Nonverbal Semantics Test (NVST)—A Novel Diagnostic Tool to Assess Semantic Processing Deficits: Application to Persons with Aphasia after Cerebrovascular Accident
Abstract
1. Introduction
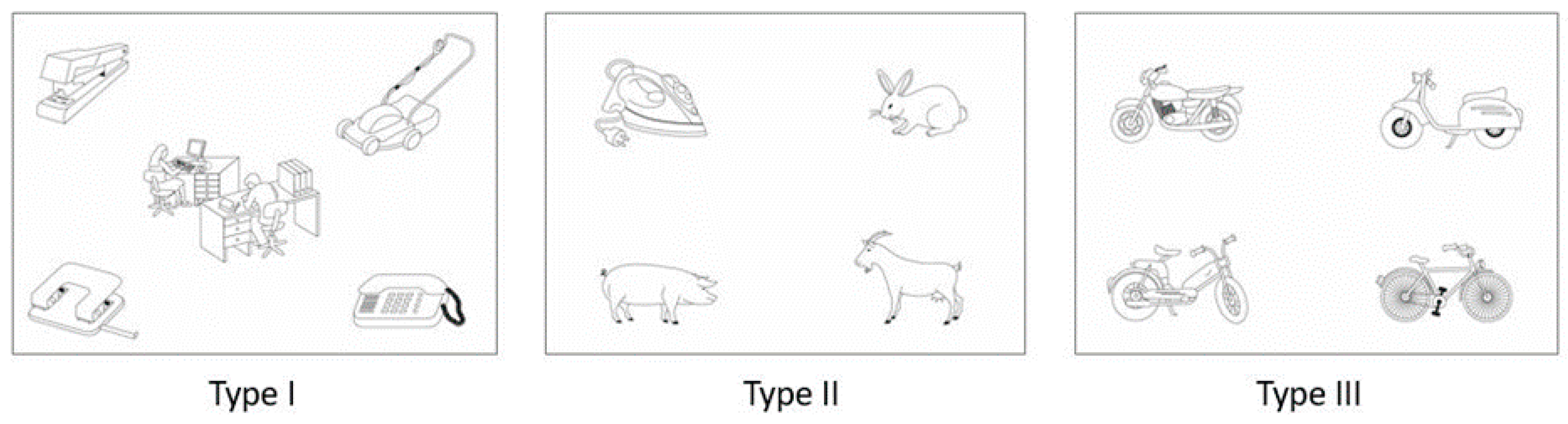
2. Materials and Methods
3. Results
3.1. Performance in the NVST Subtests
3.1.1. Degrees of Impairment in the NVST Subtests
3.1.2. Relationships between NVST Subtests
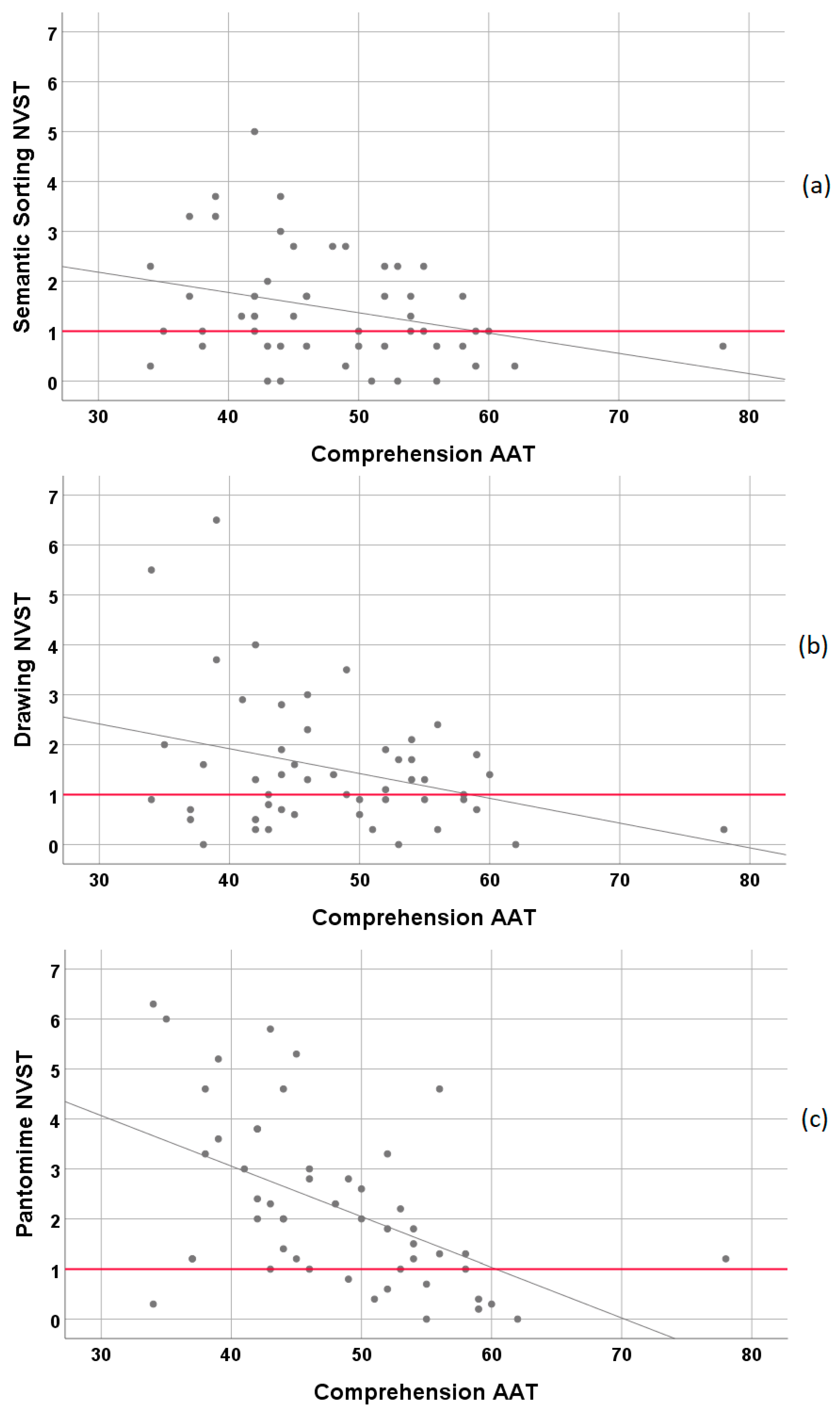
3.2. Relationship of NVST Subtests with Standard Neurolinguistic Measures (AAT)
3.2.1. Correlations between the Subtests of the NVST and the Subtests of the AAT
3.2.2. Linear Regression Models
3.2.3. Principal Component Analysis
4. Discussion
4.1. How Do Persons with Moderate to Severe Aphasia Perform in the NVST and How Are the Subtests Related to Each Other?
4.2. How Do the NVST Subtests Relate to Standard Neurolinguistic Measures?
4.3. Why Is Pantomime more Impaired than Semantic Sorting and Drawing?
4.4. Implications for Treatment Planning
4.5. Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
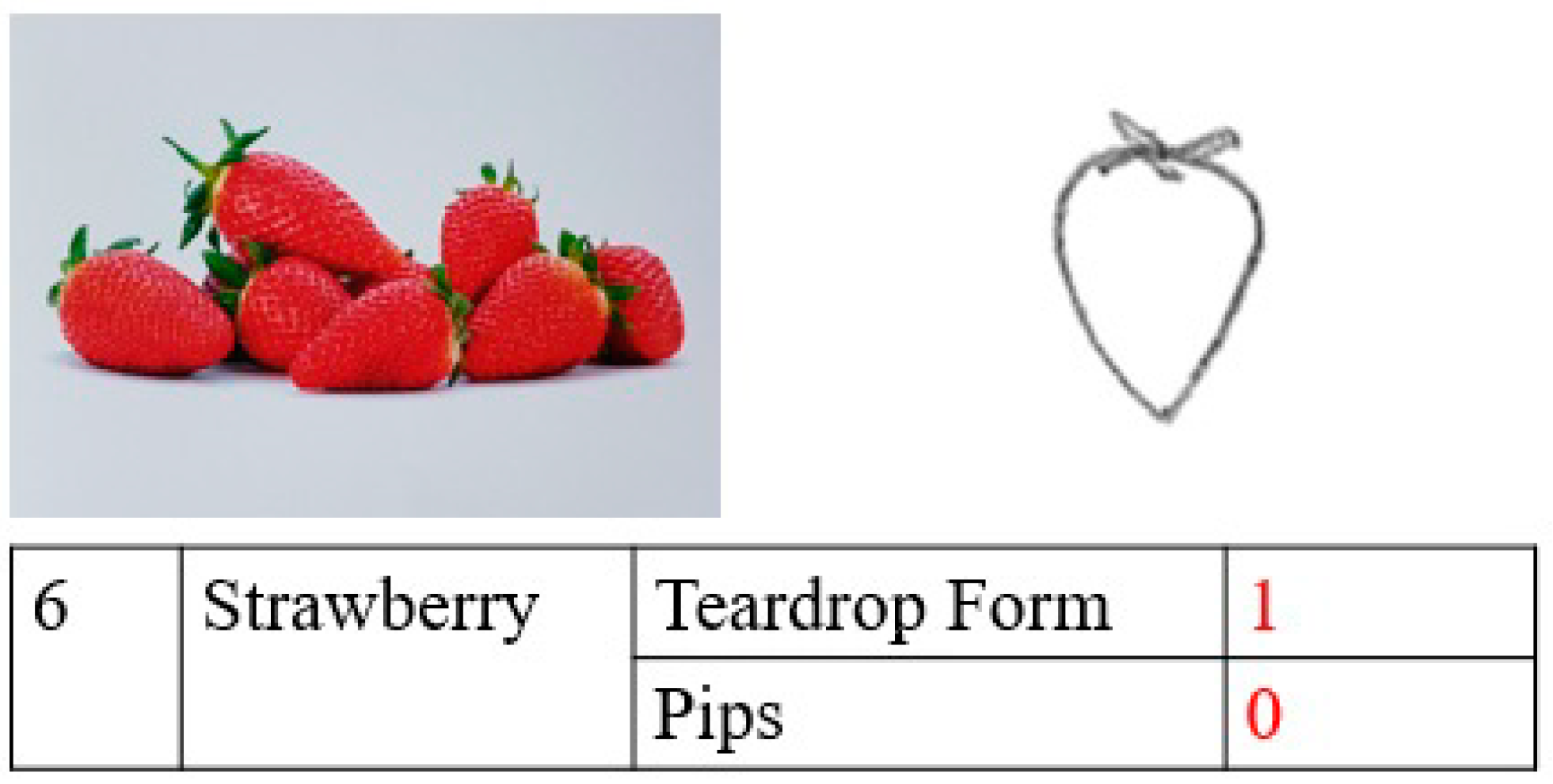
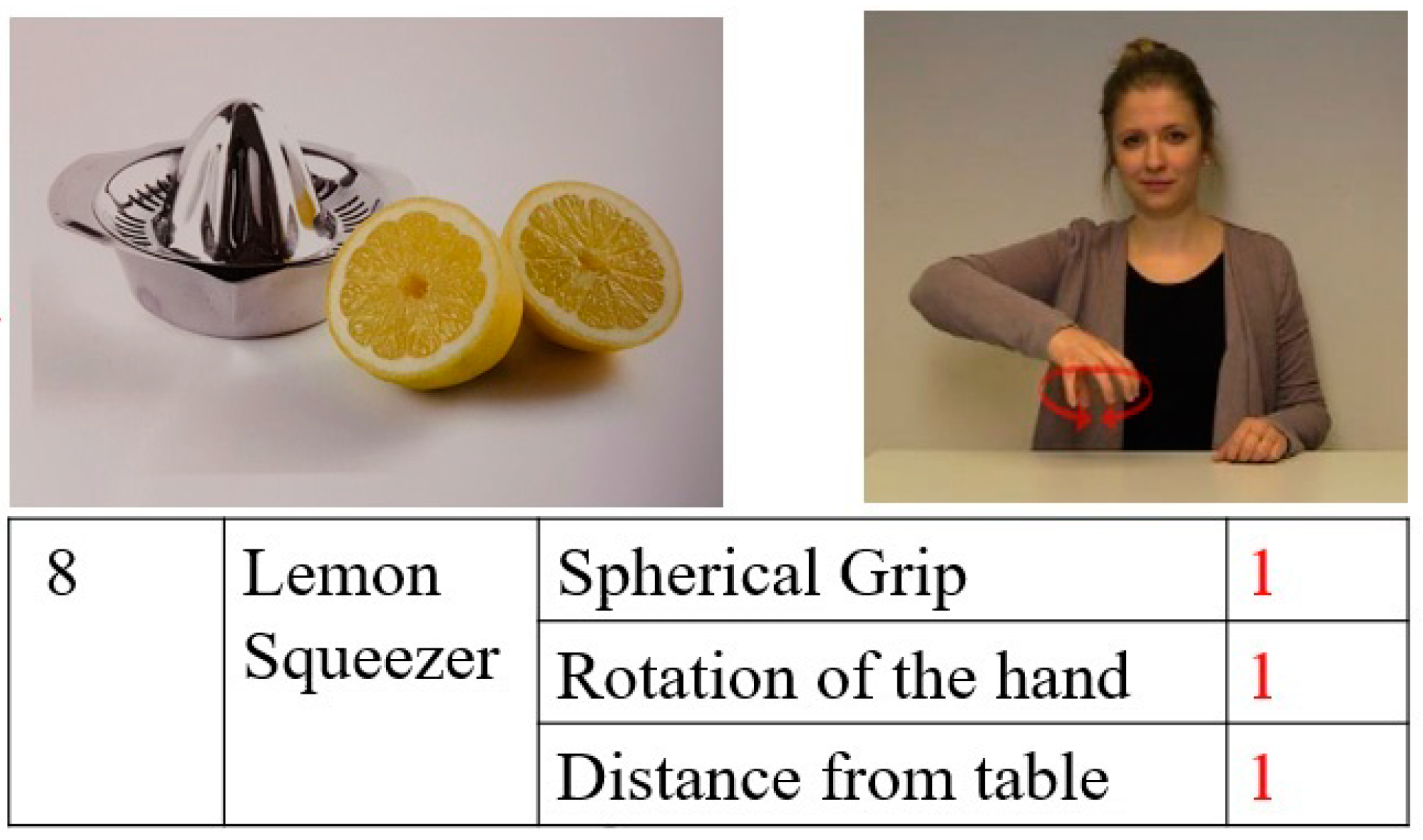
| Normalized Scores | Interpretation |
|---|---|
| <1 | No impairment |
| ≥1 and <2 | Mild impairment |
| ≥2 and <4 | Moderate impairment |
| ≥4 | Severe impairment |
References
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Huber, W.; Poeck, K.; Weniger, D.; Willmes, K. Aachener Aphasie Test; Hogrefe: Göttingen, Germany, 1983. [Google Scholar]
- Kertesz, A. The Western Aphasia Battery—Revised; Grune & Stratton: New York, NY, USA, 2007. [Google Scholar]
- Blumstein, S.; Baker, E.; Goodglass, H. Phonological factors in auditory comprehension in aphasia. Neuropsychologia 1977, 15, 19–30. [Google Scholar] [CrossRef]
- Robson, H.R.; Griffiths, T.D.; Grube, M.; Woollams, A.M. Auditory, phonological, and semantic factors in the recovery from Wernicke’s Aphasia poststroke: Predictive value and implications for rehabilitation. Neurorehabilit. Neural Repair 2019, 22, 800–812. [Google Scholar] [CrossRef]
- Howard, D.; Patterson, K. Pyramids and Palmtree Test; Thames Valley Test Company: Bury St. Edmunds, UK, 1992. [Google Scholar]
- Bozeat, S.; Lambon Ralph, M.A.; Patterson, K.; Garrard, P.; Hodges, J.R. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 2000, 38, 1207–1215. [Google Scholar] [CrossRef]
