Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review
Abstract
1. Introduction
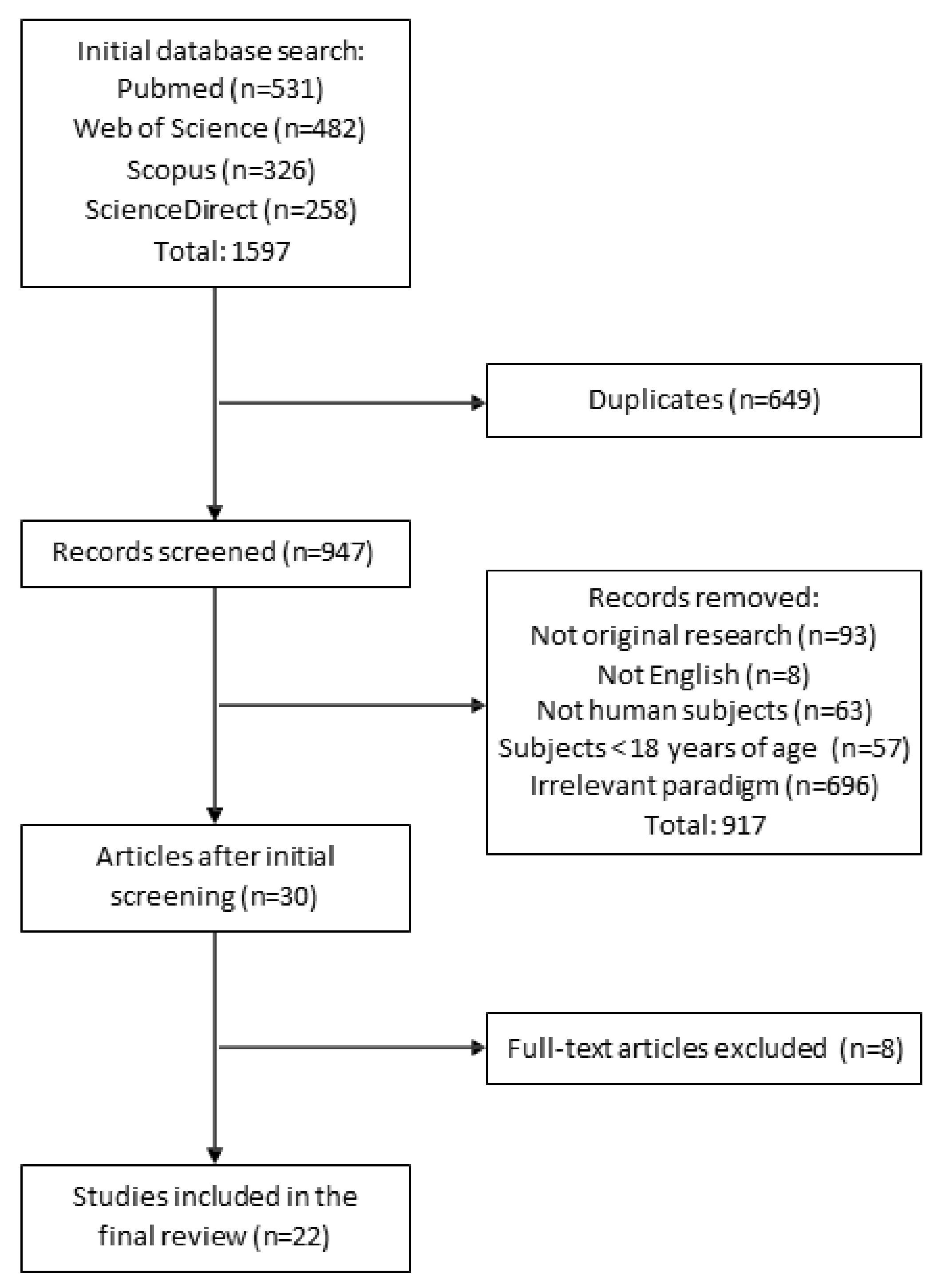
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Evaluation
3. Results
3.1. Methodological Characteristics and Assessment of Selected Studies
3.2. Correlations between ASSR and Cognitive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klimesch, W. Memory Processes, Brain Oscillations and EEG Synchronization. Int. J. Psychophysiol. 1996, 24, 61–100. [Google Scholar] [CrossRef]
- Malmo, R.B. Cognitive Factors in Impairment: A Neuropsychological Study of Divided Set. J. Exp. Psychol. 1966, 71, 184–189. [Google Scholar] [CrossRef]
- Legget, K.T.; Hild, A.K.; Steinmetz, S.E.; Simon, S.T.; Rojas, D.C. MEG and EEG Demonstrate Similar Test-Retest Reliability of the 40 Hz Auditory Steady-State Response. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2017, 114, 16–23. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-Related EEG/MEG Synchronization and Desynchronization: Basic Principles. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Bosman, C.A.; Lansink, C.S.; Pennartz, C.M.A. Functions of Gamma-Band Synchronization in Cognition: From Single Circuits to Functional Diversity across Cortical and Subcortical Systems. Eur. J. Neurosci. 2014, 39, 1982–1999. [Google Scholar] [CrossRef]
- Engel, J.; da Silva, F.L. High-Frequency Oscillations—Where We Are and Where We Need to Go. Prog. Neurobiol. 2012, 98, 316–318. [Google Scholar] [CrossRef]
- Harvey, P.D. Domains of Cognition and Their Assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef]
- Başar, E. A Review of Gamma Oscillations in Healthy Subjects and in Cognitive Impairment. Int. J. Psychophysiol. 2013, 90, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Fründ, I.; Lenz, D. Human Gamma-Band Activity: A Review on Cognitive and Behavioral Correlates and Network Models. Neurosci. Biobehav. Rev. 2010, 34, 981–992. [Google Scholar] [CrossRef]
- Bora, E.; Yücel, M.; Pantelis, C. Cognitive Impairment in Schizophrenia and Affective Psychoses: Implications for DSM-V Criteria and Beyond. Schizophr. Bull. 2010, 36, 36–42. [Google Scholar] [CrossRef]
- Mortamais, M.; Ash, J.A.; Harrison, J.; Kaye, J.; Kramer, J.; Randolph, C.; Pose, C.; Albala, B.; Ropacki, M.; Ritchie, C.W.; et al. Detecting Cognitive Changes in Preclinical Alzheimer’s Disease: A Review of Its Feasibility. Alzheimers Dement. J. Alzheimers Assoc. 2017, 13, 468–492. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Y.; Wang, X.; Yang, H.; Yu, X.; Wang, H. Enhanced Gamma Activity and Cross-Frequency Interaction of Resting-State Electroencephalographic Oscillations in Patients with Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef]
- Smith-Spark, J.H.; Henry, L.A.; Messer, D.J.; Zięcik, A.P. Verbal and Non-Verbal Fluency in Adults with Developmental Dyslexia: Phonological Processing or Executive Control Problems? Dyslexia Chichester Engl. 2017, 23, 234–250. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Demiralp, T. Human EEG Gamma Oscillations in Neuropsychiatric Disorders. Clin. Neurophysiol. 2005, 116, 2719–2733. [Google Scholar] [CrossRef]
- Uhlhaas, P.J.; Singer, W. Abnormal Neural Oscillations and Synchrony in Schizophrenia. Nat. Rev. Neurosci. 2010, 11, 100–113. [Google Scholar] [CrossRef]
- O’Donnell, B.F.; Vohs, J.L.; Krishnan, G.P.; Rass, O.; Hetrick, W.P.; Morzorati, S.L. Chapter 6—The auditory steady-state response (ASSR): A translational biomarker for schizophrenia. In Supplements to Clinical Neurophysiology; Başar, E., Başar-Eroĝlu, C., Özerdem, A., Rossini, P.M., Yener, G.G., Eds.; Application of Brain Oscillations in Neuropsychiatric Diseases; Elsevier: Amsterdam, The Netherlands, 2013; Volume 62, pp. 101–112. [Google Scholar]
- Galambos, R.; Makeig, S.; Talmachoff, P.J. A 40-Hz Auditory Potential Recorded from the Human Scalp. Proc. Natl. Acad. Sci. USA 1981, 78, 2643–2647. [Google Scholar] [CrossRef]
