Tactile Processing and Quality of Sleep in Autism Spectrum Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sensory Processing Pattern
2.3. Sleep Difficulties
2.4. Statistical Methods
3. Results
3.1. Patient Characteristics
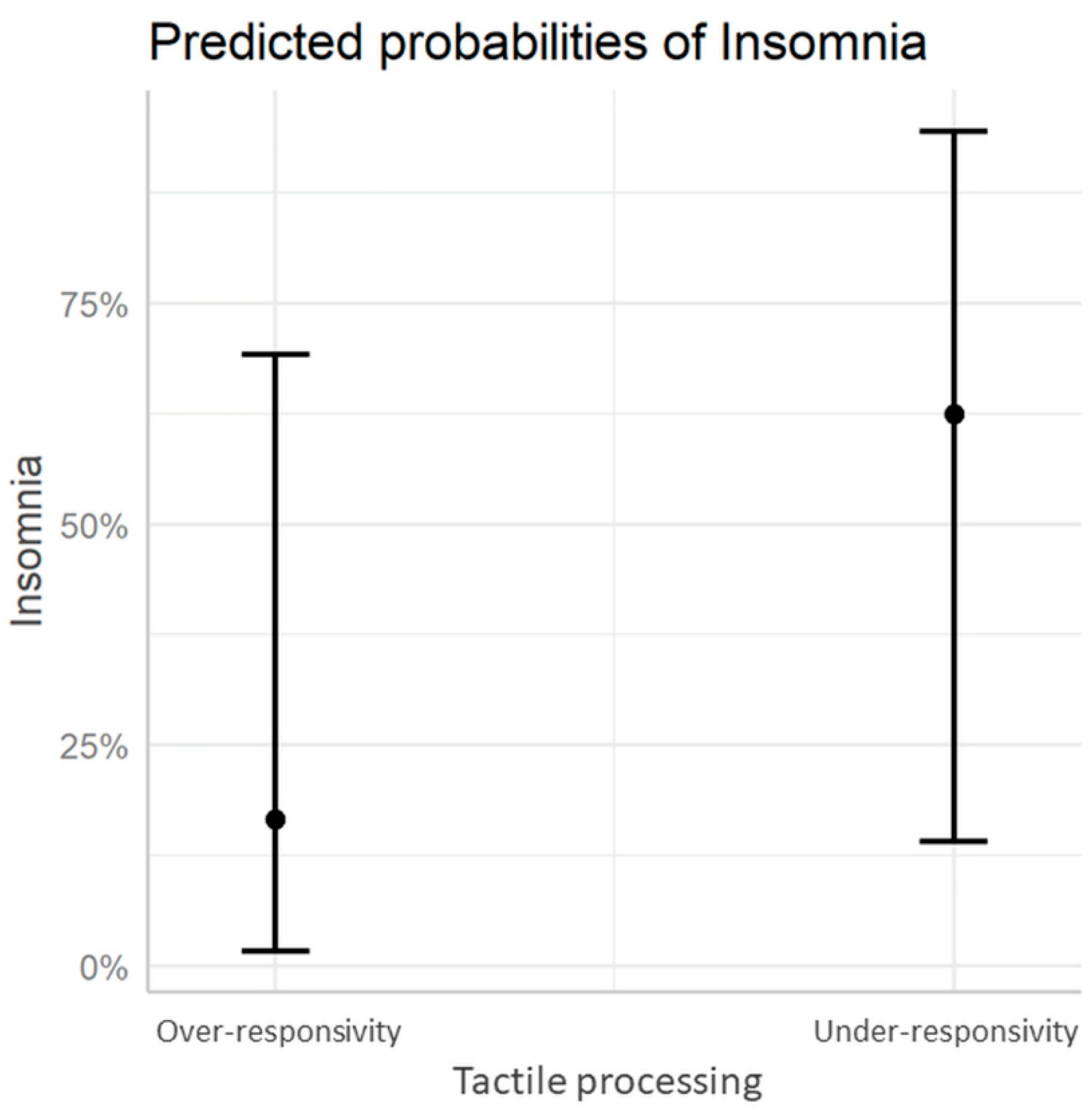
3.2. Sensory Processing Patterns and Sleep Difficulties
4. Discussion
5. Conclusions
- The potential relationship between the tactile stimuli modulation (TSM) pattern and the quality of sleep in ASD individuals.