- Adlam, A.-L.R.; Patterson, K.; Bozeat, S.; Hodges, J.R. The Cambridge Semantic Memory Test Battery: Detection of semantic deficits in semantic dementia and Alzheimer’s disease. Neurocase 2010, 16, 193–207. [Google Scholar] [CrossRef]
- Glindemann, R.; Klintwort, D.; Ziegler, W.; Goldenberg, G. Bogenhausener Semantik—Untersuchung (BOSU): Manual; Urban & Fischer: München, Germany, 2002. [Google Scholar]
- Gainotti, G. Drawing and gesturing in aphasia. Neuropsychol. Trends 2020, 27, 7–22. [Google Scholar] [CrossRef]
- Trojano, L.; Gainotti, G. Drawing disorders in alzheimer’s Disease and other forms of dementia. J. Alzheimer’s Dis. 2016, 53, 31–52. [Google Scholar] [CrossRef]
- Bozeat, S.; Lambon Ralph, M.A.; Graham, K.S.; Patterson, K.; Wilkin, H.; Rowland, J.; Rogers, T.T.; Hodges, J.R. A duck with four legs: Investigating the structure of conceptual knowledge using picture drawing in semantic dementia. Cogn. Neuropsychol. 2003, 20, 27–47. [Google Scholar] [CrossRef]
- Brantjes, M.; Bouma, A. Qualititative Analysis of the Drawings of Alzheimer’s Patients. Clin. Neuropsychol. 1991, 5, 41–52. [Google Scholar] [CrossRef]
- Pozueta, A.; Lage, C.; Martinez, M.G.; Kazimierczak, M.; Bravo, M.; Lopez-Garcia, S.; Riancho, J.; Gonzalez-Suarez, A.; Vazquez-Higuera, J.L.; de Arcocha-Torres, M.; et al. A Brief Drawing Task for the Differential Diagnosis of Semantic Dementia. J. Alzheimer’s Dis. 2019, 72, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G.; Silveri, M.C.; Villa, G.; Caltagirone, C. Drawing objects from memory in aphasia. Brain 1983, 106, 613–622. [Google Scholar] [CrossRef]
- Goldenberg, G.; Hartmann, K.; Schlott, I. Defective pantomime of object use in left brain damage: Apraxia or asymbolia? Neuropsychologia 2003, 41, 1565–1573. [Google Scholar] [CrossRef]
- Vanbellingen, T.; Kersten, B.; Van Hemelrijk, B.; Van de Winckel, A.; Bertschi, M.; Müri, R.; De Weerdt, W.; Bohlhalter, S. Comprehensive assessment of gesture production: A new test of upper limb apraxia (TULIA). Eur. J. Neurol. 2010, 17, 59–66. [Google Scholar] [CrossRef]
- Randerath, J.; Buchmann, I.; Liepert, J.; Büsching, I. Diagnostic Instrument for Limb Apraxia (DILA-S): Manual, 1st ed.; University of Konstanz and Lurija Institute: Konstanz, Germany, 2017. [Google Scholar]