- Hamm, J.P.; Gilmore, C.S.; Clementz, B.A. Augmented Gamma Band Auditory Steady-State Responses: Support for NMDA Hypofunction in Schizophrenia. Schizophr. Res. 2012, 138, 1–7. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; Alsamri, J.; John, M.S.; Purcell, D.; George, S.; Zeng, F.-G. Human Envelope Following Responses to Amplitude Modulation: Effects of Aging and Modulation Depth. Ear Hear. 2016, 37, e322–e335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Griskova-Bulanova, I.; Voicikas, A.; Dapsys, K.; Melynyte, S.; Andruskevicius, S.; Pipinis, E. Envelope Following Response to 440 Hz Carrier Chirp-Modulated Tones Show Clinically Relevant Changes in Schizophrenia. Brain Sci. 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Purcell, D.W.; John, S.M.; Schneider, B.A.; Picton, T.W. Human Temporal Auditory Acuity as Assessed by Envelope Following Responses. J. Acoust. Soc. Am. 2004, 116, 3581–3593. [Google Scholar] [CrossRef]
- Picton, T. Hearing in Time: Evoked Potential Studies of Temporal Processing. Ear Hear. 2013, 34, 385–401. [Google Scholar] [CrossRef]
- Picton, T.W.; John, M.S.; Dimitrijevic, A.; Purcell, D. Human Auditory Steady-State Responses: Respuestas Auditivas de Estado Estable En Humanos. Int. J. Audiol. 2003, 42, 177–219. [Google Scholar] [CrossRef]
- Gutschalk, A.; Mase, R.; Roth, R.; Ille, N.; Rupp, A.; Hähnel, S.; Picton, T.W.; Scherg, M. Deconvolution of 40 Hz Steady-State Fields Reveals Two Overlapping Source Activities of the Human Auditory Cortex. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 1999, 110, 856–868. [Google Scholar] [CrossRef]
- Pantev, C.; Roberts, L.E.; Elbert, T.; Ross, B.; Wienbruch, C. Tonotopic Organization of the Sources of Human Auditory Steady-State Responses. Hear. Res. 1996, 101, 62–74. [Google Scholar] [CrossRef]
- Brugge, J.F.; Nourski, K.V.; Oya, H.; Reale, R.A.; Kawasaki, H.; Steinschneider, M.; Howard, M.A. Coding of Repetitive Transients by Auditory Cortex on Heschl’s Gyrus. J. Neurophysiol. 2009, 102, 2358–2374. [Google Scholar] [CrossRef] [PubMed]
- Herdman, A.T.; Picton, T.W.; Stapells, D.R. Place Specificity of Multiple Auditory Steady-State Responses. J. Acoust. Soc. Am. 2002, 112, 1569–1582. [Google Scholar] [CrossRef]
- Ross, B.; Herdman, A.; Pantev, C. Stimulus Induced Desynchronization of Human Auditory 40-Hz Steady-State Responses. J. Neurophysiol. 2005, 94, 4082–4093. [Google Scholar] [CrossRef]
- Farahani, E.D.; Wouters, J.; van Wieringen, A. Brain Mapping of Auditory Steady-State Responses: A Broad View of Cortical and Subcortical Sources. Hum. BRAIN Mapp. 2020. [Google Scholar] [CrossRef]
- Manting, C.L.; Andersen, L.M.; Gulyas, B.; Ullén, F.; Lundqvist, D. Attentional Modulation of the Auditory Steady-State Response across the Cortex. NeuroImage 2020, 217, 116930. [Google Scholar] [CrossRef]
- Shahriari, Y.; Krusienski, D.; Dadi, Y.S.; Seo, M.; Shin, H.-S.; Choi, J.H. Impaired Auditory Evoked Potentials and Oscillations in Frontal and Auditory Cortex of a Schizophrenia Mouse Model. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry 2016, 17, 439–448. [Google Scholar] [CrossRef]
- Toader, O.; von Heimendahl, M.; Schuelert, N.; Nissen, W.; Rosenbrock, H. Suppression of Parvalbumin Interneuron Activity in the Prefrontal Cortex Recapitulates Features of Impaired Excitatory/Inhibitory Balance and Sensory Processing in Schizophrenia. Schizophr. Bull. 2020, 46, 981–989. [Google Scholar] [CrossRef]
- Manju, V.; Gopika, K.K.; Arivudai Nambi, P.M. Association of Auditory Steady State Responses with Perception of Temporal Modulations and Speech in Noise. ISRN Otolaryngol. 2014, 2014, 374035. [Google Scholar] [CrossRef] [PubMed]
- Vohs, J.L.; Chambers, R.A.; O’Donnell, B.F.; Krishnan, G.P.; Morzorati, S.L. Auditory Steady State Responses in a Schizophrenia Rat Model Probed by Excitatory/Inhibitory Receptor Manipulation. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2012, 86, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Thuné, H.; Recasens, M.; Uhlhaas, P.J. The 40-Hz Auditory Steady-State Response in Patients with Schizophrenia: A Meta-Analysis. JAMA Psychiatry 2016, 73, 1145–1153. [Google Scholar] [CrossRef]
