The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Experimental Design
2.3. Induction of Diabetes Mellitus
2.4. Sampling Protocol
Blood Samples
2.5. Tissue Samples: Brain Tissue Preparation
2.6. Biochemical Investigations
Estimation of BDNF, NT3, 5-HT, Dopamine and Glutamic Acid in the Hippocampus
2.7. Calculation of Homeostatic Model Assessment (HOMA) Index
2.8. Behavioral Tests
Elevated Plus Maze
2.9. Forced Swim Test (FST)
2.10. Statistical Analysis
3. Results
3.1. IF Increased the Hippocampal Level of NT3 and BDNF in Control and Diabetic Rats
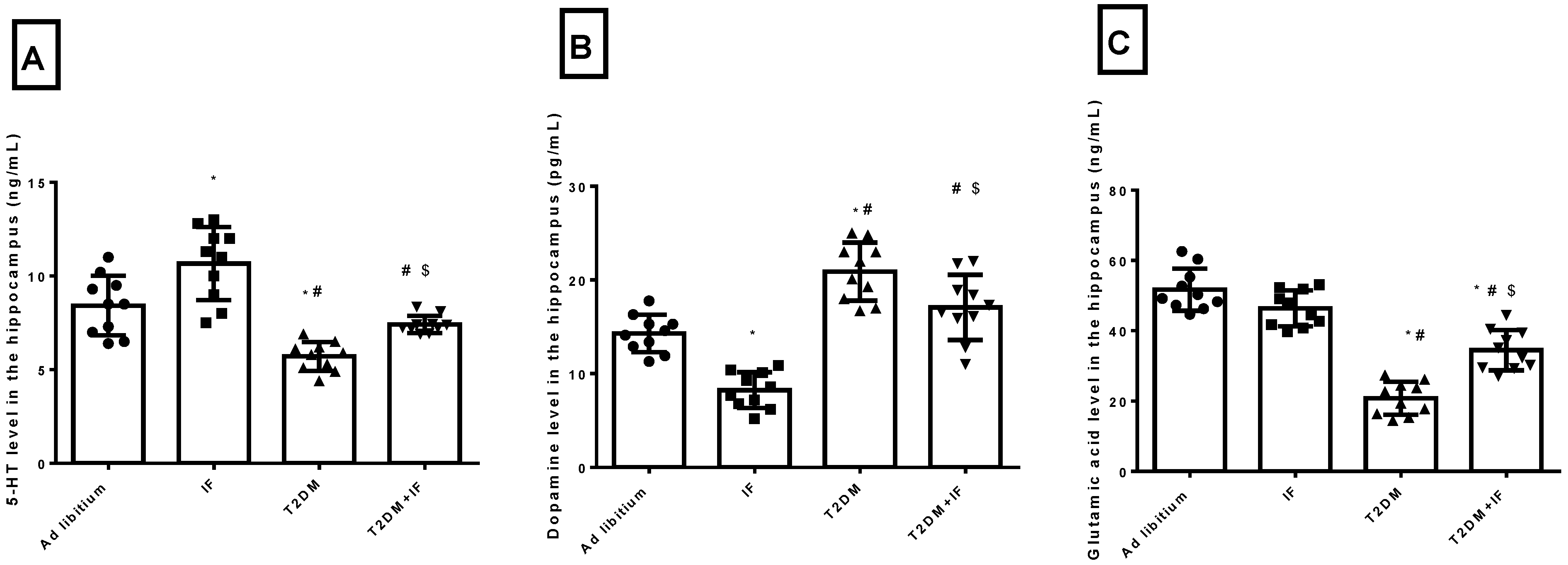
3.2. IF Modulated the Hippocampal Level of 5-HT, Dopamine and Glutamic Acid in Control and Diabetic Rats
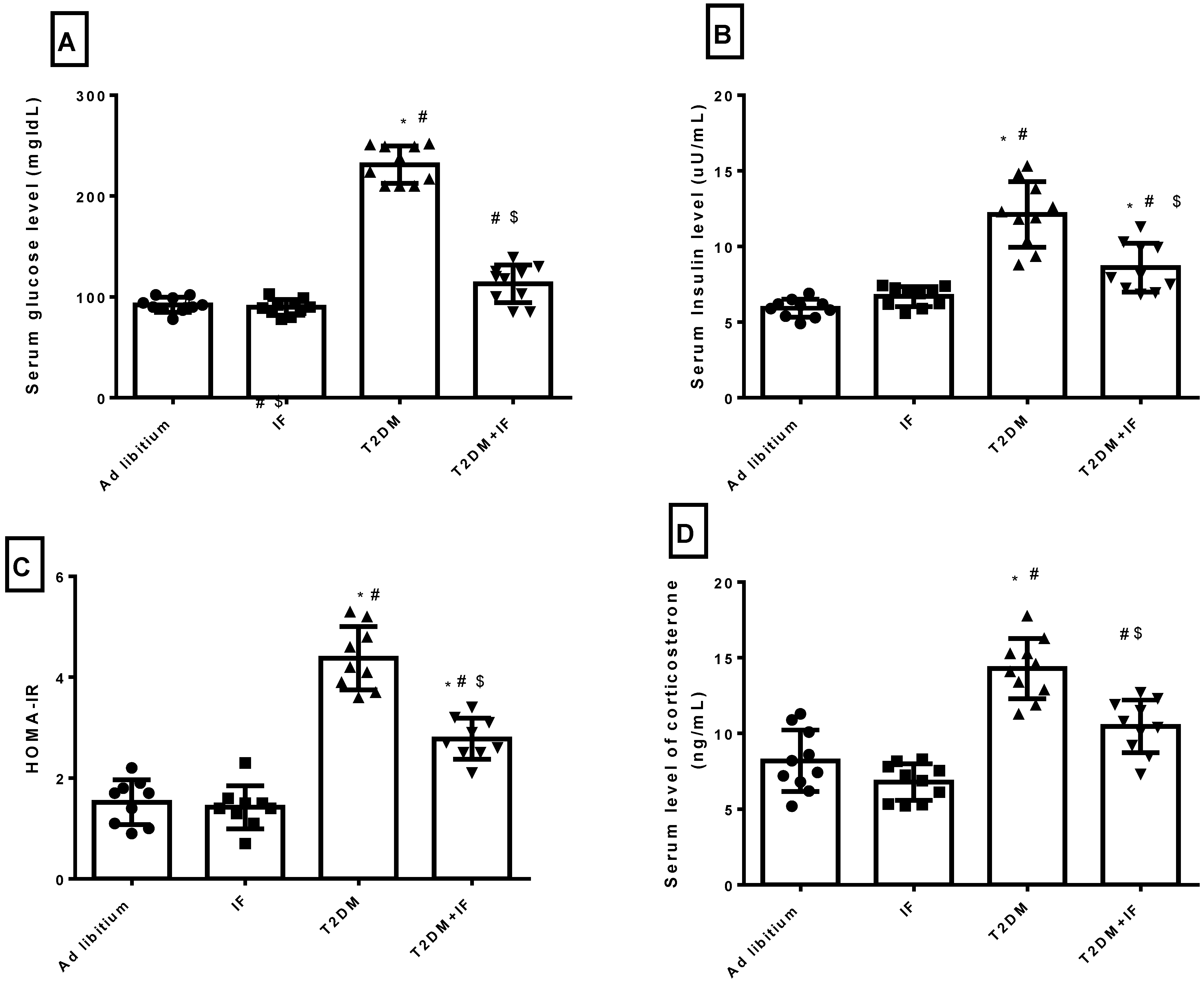
3.3. IF Modulated Glucose Homeostasis Parameters and Corticosterone in Control and Diabetic Rats
3.4. Effects of IF on the Behavioral Response in the Forced Swim Test
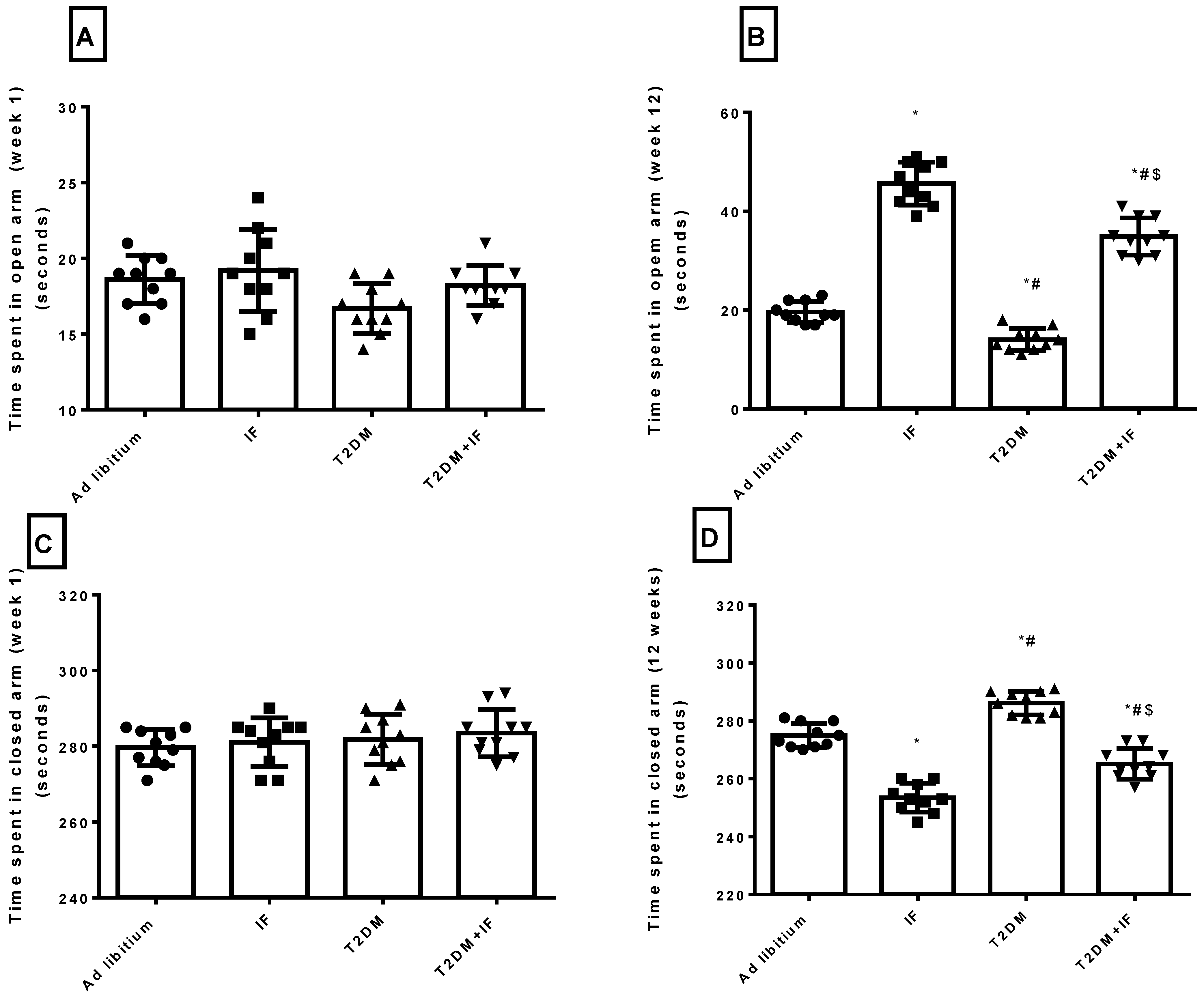
3.5. IF Reduced Anxiety in Both Control and Diabetic Rats
3.6. IF Reduced the Final Body Weight and the Percentage of Weight Gain
3.7. Correlations between Different Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zilliox, L.A.; Chadrasekaran, K.; Kwan, J.Y.; Russell, J.W. Diabetes and Cognitive Impairment. Curr. Diabetes Rep. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Jayaraman, A.; Pike, C.J. Alzheimer’s Disease and Type 2 Diabetes: Multiple Mechanisms Contribute to Interactions. Curr. Diabetes Rep. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Schneider, A.L.C.; Biessels, G.J.; Touradji, P.; Hill-Briggs, F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: A meta-analysis of six cognitive domains and the most fre-quently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc. 2014, 20, 278–291. [Google Scholar] [CrossRef]
- Berg, E.V.D.; on behalf of the Utrecht Diabetic Encephalopathy Study Group; Reijmer, Y.D.; De Bresser, J.; Kessels, R.P.C.; Kappelle, L.J.; Biessels, G.J. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetology 2009, 53, 58–65. [Google Scholar] [CrossRef]