- Goldenberg, G.; Hermsdörfer, J.; Glindemann, R.; Rorden, C.; Karnath, H.O. Pantomime of Tool Use Depends on Integrity of Left Inferior Frontal Cortex. Cereb. Cortex 2007, 17, 2769–2776. [Google Scholar] [CrossRef]
- Goldenberg, G. Facets of pantomime. J. Int. Neuropsychol. Soc. 2017, 23, 121–127. [Google Scholar] [CrossRef]
- Van Nispen, K.; van de Sandt-Koenderman, M.; Mol, L.; Krahmer, E. Pantomime production by people with aphasia: What are the influencing factors? J. Speech Lang. Hear. Res. 2016, 59, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, G. Apraxia—The Cognitive Side of Motor Control; Oxford University Press: Oxford, NY, USA, 2013. [Google Scholar]
- Davis, G.A.; Wilcox, M.J. Icorporating Parameters of Natural Conversation in Aphasia Treatment. In Language Intervention Strategies in Adult Aphasia; Chapey, R., Ed.; Williams & Wilkins: Baltimore, MD, USA, 1981; pp. 169–194. [Google Scholar]
- Nobis-Bosch, R.; Bruehl, S.; Krzok, F.; Jakob, H.; van de Sandt-Koenderman, M.; van der Meulen, I. Szenario-Test. Testung Verbaler und Non-Verbaler Aspekte Aphasischer Kommunikation; Prolog: Köln, Germany, 2020. [Google Scholar]
- Van der Meulen, I.; Van de Sandt-Koenderman, W.M.E.; Duivenvoorden, H.J.; Ribbers, G.M. Measuring verbal and non-verbal communication in aphasia: Reliability, validity, and sensitivity to change of the Scenario Test. Int. J. Lang Commun. Disord. 2010, 45, 424–435. [Google Scholar] [CrossRef]
- Van der Meulen, I.; van Gelder-Houthuizen, J.; Wiegers, J.; Wielaert, S.; van de Sandt-Koenderman, M. Scenario Test: Verbale en non-Verbale Communicatie bij Afasie. Handleiding Scenario-Test; Bohn Stafleu Van Loghum: Houten, The Netherlands, 2008. [Google Scholar]
- Glindemann, R.; Zeller, C.; Ziegler, W. Kommunikativ-Pragmatisches Screening (KOPS); Nat-Verlag: Hofheim, Germany, 2018. [Google Scholar]
- Raymer, A.M.; Singletary, F.; Rodriguez, A.; Ciampitti, M.; Heilman, K.M.; Rothi, L.J. Effects of gesture+verbal treatment for noun and verb retrieval in aphasia. J. Int. Neuropsychol. Soc. 2006, 12, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Kinney, J.; Wallace, S.E.; Schreiber, J.B. The relationship between word retrieval, drawing, and semantics in people with aphasia. Aphasiology 2020, 34, 254–274. [Google Scholar] [CrossRef]