- Oda, Y.; Onitsuka, T.; Tsuchimoto, R.; Hirano, S.; Oribe, N.; Ueno, T.; Hirano, Y.; Nakamura, I.; Miura, T.; Kanba, S. Gamma Band Neural Synchronization Deficits for Auditory Steady State Responses in Bipolar Disorder Patients. PLoS ONE 2012, 7, e39955. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.A.; Hamm, J.P.; McDowell, J.E.; Keedy, S.K.; Gershon, E.S.; Ivleva, E.I.; Pearlson, G.D.; Keshavan, M.S.; Tamminga, C.A.; Sweeney, J.A.; et al. Auditory Steady-State EEG Response across the Schizo-Bipolar Spectrum. Schizophr. Res. 2019, 209, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.M.; Salisbury, D.F.; Shenton, M.E.; McCarley, R.W. Gamma-Band Auditory Steady-State Responses Are Impaired in First Episode Psychosis. Biol. Psychiatry 2008, 64, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Tada, M.; Kirihara, K.; Koshiyama, D.; Fujioka, M.; Usui, K.; Uka, T.; Komatsu, M.; Kunii, N.; Araki, T.; Kasai, K. Gamma-Band Auditory Steady-State Response as a Neurophysiological Marker for Excitation and Inhibition Balance: A Review for Understanding Schizophrenia and Other Neuropsychiatric Disorders. Clin. EEG Neurosci. 2020, 51, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Vohs, J.L.; Chambers, R.A.; Krishnan, G.P.; O’Donnell, B.F.; Berg, S.; Morzorati, S.L. GABAergic Modulation of the 40 Hz Auditory Steady-State Response in a Rat Model of Schizophrenia. Int. J. Neuropsychopharmacol. 2010, 13, 487–497. [Google Scholar] [CrossRef]
- Sivarao, D.V.; Chen, P.; Senapati, A.; Yang, Y.; Fernandes, A.; Benitex, Y.; Whiterock, V.; Li, Y.-W.; Ahlijanian, M.K. 40 Hz Auditory Steady-State Response Is a Pharmacodynamic Biomarker for Cortical NMDA Receptors. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 2232–2240. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Timi, P.; Hong, L.E.; O’Donnell, P. Effects of NMDA and GABA-A Receptor Antagonism on Auditory Steady-State Synchronization in Awake Behaving Rats. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef]
- Light, G.A.; Hsu, J.L.; Hsieh, M.H.; Meyer-Gomes, K.; Sprock, J.; Swerdlow, N.R.; Braff, D.L. Gamma Band Oscillations Reveal Neural Network Cortical Coherence Dysfunction in Schizophrenia Patients. Biol. Psychiatry 2006, 60, 1231–1240. [Google Scholar] [CrossRef]
- Tada, M.; Nagai, T.; Kirihara, K.; Koike, S.; Suga, M.; Araki, T.; Kobayashi, T.; Kasai, K. Differential Alterations of Auditory Gamma Oscillatory Responses between Pre-Onset High-Risk Individuals and First-Episode Schizophrenia. Cereb. Cortex 2016, 26, 1027–1035. [Google Scholar] [CrossRef]
- Teale, P.; Carlson, J.; Rojas, D.; Reite, M. Reduced Laterality of the Source Locations for Generators of the Auditory Steady-State Field in Schizophrenia. Biol. Psychiatry 2003, 54, 1149–1153. [Google Scholar] [CrossRef]
- Spencer, K.M.; Niznikiewicz, M.A.; Nestor, P.G.; Shenton, M.E.; McCarley, R.W. Left Auditory Cortex Gamma Synchronization and Auditory Hallucination Symptoms in Schizophrenia. BMC Neurosci. 2009, 10, 85. [Google Scholar] [CrossRef]
- Górska, U.; Binder, M. Low and Medium Frequency Auditory Steady-State Responses Decrease during NREM Sleep. Int. J. Psychophysiol. 2019, 135, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Griskova, I.; Morup, M.; Parnas, J.; Ruksenas, O.; Arnfred, S.M. The Amplitude and Phase Precision of 40 Hz Auditory Steady-State Response Depend on the Level of Arousal. Exp. BRAIN Res. 2007, 183, 133–138. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Price, G.; Lee, J.; Iyyalol, R.; Martin-Iverson, M.T. Dexamphetamine Selectively Increases 40 Hz Auditory Steady State Response Power to Target and Nontarget Stimuli in Healthy Humans. J. Psychiatry Neurosci. 2013, 38, 24–32. [Google Scholar] [CrossRef]
- Skosnik, P.D.; Krishnan, G.P.; O’Donnell, B.F. The Effect of Selective Attention on the Gamma-Band Auditory Steady-State Response. Neurosci. Lett. 2007, 420, 223–228. [Google Scholar] [CrossRef]
- Voicikas, A.; Niciute, I.; Ruksenas, O.; Griskova-Bulanova, I. Effect of Attention on 40 Hz Auditory Steady-State Response Depends on the Stimulation Type: Flutter Amplitude Modulated Tones versus Clicks. Neurosci. Lett. 2016, 629, 215–220. [Google Scholar] [CrossRef]