- García-Casares, N.; Jorge, R.E.; García-Arnés, J.A.; Acion, L.; Berthier, M.L.; Gonzalez-Alegre, P.; Nabrozidis, A.; Gutiérrez, A.; Ariza, M.J.; Rioja, J.; et al. Cognitive Dysfunctions in Middle-Aged Type 2 Diabetic Patients and Neuroimaging Correlations: A Cross-Sectional Study. J. Alzheimer’s Dis. 2014, 42, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Arvanitakis, Z.; Macauley-Rambach, S.L.; Koenig, A.M.; Wang, H.-Y.; Ahima, R.S.; Craft, S.; Gandy, S.; Buettner, C.; Stoeckel, L.E.; et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018, 14, 168–181. [Google Scholar] [CrossRef]
- Kuhad, A.; Bishnoi, M.; Tiwari, V.; Chopra, K. Suppression of NF-kappabeta signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol. Biochem. Behav. 2009, 92, 251–259. [Google Scholar]
- Bin-Jaliah, I.; Sakr, H.F. Melatonin ameliorates brain oxidative stress and upregulates senescence marker protein-30 and osteopontin in a rat model of vascular dementia. Physiol. Int. 2018, 105, 38–52. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Redman, L.M.; Ravussin, E. Caloric Restriction in Humans: Impact on Physiological, Psychological, and Behavioral Outcomes. Antioxid. Redox Signal. 2011, 14, 275–287. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Wiglusz, M.S.; Cubała, W.J. Ketogenic diet for schizophrenia: Nutritional approach to antipsychotic treatment. Med. Hypotheses 2018, 118, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, Y.; Zhang, M.; Deng, M.; Zhang, J.-J. Intermittent Fasting Pretreatment Prevents Cognitive Impairment in a Rat Model of Chronic Cerebral Hypoperfusion. J. Nutr. 2017, 147, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, M.; Chen, Y.; Yang, Y.; Zhang, J.-J. Postoperative intermittent fasting prevents hippocampal oxidative stress and memory deficits in a rat model of chronic cerebral hypoperfusion. Eur. J. Nutr. 2018, 58, 423–432. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, Z.; Li, X.; Xing, A.; Jiang, C.; Chen, Y.; Shi, W.; An, L. Intermittent Fasting Protects against Alzheimer’s Disease Possible through Restoring Aquaporin-4 Polarity. Front. Mol. Neurosci. 2017, 10, 395. [Google Scholar] [CrossRef]
- Sakr, H.F.; Abbas, A.M.; El Samanoudy, A.Z. Effect of vitamin E on cerebral cortical oxidative stress and brain-derived neurotrophic factor gene expression induced by hypoxia and exercise in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 191–202. [Google Scholar]