- Hung, P.-F.; Ostergren, J. A comparison of drawing and writing on facilitating word retrieval in individuals with aphasia. Aphasiology 2019, 33. [Google Scholar] [CrossRef]
- Ferguson, N.F.; Evans, K.; Raymer, A.M. A comparison of intention and pantomime gesture treatment for noun retrieval in people with aphasia. Am. J. Speech Lang. Pathol. 2012, 21, S126–S139. [Google Scholar] [CrossRef]
- Marangolo, P.; Bonifazi, S.; Tomaiuolo, F.; Craighero, L.; Coccia, M.; Altoè, G.; Provinciali, L.; Cantagallo, A. Improving language without words: First evidence from aphasia. Neuropsychologia 2010, 48, 3824–3833. [Google Scholar] [CrossRef] [PubMed]
- Murteira, A.; Nickels, L. Can gesture observation help people with aphasia name actions? Cortex 2020, 123, 86–112. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.L.; Attard, M.C.; Mok, Z.; Lanyon, L.E.; Foster, A.M. Multi-modality aphasia therapy is as efficacious as a constraint-induced aphasia therapy for chronic aphasia: A phase 1 study. Aphasiology 2013, 27, 938–971. [Google Scholar] [CrossRef]
- Wallace, S.E.; Purdy, M.; Skidmore, E. A multimodal communication programm for aphasia during inpatient rehabilitation: A case study. NeuroRehabilitation 2014, 35, 615–625. [Google Scholar] [CrossRef]
- Rose, M.L.; Copland, D.; Nickels, L.; Togher, L.; Meinzer, M.; Rai, T.; Cadilhac, D.A.; Kim, J.; Foster, A.; Carragher, M.; et al. Constraint-induced or multi-modal personalized aphasia rehabilitation (COMPARE): A randomized controlled trial for stroke-related chronic aphasia. Int. J. Stroke 2019, 14, 972–976. [Google Scholar] [CrossRef]
- Roper, A.; Marshall, J.; Wilson, S. Benefits and Limitations of Computer Gesture Therapy for the Rehabilitation of Severe Aphasia. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Lambon Ralph, M.A.; Jefferies, E.; Patterson, K.; Rogers, T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2017, 18, 42–55. [Google Scholar] [CrossRef]
- Rogers, T.T.; Lambon Ralph, M.A.; Garrard, P.; Bozeat, S.; McClelland, J.L.; Hodges, J.R.; Patterson, K. Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychol. Rev. 2004, 111, 205–235. [Google Scholar] [CrossRef]
- Patterson, K.; Lambon Ralph, M.A. The hub-and-spoke hypothesis of semantic memory. In Neurobiology of Language; Hickok, G., Small, S.L., Eds.; Academic Press: Amsterdam, The Netherlands, 2016; pp. 765–775. [Google Scholar] [CrossRef]