- The need to treat sleep and sensory disruptions in ASDs, which may intensify behavioral difficulties.
- A direction for further research into non-pharmacological treatment for sleep improvement in ASDs.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, C.; Bishop, S.L. Recent Advances in Autism Research as Reflected in DSM-5 Criteria for Autism Spectrum Disorder. Annu. Rev. Clin. Psychol. 2015, 11, 53–70. [Google Scholar] [CrossRef]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, Diagnosis and Therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef]
- Christensen, D.L. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill. Summ. 2016, 65, 1. [Google Scholar] [CrossRef]
- Murray, M.L.; Hsia, Y.; Glaser, K.; Simonoff, E.; Murphy, D.G.M.; Asherson, P.J.; Eklund, H.; Wong, I.C.K. Pharmacological Treatments Prescribed to People with Autism Spectrum Disorder (ASD) in Primary Health Care. Psychopharmacology 2014, 231, 1011–1021. [Google Scholar] [CrossRef]
- King, B.H. Psychiatric Comorbidities in Neurodevelopmental Disorders. Curr. Opin. Neurol. 2016, 29, 113–117. [Google Scholar] [CrossRef]
- Mannion, A.; Leader, G. Comorbidity in Autism Spectrum Disorder: A Literature Review. Res. Autism Spectr. Disord. 2013, 7, 1595–1616. [Google Scholar] [CrossRef]
- Bangerter, A.; Chatterjee, M.; Manyakov, N.V.; Ness, S.; Lewin, D.; Skalkin, A.; Boice, M.; Goodwin, M.S.; Dawson, G.; Hendren, R.; et al. Relationship Between Sleep and Behavior in Autism Spectrum Disorder: Exploring the Impact of Sleep Variability. Front. Neurosci. 2020, 14, 211. [Google Scholar] [CrossRef]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, Sleep Deprivation, Autonomic Nervous System and Cardiovascular Diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef]
- Williams Buckley, A.; Hirtz, D.; Oskoui, M.; Armstrong, M.J.; Batra, A.; Bridgemohan, C.; Coury, D.; Dawson, G.; Donley, D.; Findling, R.L.; et al. Practice Guideline: Treatment for Insomnia and Disrupted Sleep Behavior in Children and Adolescents with Autism Spectrum Disorder: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2020, 94, 392–404. [Google Scholar] [CrossRef]
- Crocker, A.; Sehgal, A. Genetic Analysis of Sleep. Genes Dev. 2010, 24, 1220–1235. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.E. Sleep and the Developing Brain. Sleep 2007, 30, 1079–1080. [Google Scholar] [CrossRef]
- Cohen, S.; Conduit, R.; Lockley, S.W.; Rajaratnam, S.M.; Cornish, K.M. The Relationship between Sleep and Behavior in Autism Spectrum Disorder (ASD): A Review. J. Neurodev. Disord. 2014, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, F.; Giannotti, F.; Ivanenko, A.; Johnson, K. Sleep in Children with Autistic Spectrum Disorder. Sleep Med. 2010, 11, 659–664. [Google Scholar] [CrossRef]
- Cotton, S.M.; Richdale, A.L. Sleep Patterns and Behaviour in Typically Developing Children and Children with Autism, Down Syndrome, Prader-Willi Syndrome and Intellectual Disability. Res. Autism Spectr. Disord. 2010, 4, 490–500. [Google Scholar] [CrossRef]
- Elrod, M.G.; Hood, B.S. Sleep Differences among Children with Autism Spectrum Disorders and Typically Developing Peers: A Meta-Analysis. J. Dev. Behav. Pediatr. 2015, 36, 166–177. [Google Scholar] [CrossRef]
- Sadeh, A. Consequences of Sleep Loss or Sleep Disruption in Children. Sleep Med. Clin. 2007, 2, 513–520. [Google Scholar] [CrossRef]
- Richdale, A.L.; Schreck, K.A. Sleep Problems in Autism Spectrum Disorders: Prevalence, Nature, & Possible Biopsychosocial Aetiologies. Sleep Med. Rev. 2009, 13, 403–411. [Google Scholar] [CrossRef]