- Komleva, Y.; Lopatina, O.; Gorina, Y.; Chernykh, A.; Shuvaev, A.; Salmina, A. Early changes in hyppocampal neurogenesis induced by soluble Ab1-42 oligomers. Biomed. Khimiya 2018, 64, 326–333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, R.; Lakhanpal, D.; Kumar, S.; Sharma, S.; Kataria, H.; Kaur, M.; Kaur, G. Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impair-ments in male rats. Age 2012, 34, 917–933. [Google Scholar]
- Loane, D.J.; Faden, A.I. Neuroprotection for traumatic brain injury: Translational challenges and emerging therapeutic strategies. Trends Pharmacol. Sci. 2010, 31, 596–604. [Google Scholar] [CrossRef]
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, T.V.; Phillips, T.M.; Cheng, A.; Morrell, C.H.; Mattson, M.P.; Wan, R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol. 2010, 67, 41–52. [Google Scholar] [CrossRef]
- Rothman, S.M.; Griffioen, K.J.; Wan, R.; Mattson, M.P. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann. N. Y. Acad. Sci. 2012, 1264, 49–63. [Google Scholar] [CrossRef]
- Boldrini, M.; Underwood, M.D.; Hen, R.; Rosoklija, G.B.; Dwork, A.J.; Mann, J.J.; Arango, V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology 2009, 34, 2376–2389. [Google Scholar] [CrossRef]
- Brummelte, S.; Galea, L. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience 2010, 168, 680–690. [Google Scholar] [CrossRef]
- Malberg, J.E.; Eisch, A.J.; Nestler, E.J.; Duman, R.S. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. J. Neurosci. 2000, 20, 9104–9110. [Google Scholar] [CrossRef] [PubMed]
- Bessa, J.M.; Ferreira, D.; Melo, I.; Marques, F.; Cerqueira, J.J.; Palha, J.A.; Almeida, O.F.X.; Sousa, N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remod-eling. Mol. Psychiatry 2009, 14, 764–773. [Google Scholar] [PubMed]
- Xiang, X.; Wang, Z.; Zhu, Y.; Bian, L.; Yang, Y. Dosage of streptozocin in inducing rat model of type 2 diabetes mellitus. J. Hyg. Res. 2010, 39, 138–142. [Google Scholar]