- Patterson, K.; Nestor, P.J.; Rogers, T.T. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 2007, 8, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Hogrefe, K.; Glindemann, R.; Ziegler, W.; Goldenberg, G. Nonverbaler Semantiktest (NVST); Hogrefe: Göttingen, Germany, in press.
- Miller, N.; Willmes, K.; De Bleser, R. The psychometric properties of the English language version of the Aachen Aphasia Test (EAAT). Aphasiology 2000, 14, 683–722. [Google Scholar] [CrossRef]
- The R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer: New York, NY, USA, 2013. [Google Scholar]
- Revelle, W.R. Psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2020. [Google Scholar]
- Finkel, L.; Hogrefe, K.; Frey, S.H.; Goldenberg, G.; Randerath, J. It takes two to pantomime: Communication meets motor cognition. Neuroimage Clin. 2018, 19, 1008–1017. [Google Scholar] [CrossRef]
- Gentilucci, M.; Corballis, M.C. From manual gesture to speech: A gradual transition. Neurosci. Biobehav. Rev. 2006, 30, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Arbib, M.A. How the Brain Got Language: The Mirror System Hypothesis; Oxford University Press: Oxford, NY, USA, 2012. [Google Scholar]
- Tomasello, M. Origins of Human Communication; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Mirman, D.; Chen, Q.; Zhang, Y.; Wang, Z.; Faseyitan, O.K.; Coslett, H.B.; Schwartz, M.F. Neural organization of spoken language revealed by lesion-symptom mapping. Nat. Commun. 2015, 6, 6762. [Google Scholar] [CrossRef]
- Vandenberghe, R.; Price, C.J.; Wise, R.; Josephs, O.; Frackowiak, R.S.J. Functional anatomy of a common semantic system for words and pictures. Nature 1996, 383, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.J.; Patterson, K.; Hodges, J.R.; Price, C.J. Functional neuroanatomy of the semantic system: Divisible by what? J. Cogn. Neurosci. 1998, 10, 766–777. [Google Scholar] [CrossRef]
- Binder, J.R.; Desai, R.H. The neurobiology of semantic memory. Trends Cogn. Sci. 2011, 15, 527–536. [Google Scholar] [CrossRef]
- Gainotti, G. Is the difference between right and left ATLs due to the distinction between general and social cognition or between verbal and non-verbal representations? Neurosci. Biobehav. Rev. 2015, 51, 296–312. [Google Scholar] [CrossRef]