- Logue, S.F.; Gould, T.J. The Neural and Genetic Basis of Executive Function: Attention, Cognitive Flexibility, and Response Inhibition. Pharmacol. Biochem. Behav. 2014, 123, 45–54. [Google Scholar] [CrossRef]
- Farahani, E.D.; Goossens, T.; Wouters, J.; van Wieringen, A. Spatiotemporal Reconstruction of Auditory Steady-State Responses to Acoustic Amplitude Modulations: Potential Sources beyond the Auditory Pathway. NeuroImage 2017, 148, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Farahani, E.D.; Wouters, J.; van Wieringen, A. Contributions of Non-Primary Cortical Sources to Auditory Temporal Processing. NeuroImage 2019, 191, 303–314. [Google Scholar] [CrossRef]
- van Deursen, J.A.; Vuurman, E.F.P.M.; van Kranen-Mastenbroek, V.H.J.M.; Verhey, F.R.J.; Riedel, W.J. 40-Hz Steady State Response in Alzheimer’s Disease and Mild Cognitive Impairment. Neurobiol. Aging 2011, 32, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.-H.; Mueller, N.E.; Spencer, K.M.; Mallya, S.G.; Lewandowski, K.E.; Norris, L.A.; Levy, D.L.; Cohen, B.M.; Öngür, D.; Hall, M.-H. Auditory Steady State Response Deficits Are Associated with Symptom Severity and Poor Functioning in Patients with Psychotic Disorder. Schizophr. Res. 2018, 201, 278–286. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Arrondo, G.; Alegre, M.; Sepulcre, J.; Iriarte, J.; Artieda, J.; Villoslada, P. Abnormalities in Brain Synchronization Are Correlated with Cognitive Impairment in Multiple Sclerosis. Mult. Scler. Houndmills Basingstoke Engl. 2009, 15, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, L.A.; Wright, A.M.; Ma, R.E.; Hummer, T.A.; Francis, M.M.; Visco, A.C.; Mehdiyoun, N.F.; Bolbecker, A.R.; Hetrick, W.P.; Dydak, U.; et al. Relationship of Auditory Electrophysiological Responses to Magnetic Resonance Spectroscopy Metabolites in Early Phase Psychosis. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2019, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, C.; Jaekel, B.N.; Gordon-Salant, S.; Goupell, M.J.; Anderson, S. Effects of Aging on Perceptual and Electrophysiological Responses to Acoustic Pulse Trains as a Function of Rate. J. Speech Lang. Hear. Res. JSLHR 2019, 62, 1087–1098. [Google Scholar] [CrossRef]
- Hirano, Y.; Oribe, N.; Onitsuka, T.; Kanba, S.; Nestor, P.G.; Hosokawa, T.; Levin, M.; Shenton, M.E.; McCarley, R.W.; Spencer, K.M. Auditory Cortex Volume and Gamma Oscillation Abnormalities in Schizophrenia. Clin. EEG Neurosci. 2020. [Google Scholar] [CrossRef]
- Van Hirtum, T.; Ghesquière, P.; Wouters, J. Atypical Neural Processing of Rise Time by Adults with Dyslexia. Cortex 2019, 113, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, S.-K.; Kim, D.-W.; Shim, M.; Kim, Y.-W.; Im, C.-H.; Lee, S.-H. Cortical Volume and 40-Hz Auditory-Steady-State Responses in Patients with Schizophrenia and Healthy Controls. NeuroImage Clin. 2019, 22, 101732. [Google Scholar] [CrossRef]
- Kirihara, K.; Rissling, A.J.; Swerdlow, N.R.; Braff, D.L.; Light, G.A. Hierarchical Organization of Gamma and Theta Oscillatory Dynamics in Schizophrenia. Biol. Psychiatry 2012, 71, 873–880. [Google Scholar] [CrossRef]
- Koshiyama, D.; Miyakoshi, M.; Thomas, M.L.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Unique Contributions of Sensory Discrimination and Gamma Synchronization Deficits to Cognitive, Clinical, and Psychosocial Functional Impairments in Schizophrenia. Schizophr. Res. 2021, 228, 280–287. [Google Scholar] [CrossRef]
- Koshiyama, D.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. A Distributed Frontotemporal Network Underlies Gamma-Band Synchronization Impairments in Schizophrenia Patients. Neuropsychopharmacology 2020, 45, 2198–2206. [Google Scholar] [CrossRef]
- Koshiyama, D.; Thomas, M.L.; Miyakoshi, M.; Joshi, Y.B.; Molina, J.L.; Tanaka-Koshiyama, K.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; Light, G.A. Hierarchical Pathways from Sensory Processing to Cognitive, Clinical, and Functional Impairments in Schizophrenia. Schizophr. Bull. 2020. [Google Scholar] [CrossRef]