- Guo, X.-X.; Wang, Y.; Wang, K.; Ji, B.-P.; Zhou, F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J. Zhejiang Univ. Sci. B 2018, 19, 559–569. [Google Scholar] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, R.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Le Pichon, M.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nat. Cell Biol. 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Sakr, H.F.; Abbas, A.M.; Elsamanoudy, A.Z.; Ghoneim, F.M. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 515–527. [Google Scholar]
- Wang, Z.; Andrade, N.; Torp, M.; Wattananit, S.; Arvidsson, A.; Kokaia, Z.; Jørgensen, J.R.; Lindvall, O. Meteorin is a Chemokinetic Factor in Neuroblast Migration and Promotes Stroke-Induced Striatal Neurogenesis. Br. J. Pharmacol. 2011, 32, 387–398. [Google Scholar] [CrossRef]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.-Y. Leptin Increases Adult Hippocampal Neurogenesis in Vivo and in Vitro. J. Biol. Chem. 2008, 283, 18238–18247. [Google Scholar]
- Eisch, A.J.; Petrik, D. Depression and Hippocampal Neurogenesis: A Road to Remission? Science 2012, 338, 72–75. [Google Scholar] [CrossRef]
- Chalazonitis, A. Neurotrophin-3 as an essential signal for the developing nervous system. Mol. Neurobiol. 1996, 12, 39–53. [Google Scholar] [CrossRef]
- Tyler, W.J.; Perrett, S.P.; Pozzo-Miller, L.D. The Role of Neurotrophins in Neurotransmitter Release. Neuroscience 2002, 8, 524–531. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Kim, S.W.; Moehl, K.; Scheibye-Knudsen, M.; Cheng, A.; Cutler, R.; Camandola, S.; Mattson, M.P. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J. Neurochem. 2016, 139, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Bishop, R.E. Ketoacidosis—Where Do the Protons Come From? Trends Biochem. Sci. 2019, 44, 484–489. [Google Scholar] [CrossRef]
- Chowdhury, G.M.I.; Jiang, L.; Rothman, D.L.; Behar, K.L. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J. Cereb. Blood Flow Metab. 2014, 34, 1233–1242. [Google Scholar]
- McNally, M.A.; Hartman, A.L. Ketone bodies in epilepsy. J. Neurochem. 2012, 121, 28–35. [Google Scholar] [CrossRef]
- Greco, T.; Glenn, T.C.; A Hovda, D.; Prins, M.L. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. Br. J. Pharmacol. 2016, 36, 1603–1613. [Google Scholar] [CrossRef]