- Swabey, K. Child development: Approaches to learning. In Learning to Teach in the Primary School; VIC Cambridge University Press: New York, NY, USA, 2013; pp. 1–17. ISBN 978-1-107-67282-6. [Google Scholar]
- Kranowitz, C. The Out-of-Sync Child—Recognizing and Doping with Sensory Processing Disorder, 1st ed.; Harmonia: Gdańsk, Poland, 2012; ISBN 978-83-7744-015-5. [Google Scholar]
- Kanner, L. Autistic Disturbances of Affective Contact. Acta Paedopsychiatr. 1968, 35, 100–136. [Google Scholar]
- Tavassoli, T.; Miller, L.J.; Schoen, S.A.; Nielsen, D.M.; Baron-Cohen, S. Sensory Over-Responsivity in Adults with Autism Spectrum Conditions. Autism 2014, 18, 428–432. [Google Scholar] [CrossRef]
- Tomchek, S.D.; Huebner, R.A.; Dunn, W. Patterns of Sensory Processing in Children with an Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2014, 8, 1214–1224. [Google Scholar] [CrossRef]
- Hazen, E.P.; Stornelli, J.L.; O’Rourke, J.A.; Koesterer, K.; McDougle, C.J. Sensory Symptoms in Autism Spectrum Disorders. Harv. Rev. Psychiatry 2014, 22, 112–124. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, TX, USA, 2013. [Google Scholar]
- Ben-Sasson, A.; Soto, T.W.; Martínez-Pedraza, F.; Carter, A.S. Early Sensory Over-Responsivity in Toddlers with Autism Spectrum Disorders as a Predictor of Family Impairment and Parenting Stress. J. Child Psychol. Psychiatry 2013, 54, 846–853. [Google Scholar] [CrossRef]
- Baranek, G.T.; Foster, L.G.; Berkson, G. Tactile Defensiveness and Stereotyped Behaviors. Am. J. Occup. Ther. 1997, 51, 91–95. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Wodka, E.L.; Mostofsky, S.H.; Puts, N.A.J. Autism Spectrum Disorder in the Scope of Tactile Processing. Dev. Cogn. Neurosci. 2018, 29, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Tomchek, S.D. Characterizing Sensory Processing in Autism Spectrum Disorders. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2005. [Google Scholar]
- Kelmanson, I.A.; Adulas, E.I. Massage Therapy and Sleep Behaviour in Infants Born with Low Birth Weight. Complement. Ther. Clin. Pract. 2006, 12, 200–205. [Google Scholar] [CrossRef]
- Miller, L.J.; Anzalone, M.E.; Lane, S.J.; Cermak, S.A.; Osten, E.T. Concept Evolution in Sensory Integration: A Proposed Nosology for Diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef]
- Ayres, A. Sensory Integration and Praxis Tests (SIPT); Western Psychological Services: Los Angeles, CA, USA, 1989. [Google Scholar]
- Fornal-Pawłowska, M.; Wołyńczyk-Gmaj, D.; Szelenberger, W. Validation of the Polish version of the Athens Insomnia Scale. Psychiatr. Pol. 2011, 45, 211–221. [Google Scholar]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. The Diagnostic Validity of the Athens Insomnia Scale. J. Psychosom. Res. 2003, 55, 263–267. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-Test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Freeman, J.V.; Julious, S.A. The Analysis of Categorical Data. Scope 2007, 16, 18–21. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical Power Analyses Using G*Power 3.1: Tests for Correlation and Regression Analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Mannion, A.; Leader, G.; Healy, O. An Investigation of Comorbid Psychological Disorders, Sleep Problems, Gastrointestinal Symptoms and Epilepsy in Children and Adolescents with Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2013, 7, 35–42. [Google Scholar] [CrossRef]