- Lehongre, K.; Ramus, F.; Villiermet, N.; Schwartz, D.; Giraud, A.-L. Altered Low-Gamma Sampling in Auditory Cortex Accounts for the Three Main Facets of Dyslexia. Neuron 2011, 72, 1080–1090. [Google Scholar] [CrossRef]
- Leonhardt, B.L.; Vohs, J.L.; Bartolomeo, L.A.; Visco, A.; Hetrick, W.P.; Bolbecker, A.R.; Breier, A.; Lysaker, P.H.; O’Donnell, B.F. Relationship of Metacognition and Insight to Neural Synchronization and Cognitive Function in Early Phase Psychosis. Clin. EEG Neurosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Ramakrishnan, N.; Walker, C.P.; Polizzotto, N.R.; Cho, R.Y. Intact Auditory Cortical Cross-Frequency Coupling in Early and Chronic Schizophrenia. Front. Psychiatry 2020, 11, 507. [Google Scholar] [CrossRef]
- Parciauskaite, V.; Voicikas, A.; Jurkuvenas, V.; Tarailis, P.; Kraulaidis, M.; Pipinis, E.; Griskova-Bulanova, I. 40-Hz Auditory Steady-State Responses and the Complex Information Processing: An Exploratory Study in Healthy Young Males. PLoS ONE 2019, 14, e0223127. [Google Scholar] [CrossRef]
- Puvvada, K.C.; Summerfelt, A.; Du, X.; Krishna, N.; Kochunov, P.; Rowland, L.M.; Simon, J.Z.; Hong, L.E. Delta Vs Gamma Auditory Steady State Synchrony in Schizophrenia. Schizophr. Bull. 2018, 44, 378–387. [Google Scholar] [CrossRef]
- Rass, O.; Krishnan, G.; Brenner, C.A.; Hetrick, W.P.; Merrill, C.C.; Shekhar, A.; O’Donnell, B.F. Auditory Steady State Response in Bipolar Disorder: Relation to Clinical State, Cognitive Performance, Medication Status, and Substance Disorders. Bipolar Disord. 2010, 12, 793–803. [Google Scholar] [CrossRef]
- Rass, O.; Forsyth, J.; Krishnan, G.; Hetrick, W.P.; Klaunig, M.; Breier, A.; O’Donnell, B.F.; Brenner, C.A. Auditory Steady State Response in the Schizophrenia, First-Degree Relatives, and Schizotypal Personality Disorder. Schizophr. Res. 2012, 136, 143–149. [Google Scholar] [CrossRef]
- Rojas, D.C.; Teale, P.D.; Maharajh, K.; Kronberg, E.; Youngpeter, K.; Wilson, L.B.; Wallace, A.; Hepburn, S. Transient and Steady-State Auditory Gamma-Band Responses in First-Degree Relatives of People with Autism Spectrum Disorder. Mol. Autism 2011, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhou, P.; Wang, C.; Fan, Y.; Tian, Q.; Dong, F.; Zhou, F.; Wang, C. Defects of Gamma Oscillations in Auditory Steady-State Evoked Potential of Schizophrenia. Shanghai Arch. Psychiatry 2018, 30, 27. [Google Scholar]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-119-96479-7. [Google Scholar]
- Gandal, M.J.; Edgar, J.C.; Klook, K.; Siegel, S.J. Gamma Synchrony: Towards a Translational Biomarker for the Treatment-Resistant Symptoms of Schizophrenia. Neuropharmacology 2012, 62, 1504–1518. [Google Scholar] [CrossRef]
- McNally, J.M.; McCarley, R.W. Gamma Band Oscillations: A Key to Understanding Schizophrenia Symptoms and Neural Circuit Abnormalities. Curr. Opin. Psychiatry 2016, 29, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.J.; McGrew, K.S. The Cattell-Horn-Carroll model of intelligence. In Contemporary Intellectual Assessment: Theories, Tests, and Issues, 3rd ed.; The Guilford Press: New York, NY, USA, 2012; pp. 99–144. ISBN 978-1-60918-995-2. [Google Scholar]
- Carroll, J.B. Human Cognitive Abilities: A Survey of Factor-Analytic Studies; Cambridge University Press: Cambridge, UK, 1993; ISBN 978-0-521-38712-5. [Google Scholar]
- Carroll, J.B. Chapter 1—The Higher-stratum Structure of Cognitive Abilities: Current Evidence Supports g and About Ten Broad Factors. In The Scientific Study of General Intelligence; Nyborg, H., Ed.; Pergamon: Oxford, UK, 2003; pp. 5–21. ISBN 978-0-08-043793-4. [Google Scholar]
- Deák, G.O.; Wiseheart, M. Cognitive Flexibility in Young Children: General or Task-Specific Capacity? J. Exp. Child Psychol. 2015, 138, 31–53. [Google Scholar] [CrossRef]
- Suchy, Y. Executive Functioning: Overview, Assessment, and Research Issues for Non-Neuropsychologists. Ann. Behav. Med. 2009, 37, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Cazalis, F.; Valabrègue, R.; Pélégrini-Issac, M.; Asloun, S.; Robbins, T.W.; Granon, S. Individual Differences in Prefrontal Cortical Activation on the Tower of London Planning Task: Implication for Effortful Processing. Eur. J. Neurosci. 2003, 17, 2219–2225. [Google Scholar] [CrossRef]