- Butler, C.R.; Brambati, S.M.; Miller, B.L.; Gorno-Tempini, M.L. The neural correlates of verbal and non-verbal semantic processing deficits in neudegenerative disease. Cogn. Behav. Neurol. 2009, 22, 73–80. [Google Scholar] [CrossRef]
- Snowden, J.S.; Thompson, J.C.; Neary, D. Famous people knowledge and the right and left temporal lobes. Behav. Neurol. 2012, 25, 35–44. [Google Scholar] [CrossRef]
- Woollams, A.M.; Patterson, K. Cognitive consequences of the left-right assymetry of atrophy in semantic dementia. Cortex 2018, 107, 64–77. [Google Scholar] [CrossRef]
- Harrington, G.S.; Farias, D.; Davis, C. The neural basis for simulated drawing and the semantic implications. Cortex 2009, 45, 386–393. [Google Scholar] [CrossRef]
- Farias, D.; Davis, C.; Harrington, G. Drawing: Its contribution to naming in aphasia. Brain Lang. 2006, 97, 53–63. [Google Scholar] [CrossRef]
- Makuuchi, M.; Kaminaga, T.; Sugishita, M. Both parietal lobes are involved in drawing: A functional MRI study and implications for constructional apraxia. Cogn. Brain Res. 2003, 16, 338–347. [Google Scholar] [CrossRef]
- Harrington, G.S.; Farias, D.; Davis, C.H.; Buonocore, M.H. Comparison of the neural basis for imagined writing and drawing. Hum. Brain Mapp. 2007, 28, 450–459. [Google Scholar] [CrossRef]
- Grossmann, M. Drawing deficits in brain-damaged patients’ freehand pictures. Brain Cogn. 1988, 8, 189–205. [Google Scholar] [CrossRef]
- Lausberg, H.; Zaidel, E.; Cruz, R.F.; Ptito, A. Speech-independent production of communicative gestures: Evidence from patients with complete callosal disconnection. Neuropsychologia 2007, 45, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Hogrefe, K.; Rein, R.; Skomroch, H.; Lausberg, H. Co-speech hand movement behaviour in narrations: What is the impact of right vs. left hemisphere damage? Neuropsychologia 2016, 93, 176–188. [Google Scholar] [CrossRef]
- Blonder, L.X.; Burns, A.F.; Bowers, D.; Moore, R.W.; Heilman, K.M. Spontaneous gestures following right hemisphere infarct. Neuropsychologia 1995, 33, 203–213. [Google Scholar] [CrossRef]
- Cocks, N.; Hird, K.; Kirsner, K. The relationship between right hemisphere damage and gesture in spontaneous discourse. Aphasiology 2007, 21, 299–319. [Google Scholar] [CrossRef]