- Lai, M.-C.; Kassee, C.; Besney, R.; Bonato, S.; Hull, L.; Mandy, W.; Szatmari, P.; Ameis, S.H. Prevalence of Co-Occurring Mental Health Diagnoses in the Autism Population: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2019, 6, 819–829. [Google Scholar] [CrossRef]
- Picchioni, D.; Reith, R.M.; Nadel, J.L.; Smith, C.B. Sleep, Plasticity and the Pathophysiology of Neurodevelopmental Disorders: The Potential Roles of Protein Synthesis and Other Cellular Processes. Brain Sci. 2014, 4, 150–201. [Google Scholar] [CrossRef]
- Devnani, P.A.; Hegde, A.U. Autism and Sleep Disorders. J. Pediatr. Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef]
- Sivertsen, B.; Posserud, M.-B.; Gillberg, C.; Lundervold, A.J.; Hysing, M. Sleep Problems in Children with Autism Spectrum Problems: A Longitudinal Population-Based Study. Autism 2012, 16, 139–150. [Google Scholar] [CrossRef]
- Souders, M.C.; Mason, T.B.A.; Valladares, O.; Bucan, M.; Levy, S.E.; Mandell, D.S.; Weaver, T.E.; Pinto-Martin, J. Sleep Behaviors and Sleep Quality in Children with Autism Spectrum Disorders. Sleep 2009, 32, 1566–1578. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goodlin-Jones, B.; Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.L. Sleep Problems in Children with Autism Spectrum Disorders, Developmental Delays, and Typical Development: A Population-Based Study. J. Sleep Res. 2008, 17, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hubbard, J.A.; Fabes, R.A.; Adam, J.B. Sleep Disturbances and Correlates of Children with Autism Spectrum Disorders. Child Psychiatry Hum. Dev. 2006, 37, 179–191. [Google Scholar] [CrossRef]
- Taira, M.; Takase, M.; Sasaki, H. Sleep Disorder in Children with Autism. Psychiatry Clin. Neurosci. 1998, 52, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Turner, K.S.; Foldes, E.; Brooks, M.M.; Kronk, R.; Wiggs, L. Behavioral Parent Training to Address Sleep Disturbances in Young Children with Autism Spectrum Disorder: A Pilot Trial. Sleep Med. 2013, 14, 995–1004. [Google Scholar] [CrossRef]
- Sikora, D.M.; Johnson, K.; Clemons, T.; Katz, T. The Relationship between Sleep Problems and Daytime Behavior in Children of Different Ages with Autism Spectrum Disorders. Pediatrics 2012, 130 (Suppl. 2), S83–S90. [Google Scholar] [CrossRef]
- Ming, X.; Gordon, E.; Kang, N.; Wagner, G.C. Use of Clonidine in Children with Autism Spectrum Disorders. Brain Dev. 2008, 30, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, S.; Lane, S.J.; Thacker, L. Sensory Processing, Physiological Stress, and Sleep Behaviors in Children with and without Autism Spectrum Disorders. OTJR Occup. Particip. Health 2012, 32, 246–257. [Google Scholar] [CrossRef]
- Park, J.H.; An, H.; Jang, E.S.; Chung, S. The Influence of Personality and Dysfunctional Sleep-Related Cognitions on the Severity of Insomnia. Psychiatry Res. 2012, 197, 275–279. [Google Scholar] [CrossRef]
- Demopoulos, C.; Brandes-Aitken, A.N.; Desai, S.S.; Hill, S.S.; Antovich, A.D.; Harris, J.; Marco, E.J. Shared and Divergent Auditory and Tactile Processing in Children with Autism and Children with Sensory Processing Dysfunction Relative to Typically Developing Peers. J. Int. Neuropsychol. Soc. 2015, 21, 444–454. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A Meta-Analysis of Sensory Modulation Symptoms in Individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef]