- Alaerts, J.; Luts, H.; Hofmann, M.; Wouters, J. Cortical Auditory Steady-State Responses to Low Modulation Rates. Int. J. Audiol. 2009, 48, 582–593. [Google Scholar] [CrossRef]
- Dimitrijevic, A.; John, M.S.; Picton, T.W. Auditory Steady-State Responses and Word Recognition Scores in Normal-Hearing and Hearing-Impaired Adults. Ear Hear. 2004, 25, 68–84. [Google Scholar] [CrossRef]
- Baddeley, A. Working Memory and Language: An Overview. J. Commun. Disord. 2003, 36, 189–208. [Google Scholar] [CrossRef]
- Archibald, L.M. Working Memory and Language Learning: A Review. Child Lang. Teach. Ther. 2017, 33, 5–17. [Google Scholar] [CrossRef]
- Baltus, A.; Herrmann, C.S. Auditory Temporal Resolution Is Linked to Resonance Frequency of the Auditory Cortex. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2015, 98, 1–7. [Google Scholar] [CrossRef]
- Zaehle, T.; Lenz, D.; Ohl, F.W.; Herrmann, C.S. Resonance Phenomena in the Human Auditory Cortex: Individual Resonance Frequencies of the Cerebral Cortex Determine Electrophysiological Responses. Exp. BRAIN Res. 2010, 203, 629–635. [Google Scholar] [CrossRef]
- Binder, M.; Górska, U.; Pipinis, E.; Voicikas, A.; Griskova-Bulanova, I. Auditory Steady-State Response to Chirp-Modulated Tones: A Pilot Study in Patients with Disorders of Consciousness. NeuroImage Clin. 2020, 27, 102261. [Google Scholar] [CrossRef]
- Munglani, R.; Andrade, J.; Sapsford, D.J.; Baddeley, A.; Jones, J.G. A measure of consciousness and memory during isoflurane administration: The coherent frequency. Br. J. Anaesth. 1993, 71, 633–641. [Google Scholar] [CrossRef]
- Andrade, J.; Sapsford, D.J.; Jeevaratnum, D.; Pickworth, A.J.; Jones, J.G. The Coherent Frequency in the Electroencephalogram as an Objective Measure of Cognitive Function during Propofol Sedation. Anesth. Analg. 1996, 83, 1279–1284. [Google Scholar] [CrossRef]
- Halpern, D.F.; LaMay, M.L. The Smarter Sex: A Critical Review of Sex Differences in Intelligence. Educ. Psychol. Rev. 2000, 12, 229–246. [Google Scholar] [CrossRef]
- Verhaeghen, P. The Elements of Cognitive Aging: Meta-Analyses of Age-Related Differences in Processing Speed and Their Consequences; Oxford University Press: Oxford, UK, 2013; ISBN 978-0-19-936917-1. [Google Scholar]
- Griskova-Bulanova, I.; Dapsys, K.; Maciulis, V. Does Brain Ability to Synchronize with 40 Hz Auditory Stimulation Change with Age? Acta Neurobiol. Exp. 2013, 73, 564–570. [Google Scholar]
- Melynyte, S.; Pipinis, E.; Genyte, V.; Voicikas, A.; Rihs, T.; Griskova-Bulanova, I. 40 Hz Auditory Steady-State Response: The Impact of Handedness and Gender. Brain Topogr. 2018, 31, 419–429. [Google Scholar] [CrossRef]
- Griskova-Bulanova, I.; Dapsys, K.; Maciulis, V.; Arnfred, S.M. Closed Eyes Condition Increases Auditory Brain Responses in Schizophrenia. Psychiatry Res. Neuroimaging 2013, 211, 183–185. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Curtin, A.; Chan, R.C.K.; Wang, Y.; Li, H.; Zhang, T.; Qian, Z.; Guo, Q.; Li, Y.; et al. Abnormal Auditory-Evoked Gamma Band Oscillations in First-Episode Schizophrenia during Both Eye Open and Eye Close States. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 279–286. [Google Scholar] [CrossRef]
- Hong, L.; Summerfelt, A.; McMahon, R.; Adami, H.; Francis, G.; Elliott, A.; Buchanan, R.; Thaker, G. Evoked Gamma Band Synchronization and the Liability for Schizophrenia. Schizophr. Res. 2004, 70, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Lepock, J.R.; Mizrahi, R.; Bagby, R.M.; Gerritsen, C.J.; Korostil, M.; Light, G.A.; Kiang, M. Decreased Gamma Auditory Steady-State Response Is Associated with Impaired Real-World Functioning in Unmedicated Patients at Clinical High Risk for Psychosis. Clin. EEG Neurosci. 2020. [Google Scholar] [CrossRef]
- Hipp, J.F.; Siegel, M. Dissociating Neuronal Gamma-Band Activity from Cranial and Ocular Muscle Activity in EEG. Front. Hum. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
