.F.; Atherton, J.F.; Bevan, M.D. Loss of Hyperdirect Pathway Cortico-Subthalamic Inputs Following Degeneration of Midbrain Dopamine Neurons. Neuron 2017, 95, 1306–1318.e5. [Google Scholar] [CrossRef]
- Young, M.S.; Triggs, W.J.; Bowers, D.; Greer, M.; Friedman, W.A. Stereotactic Pallidotomy Lengthens the Transcranial Magnetic Cortical Stimulation Silent Period in Parkinson’s Disease. Neurology 1997, 49, 1278–1283. [Google Scholar] [CrossRef]
- Greenhouse, I.; Gould, S.; Houser, M.; Aron, A.R. Stimulation of Contacts in Ventral but Not Dorsal Subthalamic Nucleus Normalizes Response Switching in Parkinson’s Disease. Neuropsychologia 2013, 51, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Takada, M.; Inase, M.; Tokuno, H. Dual Somatotopical Representations in the Primate Subthalamic Nucleus: Evidence for Ordered but Reversed Body-Map Transformations from the Primary Motor Cortex and the Supplementary Motor Area. J. Neurosci. 1996, 16, 2671–2683. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Limousin, P.; Krack, P.; Pollak, P.; Benazzouz, A.; Ardouin, C.; Hoffmann, D.; Benabid, A.-L. Electrical Stimulation of the Subthalamic Nucleus in Advanced Parkinson’s Disease. N. Engl. J. Med. 1998, 339, 1105–1111. [Google Scholar] [CrossRef]
- Schroll, H.; Beste, C.; Hamker, F.H. Combined Lesions of Direct and Indirect Basal Ganglia Pathways but Not Changes in Dopamine Levels Explain Learning Deficits in Patients with Huntington’s Disease. Eur. J. Neurosci. 2015, 41, 1227–1244. [Google Scholar] [CrossRef]
- Beaumont, V.; Zhong, S.; Lin, H.; Xu, W.; Bradaia, A.; Steidl, E.; Gleyzes, M.; Wadel, K.; Buisson, B.; Padovan-Neto, F.E.; et al. Phosphodiesterase 10A Inhibition Improves Cortico-Basal Ganglia Function in Huntington’s Disease Models. Neuron 2016, 92, 1220–1237. [Google Scholar] [CrossRef]
- Vitek, J.L. Pathophysiology of Dystonia: A Neuronal Model. Mov. Disord. Off. J. Mov. Disord. Soc. 2002, 17, S49–S62. [Google Scholar] [CrossRef]
- Granert, O.; Peller, M.; Jabusch, H.-C.; Altenmüller, E.; Siebner, H.R. Sensorimotor Skills and Focal Dystonia Are Linked to Putaminal Grey-Matter Volume in Pianists. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1225–1231. [Google Scholar] [CrossRef]
- Silberstein, P.; KuÈhn, A.A.; Kupsch, A.; Trottenberg, T.; Krauss, J.K.; WoÈhrle, J.C.; Mazzone, P.; Insola, A.; Di Lazzaro, V.; Oliviero, A. Patterning of Globus Pallidus Local Field Potentials Differs between Parkinson’s Disease and Dystonia. Brain 2003, 126, 2597–2608. [Google Scholar] [CrossRef]
- Zeuner, K.E.; Knutzen, A.; Granert, O.; Götz, J.; Wolff, S.; Jansen, O.; Dressler, D.; Hefter, H.; Hallett, M.; Deuschl, G. Increased Volume and Impaired Function: The Role of the Basal Ganglia in Writer’s Cramp. Brain Behav. 2015, 5, e00301. [Google Scholar] [CrossRef]
- Simonyan, K.; Cho, H.; Hamzehei Sichani, A.; Rubien-Thomas, E.; Hallett, M. The Direct Basal Ganglia Pathway Is Hyperfunctional in Focal Dystonia. Brain 2017, 140, 3179–3190. [Google Scholar] [CrossRef] [PubMed]
- Cotroneo, M.; Ciacciarelli, A.; Cosenza, D.; Casella, C.; Dell’Aera, C.; Grillo, F.; Fazio, M.C.; La Spina, P.; Musolino, R.F. Hemiballism: Unusual Clinical Manifestation in Three Patients with Frontoparietal Infarct. Clin. Neurol. Neurosurg. 2020, 188, 105612. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeugin, D.; Ionta, S. Anatomo-Functional Origins of the Cortical Silent Period: Spotlight on the Basal Ganglia. Brain Sci. 2021, 11, 705. https://doi.org/10.3390/brainsci11060705
Zeugin D, Ionta S. Anatomo-Functional Origins of the Cortical Silent Period: Spotlight on the Basal Ganglia. Brain Sciences. 2021; 11(6):705. https://doi.org/10.3390/brainsci11060705
Chicago/Turabian StyleZeugin, David, and Silvio Ionta. 2021. "Anatomo-Functional Origins of the Cortical Silent Period: Spotlight on the Basal Ganglia" Brain Sciences 11, no. 6: 705. https://doi.org/10.3390/brainsci11060705
APA StyleZeugin, D., & Ionta, S. (2021). Anatomo-Functional Origins of the Cortical Silent Period: Spotlight on the Basal Ganglia. Brain Sciences, 11(6), 705. https://doi.org/10.3390/brainsci11060705