- Fernández-Andrés, M.I.; Pastor-Cerezuela, G.; Sanz-Cervera, P.; Tárraga-Mínguez, R. A Comparative Study of Sensory Processing in Children with and without Autism Spectrum Disorder in the Home and Classroom Environments. Res. Dev. Disabil. 2015, 38, 202–212. [Google Scholar] [CrossRef]
- Maas, V. Sensory Integration and Neuroscience—from Birth to Old Age, 1st ed.; Foundation for Innovation and Warsaw School of Social and Economic Studies: Warsaw, Poland, 2007; ISBN 83-86169-52-4. [Google Scholar]
- Przyrowski, Z. Sensometry Integration Therapy. In Speech-Supporting Methods in Various Training Sessions; DiG: Warsaw, Poland, 2002; pp. 204–212. ISBN 83-7181-247-7. [Google Scholar]
- Puts, N.A.J.; Wodka, E.L.; Tommerdahl, M.; Mostofsky, S.H.; Edden, R.A.E. Impaired Tactile Processing in Children with Autism Spectrum Disorder. J. Neurophysiol. 2014, 111, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.-J.; Tavassoli, T.; Calò, S.; Thomas, R.M.; Catmur, C.; Frith, U.; Haggard, P. Tactile Sensitivity in Asperger Syndrome. Brain Cogn. 2006, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cascio, C.; McGlone, F.; Folger, S.; Tannan, V.; Baranek, G.; Pelphrey, K.A.; Essick, G. Tactile Perception in Adults with Autism: A Multidimensional Psychophysical Study. J. Autism Dev. Disord. 2008, 38, 127–137. [Google Scholar] [CrossRef]
- Güçlü, B.; Tanidir, C.; Mukaddes, N.M.; Unal, F. Tactile Sensitivity of Normal and Autistic Children. Somatosens. Mot. Res. 2007, 24, 21–33. [Google Scholar] [CrossRef]
- O’Riordan, M.; Passetti, F. Discrimination in Autism within Different Sensory Modalities. J. Autism Dev. Disord. 2006, 36, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Auld, M.L.; Boyd, R.N.; Moseley, G.L.; Johnston, L.M. Tactile Assessment in Children with Cerebral Palsy: A Clinimetric Review. Phys. Occup. Ther. Pediatr. 2011, 31, 413–439. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-F.; Li, W.-L.; Han, Y.; Gao, L.; Dai, W.; Su, Y.-Y.; Zhang, X. Sensory Processing Problems and Comorbidities in Chinese Preschool Children with Autism Spectrum Disorders. J. Autism Dev. Disord. 2019, 49, 4097–4108. [Google Scholar] [CrossRef] [PubMed]
- Hollway, J.A.; Aman, M.G.; Butter, E. Correlates and Risk Markers for Sleep Disturbance in Participants of the Autism Treatment Network. J. Autism Dev. Disord. 2013, 43, 2830–2843. [Google Scholar] [CrossRef]
- Ghanbari, S.; Rezaei, A. The Relationship between Sensory-Processing Disorders and Sleep Disturbances in School-Aged Autistic Children in Shiraz. 2015. Available online: https://sites.kowsarpub.com/jjcdc/articles/21813.html#abstract (accessed on 8 January 2021).
- Vasak, M.; Williamson, J.; Garden, J.; Zwicker, J.G. Sensory Processing and Sleep in Typically Developing Infants and Toddlers. Am. J. Occup. Ther. 2015, 69, 6904220040. [Google Scholar] [CrossRef]
- Roth, E. The Relationship between Sensory Processing Patterns and Sleep in Infants; The University of Toledo Digital Repository: Toledo, Spain, 2012. [Google Scholar]
- Shochat, T.; Tzischinsky, O.; Engel-Yeger, B. Sensory Hypersensitivity as a Contributing Factor in the Relation between Sleep and Behavioral Disorders in Normal Schoolchildren. Behav. Sleep Med. 2009, 7, 53–62. [Google Scholar] [CrossRef]
- Mazurek, M.O.; Petroski, G.F. Sleep Problems in Children with Autism Spectrum Disorder: Examining the Contributions of Sensory over-Responsivity and Anxiety. Sleep Med. 2015, 16, 270–279. [Google Scholar] [CrossRef]