| Article | Sample Size; Males/Females; Mean Age or Age Range in Years (Standard Deviation) | Neuropsychological Tasks Used | Stimuli Frequencies; Type; Train Duration; Number of Stimuli | ASSR Measures and Site | Correlations | |
|---|---|---|---|---|---|---|
| 1 | Arrondo et al. 2009 [58] | Healthy controls: 22; n/a (similar) Patients with multiple sclerosis: 27; 10/17; 44.11 (11.45) | Brief repeatable battery-neuropsychological (BRB-N): Bushke selective reminding test (SRT); 10/36 Spatial recall test (SPART); Oral version of the symbol digit modalities test (SDMT); Paced auditory serial addition task with a 3 s interval (PASAT-3); Semantic word list generation (WLG) | 1–120 Hz; chirp; 1.61 s; 500 sweeps | EEG Frequency and amplitude of the maximal response; at Fz and Cz | Healthy controls: n.s. Patients with multiple sclerosis: SDMT and the frequency of the maximal amplitude-following responses around 40-Hz (r = 0.524, p = 0.010); PASAT-3 and the frequency of the maximal amplitude-following responses around 40-Hz (r = 0.483, p = 0.012); WLG and the frequency of the maximal amplitude-following responses around 40-Hz (r = 0.437, p = 0.023) |
| 2 | Bartolomeo et al. 2019 [59] | Healthy controls: 16; 14/5; 22.9 (3.6) Early phase of psychosis: 34; 24/7; 22.0 (4.3) | The Brief Assessment of Cognition in Schizophrenia (BACS) | 40 Hz; clicks; 500 ms; 8 trials | EEG Power; at Fz | Healthy controls: n.s. Early phase of psychosis: n.s. |
| 3 | Gaskins et al. 2019 [60] | Healthy younger subjects: 15; n/a; 22.3 (2.7) Healthy older subjects: 15; n/a; 70.3 (3.8) | Digit Symbol Coding and Digit Symbol Search | 40, 80 Hz; AM tone; 300 ms; 1024 sweeps | EEG SNR; at Cz | Healthy younger subjects: n.s. for 80-Hz ASSR; n/a for 40-Hz ASSR Healthy older subjects: n.s. for 80-Hz ASSR; n/a for 40-Hz ASSR |
| 4 | Hirano et al. 2020 [61] | Healthy controls: 24; 20/4; 44.1 (7.3) Chronic stage schizophrenia: 23; 19/4; 45.6 (9.1) | Information subscale of the Wechsler Adult Intelligence Scale–Fourth Edition (WAIS-IV) | 20, 30, and 40 Hz; clicks; 500 ms | EEG PLF, evoked power, induced power; tangential and radial dipoles in each hemisphere above primary auditory cortex | Healthy control: n.s. Chronic stage schizophrenia: n.s. |
| 5 | Hirtum et al. 2019 [62] | Healthy controls: 18; 8/10; 18–25 years Dyslexia: 20; 10/10; 18–25 years | Literacy; Spoonerisms task; Random Automatized Naming (RAN) | 40 Hz; AM tone; 300 ms; 300 epochs | EEG SNR; temporal-parietal and occipital regions: left (TP7, P1, P3, P5, P7, P9, PO3, PO7, O1) and right (TP8, P2, P4, P6, P8, P10, PO4, PO8, O2) | Healthy controls: n.s. Dyslexia: Literacy and 40-Hz neural background activity in the right hemisphere (r = −0.35, p = 0.033); Spoonerisms task and 40-Hz neural background activity in the right hemisphere (r = −0.39, p = 0.017); RAN and 40-Hz neural background activity in the right hemisphere (r = −0.39, p = 0.017) |
| 6 | Kim et al. 2019 [63] | Healthy controls: 30; 13/17; 43.33 (12.95) Schizophrenia: 33; 16/17; 42.21 (10.99) | Trail Making Test-A and B; verbal fluency test; Korean-Auditory Verbal Learning Test (K-AVLT) | 40 Hz; clicks; 500 ms; 150 trials | EEG Mean evoked power, ITC; at Cz | Healthy controls: n.s. Schizophrenia: Verbal fluency mean evoked power at 40-Hz (r = 0.223, p = 0.019) |
| 7 | Kirihara et al. 2012 [64] | Healthy controls: 188; 94/94; 43.9 (11.1) Schizophrenia: 234;182/52; 44.5 (8.8) | The Wide Range Achievement Test 3 Reading subtest; California Verbal Learning Test (CVLT-2 list A, 1–5 total score and the delayed free recall indices); Wisconsin Card Sorting Test (WCST-64); Letter-Number Sequencing (LNS) test forward and reordering conditions | 30, 40 Hz; clicks; 500 ms; 200 trials | EEG Amplitude, ITC, cross frequency coupling, modulation index; at FCz | Healthy controls: n.s. Schizophrenia: n.s. |