- Sampaio, L.P.D.B. Ketogenic diet for epilepsy treatment. Arq. Neuro-Psiquiatr. 2016, 74, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Han, P.; Tang, Z.; Liu, Q.; Shi, J. Sirtuin 3 Mediates Neuroprotection of Ketones against Ischemic Stroke. Br. J. Pharmacol. 2015, 35, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- VanItallie, T.B.; Nonas, C.; Di Rocco, A.; Boyar, K.; Hyams, K.; Heymsfield, S.B. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology 2005, 64, 728–730. [Google Scholar] [CrossRef] [PubMed]
- Śmieszek, A.; Stręk, Z.; Kornicka, K.; Grzesiak, J.; Weiss, C.; Marycz, K. Antioxidant and Anti-Senescence Effect of Metformin on Mouse Olfactory Ensheathing Cells (mOECs) May Be Associated with Increased Brain-Derived Neurotrophic Factor Levels—An Ex Vivo Study. Int. J. Mol. Sci. 2017, 18, 872. [Google Scholar]
- Ruud, J.; Steculorum, S.M.; Brüning, J.C. Neuronal control of peripheral insulin sensitivity and glucose metabolism. Nat. Commun. 2017, 8, 15259. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Nunes, S.; Rolo, A.P.; Reis, F.; Palmeira, C.M. Therapeutic Options Targeting Oxidative Stress, Mitochondrial Dysfunction and Inflammation to Hinder the Progression of Vascular Complications of Diabetes. Front. Physiol. 2019, 9, 1857. [Google Scholar] [CrossRef]
- An, Y.; Varma, R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C.W.; Egan, J.M.; Ferrucci, L.; Troncoso, J.; et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimers Dement. 2018, 14, 318–329. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M. Type 3 diabetes is sporadic Alzheimer’s disease: Mini-review. Eur. Neuropsychopharmacol. 2014, 24, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Zhao, Y.; Zhang, X.; Li, B.; Cui, R. The Effects of Calorie Restriction in Depression and Potential Mechanisms. Curr. Neuropharmacol. 2015, 13, 536–542. [Google Scholar] [PubMed]
- Ishida, A.; Nakajima, W.; Takada, G. Short-term fasting alters neonatal rat striatal dopamine levels and serotonin metabolism: An in vivo microdialysis study. Dev. Brain Res. 1997, 104, 131–136. [Google Scholar] [CrossRef]
- Huether, G.; Zhou, D. Long-term modulation of presynaptic 5-HT-output: Experimentally induced changes in cortical 5-HT-transporter density, tryptophan hydroxylase content and 5-HT innervation density. J. Neural Transm. 1997, 104, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Herrera, R.; Manjarrez, G.; Hernandez, J. Inhibition and kinetic changes of brain tryptophan-5-hydroxylase during insulin-dependent diabetes mellitus in the rat. Nutr. Neurosci. 2005, 8, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef]