- Akbiyik, S.; Karaduman, A.; Göksun, T.; Chatterjee, A. The relationship between co-speech gesture production and macrolinguistic disourse abilities in people with focal brain injury. Neuropsychologia 2018, 117, 440–453. [Google Scholar] [CrossRef]
- Buxbaum, L.J.; Shapiro, A.; Coslett, H.B. Critical brain regions for tool related and imitative actions: A componential analysis. Brain 2014, 137, 1971–1985. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, G.; Randerath, J. Shared neural substrates of aphasia and apraxia. Neuropsychologia 2015, 75, 40–49. [Google Scholar] [CrossRef]
- Hogrefe, K.; Ziegler, W.; Glindemann, R.; Klonowski, M.; Wagner-Sonntag, E.; Klingenberg, G.; Diehl-Schmid, J.; Roßmeier, C.; Danek, A.; Levin, J.; et al. Application of the Nonverbal Semantics Test (NVST) to persons with aphasia after stroke and persons with dementia. Stem Spraak Taalpathol. 2019, 24, 132–135. [Google Scholar]
- Hogrefe, K.; Ziegler, W.; Weidinger, N.; Goldenberg, G. Non-verbal communication in severe aphasia: Influence of aphasia, apraxia, or semantic processing? Cortex 2012, 48, 952–962. [Google Scholar] [CrossRef]
- Hogrefe, K.; Ziegler, W.; Weidinger, N.; Goldenberg, G. Comprehensibility and neural substrate of communicative gestures in severe aphasie. Brain Lang. 2017, 171, 62–71. [Google Scholar] [CrossRef]
- Hogrefe, K.; Ziegler, W.; Wiesmayer, S.; Weidinger, N.; Goldenberg, G. The actual and potential use of gestures for communication in aphasia. Aphasiology 2013, 27, 1070–1089. [Google Scholar] [CrossRef]
- Caute, A.; Pring, T.; Cocks, N.; Cruice, M.; Best, W.; Marshall, J. Enhancing communication through gesture and naming therapy. J. Speech Lang. Hear. Res. 2013, 56. [Google Scholar] [CrossRef]
- Bauer, A.; Kaiser, G. Drawing on drawings. Aphasiology 1995, 9, 68–78. [Google Scholar] [CrossRef]
- Warrington, E.K.; Cipolotti, L. Word comprehension. The distinction between refractory and storage impairments. Brain 1996, 119, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, E.; Lambon Ralph, M.A. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain 2006, 129, 2132–2147. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.A.; Hasan, O.; Schulz, P.E.; Martin, R.C. Evaluating the distinction between semantic knowledge and semantic access: Evidence from semantic dementia and comprehension-impaired stroke aphasia. Psychon. Bull. Rev. 2020, 27, 607–639. [Google Scholar] [CrossRef]