- Liss, M.; Saulnier, C.; Fein, D.; Kinsbourne, M. Sensory and Attention Abnormalities in Autistic Spectrum Disorders. Autism 2006, 10, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E. Cellular mechanisms of learning and the biological basis of individuality. In Principles of Neural Science; Appleton and Lange: Norwalk, CT, USA, 1991; pp. 1009–1031. ISBN 978-0-07-139011-8. [Google Scholar]
- Dunn, W. The Sensations of Everyday Life: Empirical, Theoretical, and Pragmatic Considerations. Am. J. Occup. Ther. 2001, 55, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Tzischinsky, O.; Meiri, G.; Manelis, L.; Bar-Sinai, A.; Flusser, H.; Michaelovski, A.; Zivan, O.; Ilan, M.; Faroy, M.; Menashe, I.; et al. Sleep Disturbances Are Associated with Specific Sensory Sensitivities in Children with Autism. Mol. Autism 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Appleyard, K.; Schaughency, E.; Taylor, B.; Sayers, R.; Haszard, J.; Lawrence, J.; Taylor, R.; Galland, B. Sleep and Sensory Processing in Infants and Toddlers: A Cross-Sectional and Longitudinal Study. Am. J. Occup. Ther. 2020, 74, 7406205010p1–7406205010p12. [Google Scholar] [CrossRef]

| Variable | Caesarian Section (n = 13) n (%) | Vaginal Delivery (n = 14) n (%) | p * | p (FDR) |
|---|---|---|---|---|
| Breastfeeding (yes/no) | 9 (69.2)/4 (30.8) | 11 (78.6)/3 (21.4) | 0.58 | 0.725 |
| Sucking problems during breastfeeding (yes/no) | 9 (69.2)/4 (30.8) | 6 (42.8)/8 (57.2) | 0.16 | 0.266 |
| Artificial ventilation (yes/no) | 5 (38.5)/8 (61.5) | 2 (14.3)/12 (85.7) | 0.15 | 0.266 |
| Intraventricular hemorrhages (yes/no) | 3 (23.1)/10 (76.9) | 3 (21.4)/11 (78.6) | 0.91 | 0.91 |
| Unrelenting anxiety during care activities (yes/no) | 4 (30.8)/9 (69.2) | 8 (57.1)/6 (42.9) | 0.15 | 0.266 |
| Variable | Over-Responsivity n (%) | Under-Responsivity n (%) | p * | p (FDR) |
|---|---|---|---|---|
| Breastfeeding (yes/no) | 16 (80)/4 (20) | 4 (57.1)/3 (42.9) | 0.244 | 0.59 |
| Sucking problems during breastfeeding (yes/no) | 10 (50)/10 (50) | 5 (71.4)/2 (28.6) | 0.335 | 0.59 |
| Artificial ventilation (yes/no) | 5 (25)/15 (75) | 2 (28.6)/5 (71.4) | 0.856 | 0.59 |
| Intraventricular hemorrhages (yes/no) | 3 (15)/17 (85) | 3 (42.9)/4 (57.1) | 0.134 | 0.59 |
| Pharmacotherapy (yes/no) | 9 (45)/11 (55) | 2 (28.6)/5 (71.4) | 0.455 | 0.59 |
| Type of birth (vaginal/vaginal with oxytocin induction/caesarean section) | 7 (35)/2 (10)/11 (55) | 2 (28.6)/2 (28.6)/3 (42.9) | 0.492 | 0.59 |
| Variable | Normal Sleep n = 16 n (%) | Insomnia n = 11 n (%) | p * |
|---|---|---|---|
| Under-responsivity n = 7 | 2 (28.6%) | 5 (71.4%) | 0.08 |
| Over-responsivity n = 20 | 14 (70%) | 6 (30%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamioł-Milc, D.; Bloch, M.; Liput, M.; Stachowska, L.; Skonieczna-Żydecka, K. Tactile Processing and Quality of Sleep in Autism Spectrum Disorders. Brain Sci. 2021, 11, 362. https://doi.org/10.3390/brainsci11030362
Jamioł-Milc D, Bloch M, Liput M, Stachowska L, Skonieczna-Żydecka K. Tactile Processing and Quality of Sleep in Autism Spectrum Disorders. Brain Sciences. 2021; 11(3):362. https://doi.org/10.3390/brainsci11030362
Chicago/Turabian StyleJamioł-Milc, Dominika, Mirosława Bloch, Magdalena Liput, Laura Stachowska, and Karolina Skonieczna-Żydecka. 2021. "Tactile Processing and Quality of Sleep in Autism Spectrum Disorders" Brain Sciences 11, no. 3: 362. https://doi.org/10.3390/brainsci11030362
APA StyleJamioł-Milc, D., Bloch, M., Liput, M., Stachowska, L., & Skonieczna-Żydecka, K. (2021). Tactile Processing and Quality of Sleep in Autism Spectrum Disorders. Brain Sciences, 11(3), 362. https://doi.org/10.3390/brainsci11030362