- Bastani, A.; Rajabi, S.; Kianimarkani, F. The Effects of Fasting during Ramadan on the Concentration of Serotonin, Dopamine, Brainderived Neurotrophic Factor and Nerve Growth Factor. Neurol. Int. 2017, 9, 29–33. [Google Scholar] [CrossRef]
- Del Arco, A.; Segovia, G.; De Blas, M.; Garrido, P.; Acuña-Castroviejo, D.; Pamplona, R.; Mora, F. Prefrontal cortex, caloric restriction and stress during aging: Studies on dopamine and acetylcholine release, BDNF and working memory. Behav. Brain Res. 2011, 216, 136–145. [Google Scholar] [CrossRef]
- Woolcott, O.O.; Ader, M.; Bergman, R.N. Glucose Homeostasis during Short-term and Prolonged Exposure to High Altitudes. Endocr. Rev. 2015, 36, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Fernando, H.A.; Zibellini, J.; Harris, R.A.; Seimon, R.V.; Sainsbury, A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analysis. Nutriton 2019, 11, 478. [Google Scholar] [CrossRef] [PubMed]
- Rios, M. BDNF and the central control of feeding: Accidental bystander or essential player? Trends Neurosci. 2013, 36, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 2016, 17, 282–292. [Google Scholar] [CrossRef]
- Ben Salem, L.; Bchir, S.; Bouguerra, R.; Ben Slama, C. Cortisol rhythm during the month of Ramadan. East. Mediterr. Health J. 2006, 9, 1093–1098. [Google Scholar]
- Duclos, M.; Bouchet, M.; Vettier, A.; Richard, D. Genetic Differences in Hypothalamic-Pituitary-Adrenal Axis Activity and Food Restriction-induced Hyperactivity in Three Inbred Strains of Rats. J. Neuroendocr. 2005, 17, 740–752. [Google Scholar] [CrossRef]
- Jahng, J.W.; Lee, J.Y.; Yoo, S.B.; Kim, Y.M.; Ryu, V.; Kang, D.W.; Lee, J.-H. Refeeding-induced expression of neuronal nitric oxide synthase in the rat paraventricular nucleus. Brain Res. 2005, 1048, 185–192. [Google Scholar] [CrossRef]
- Manchishi, S.M.; Cui, R.J.; Zou, X.H.; Cheng, Z.Q.; Li, B.J. Effect of caloric restriction on depression. J. Cell. Mol. Med. 2018, 22, 2528–2535. [Google Scholar]