- Kelter, S.; Cohen, R.; Engel, D.; List, G.; Strohner, H. Aphasic disorders in matching tasks involving conceptual analysis and covert naming. Cortex 1976, 12, 383–394. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]


| Mean (sd) | No Impairment | Mild Impairment | Moderate Impairment | Severe Impairment | |
|---|---|---|---|---|---|
| Semantic Sorting | 1.45(1.12) | 18 | 19 | 13 | 1 |
| Drawing | 1.52(1.32) | 21 | 18 | 9 | 3 |
| Pantomime | 2.24 (1.67) | 10 | 15 | 18 | 8 |
| Semantic Sorting | Drawing | Pantomime | |
|---|---|---|---|
| Token Test | r = −0.18, p = 0.21 | r = −0.20, p = 0.15 | r = −0.43, p < 0.01 |
| Repetition | r = −0.04, p = 0.81 | r = −0.07, p = 0.68 | r = −0.38, p < 0.01 |
| Written Language | r = −0.13, p = 0.37 | r = −0.35, p < 0.05 | r = −0.46, p < 0.01 |
| Naming | r = −0.15, p = 0.29 | r = −0.24, p = 0.09 | r = −0.48, p < 0.01 |
| Comprehension | r = −0.31, p < 0.05 | r = −0.32, p < 0.05 | r = −0.52, p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hogrefe, K.; Goldenberg, G.; Glindemann, R.; Klonowski, M.; Ziegler, W. Nonverbal Semantics Test (NVST)—A Novel Diagnostic Tool to Assess Semantic Processing Deficits: Application to Persons with Aphasia after Cerebrovascular Accident. Brain Sci. 2021, 11, 359. https://doi.org/10.3390/brainsci11030359
Hogrefe K, Goldenberg G, Glindemann R, Klonowski M, Ziegler W. Nonverbal Semantics Test (NVST)—A Novel Diagnostic Tool to Assess Semantic Processing Deficits: Application to Persons with Aphasia after Cerebrovascular Accident. Brain Sciences. 2021; 11(3):359. https://doi.org/10.3390/brainsci11030359
Chicago/Turabian StyleHogrefe, Katharina, Georg Goldenberg, Ralf Glindemann, Madleen Klonowski, and Wolfram Ziegler. 2021. "Nonverbal Semantics Test (NVST)—A Novel Diagnostic Tool to Assess Semantic Processing Deficits: Application to Persons with Aphasia after Cerebrovascular Accident" Brain Sciences 11, no. 3: 359. https://doi.org/10.3390/brainsci11030359
APA StyleHogrefe, K., Goldenberg, G., Glindemann, R., Klonowski, M., & Ziegler, W. (2021). Nonverbal Semantics Test (NVST)—A Novel Diagnostic Tool to Assess Semantic Processing Deficits: Application to Persons with Aphasia after Cerebrovascular Accident. Brain Sciences, 11(3), 359. https://doi.org/10.3390/brainsci11030359