- Moore, M.N.; Shaw, J.P.; Adams, D.R.F.; Viarengo, A. Anti-oxidative cellular protection effect of fasting-induced autophagy as a mechanism for hormesis. Mar. Environ. Res. 2015, 107, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.M.; Walker, B.R.; Syddall, H.E.; Whorwood, C.B.; Wood, P.J.; Phillips, D.I. Elevated plasma cortisol in glucose-intolerant men: Differences in responses to glucose and habituation to venepuncture. J. Clin. Endocrinol. Metab. 2001, 86, 1149–1153. [Google Scholar]
- Wan, R.; Camandola, S.; Mattson, M.P. Intermittent Food Deprivation Improves Cardiovascular and Neuroendocrine Responses to Stress in Rats. J. Nutr. 2003, 133, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Chausse, B.; Solon, C.; Da Silva, C.C.C.; Dos Reis, I.G.M.; Manchado-Gobatto, F.B.; Gobatto, C.A.; Velloso, L.A.; Kowaltowski, A.J. Intermittent Fasting Induces Hypothalamic Modifications Resulting in Low Feeding Efficiency, Low Body Mass and Overeating. Endocrinology 2014, 155, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- A Varady, K.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [PubMed]



| Ad Libitum | IF | T2DM | T2DM+IF | |
|---|---|---|---|---|
| Initial body weight | 355.1 ± 8.8 | 347.4 ± 8.2 | 342.6 ± 7.4 | 367.6 ± 9.1 |
| Final body weight | 438.5 ± 9.9 | 402.8 ± 8.3 * | 471.7 ± 8.4 *# | 413.4 ± 7.3 *$ |
| Weight gain (%) | 39.08 ± 3.6 | 17.57 ± 5.8 * | 36.29 ± 6.2 *# | 10.89 ± 3.5 *$ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elesawy, B.H.; Raafat, B.M.; Muqbali, A.A.; Abbas, A.M.; Sakr, H.F. The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus. Brain Sci. 2021, 11, 242. https://doi.org/10.3390/brainsci11020242
Elesawy BH, Raafat BM, Muqbali AA, Abbas AM, Sakr HF. The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus. Brain Sciences. 2021; 11(2):242. https://doi.org/10.3390/brainsci11020242
Chicago/Turabian StyleElesawy, Basem H., Bassem M. Raafat, Aya Al Muqbali, Amr M. Abbas, and Hussein F. Sakr. 2021. "The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus" Brain Sciences 11, no. 2: 242. https://doi.org/10.3390/brainsci11020242
APA StyleElesawy, B. H., Raafat, B. M., Muqbali, A. A., Abbas, A. M., & Sakr, H. F. (2021). The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus. Brain Sciences, 11(2), 242. https://doi.org/10.3390/brainsci11020242