| 8 | Koshiyama et al. 2020a [65] | Healthy controls: 283; 139/144; 44.5 (11.4) Schizophrenia: 428; 309/119; 44.5 (9.5) | Letter-Number Sequencing (LNS), California verbal learning test (CVLT-2 list A 1–5 total score), Wisconsin Card Sorting Test (WCST, perseverative responses) | 40 Hz; clicks; 500 ms; 200 trains | EEG ERSP at Fz | Healthy control: n.s. Schizophrenia: 40-Hz ASSR predicted LNS scores (standardized coefficient β = 0.17, p < 5.5 × 10−4) and CVLT scores (β = 0.16, p < 1.1 × 10−3) |
| 9 | Koshiyama et al. 2020b [66] | Healthy controls: 293; 141/152; 44.7 (11.4) Schizophrenia: 427; 309/118; 45.5 (9.5) | Letter-Number Sequencing (LNS), Letter-Number Span (LN Span), California verbal learning test (CVLT-2 list A 1–5 total score), Reading subtest of the Wide Range Achievement Test-3 (WRAT) | 40 Hz; clicks; 500 ms; 200 trains | EEG ITC, 8 dipoles above primary auditory cortex | Healthy control: n/a Schizophrenia: LN Span scores with ITC of 40-Hz ASSR in the right temporal cortex (r = 0.16, p = 0.01); LNS scores with ITC of 40-Hz ASSR in the right temporal cortex (r = 0.13, p = 0.046) and left temporal cortex (r = 0.17, p = 0.02); WRAT scores with ITC of 40-Hz ASSR in the left temporal cortex (r = 0.17, p = 0.01); CVLT scores with ITC of 40-Hz ASSR in the left temporal cortex (r = 0.14, p = 0.04) and left superior frontal cortex (r = 0.17, p = 0.02) |
| 10 | Koshiyama et al. 2020c [67] | Healthy controls: 503; 234/269; 43.7 (12.8) Schizophrenia: 695; 477/218; 45.5 (10.2) | Letter-Number Sequencing (LNS), Letter-Number Span (LN Span), California verbal learning test (CVLT-2) list A 1–5 total score and Recognition Hits subscales, Reading subtest of the Wide Range Achievement Test-3 (WRAT) | 40 Hz; clicks; 500 ms; 200 trains | EEG ITC and ERSP at Fz | 40-Hz ITC and ERSP with LNS (p < 0.00042) |
| 11 | Lehongre et al. 2011 [68] | Healthy controls: 21; 11/10; 24.38 (3.85) Dyslexic: 23; 14/9; 24.61 (4.57) | Alouette test (reading fluency); Random Automatized Naming (RAN); composite measure of phonology (PHONO); the Wechsler Adult Intelligence Scale (WAIS-III), Digit span, Spoonerism tasks, Nonword repetition test | 10–80 Hz; chirp; 5.4 s; 40 trials in 2 sessions, each with 80 sweeps | MEG Power, power asymmetry (left-right) at planum temporale (PT), superior temporal sulcus | Healthy controls: Reading speed and 30-Hz ASSRs power (p < 0.05) in left and right PT; Verbal fluency mean evoked power at 40-Hz (r = 0.223, p = 0.019). Composite measure of phonology and 30-Hz ASSRs power (p < 0.05) in left PT Dyslexic: Spoonerism task and 30-Hz ASSR power asymmetry (left minus right) (r = −0.450, p = 0.047) (effect was mostly driven by nonword repetition (r = 0.44, p = 0.04); RAN and 30-Hz ASSR power asymmetry (r = 0.552, p = 0.006); Digit span and 45–65 Hz magnitude in left PT (at 58-Hz r = −0.542, p = 0.009), left PFC (r = −0.486, p = 0.022) and left STS (r = −0.511, p = 0.015) |
| 12 | Leonhardt et al. 2019 [69] | Schizophrenia or schizoaffective disorder: 17; 14/3; 21.5 (3.8) | Brief Assessment of Cognition in Schizophrenia (BACS): Composite score | 40 Hz; clicks; 500 ms; 80 trials | EEG Power; at Fz and Cz | Schizophrenia or schizoaffective disorder: n.s. |
| 13 | Light et al. 2006 [43] | Healthy controls: 80; n/a; 33.6 (9.95) Schizophrenia: 100; n/a; 42.5 (8.31) | The Wide Range Achievement Test 3 Reading subtest; California Verbal Learning Test (CVLT-2 list A, 1–5 total score and the delayed free recall indices); Wisconsin Card Sorting Test (WCST-64); Letter-Number Sequencing (LNS) test forward and reordering conditions | 30, 40 Hz; clicks; 500 ms; 200 trials | EEG Evoked power and ITPC; at FCz | Healthy controls: n.s. Schizophrenia: LNS and power at 40-Hz (r = 0.32, p < 0.01) |
| 14 | Murphy et al. 2020 [70] | Healthy controls: 17; 9/8; 28.87 (5.98) Early-stage schizophrenia: 12; 12/0; 27.5 (6.89) Chronic-stage schizophrenia: 16; 13/3; 33.63 (6.94) | Brief Assessment of Cognition in Schizophrenia (BACS): Composite score | 20 Hz, 30 Hz, 40 Hz; clicks; 1000 ms; 100 trails each of 10 block | MEG Amplitude and phase-amplitude coupling at combined region of bilateral transverse temporal cortex and superior temporal gyrus | Healthy controls: n.s. Early stage schizophrenia: n.s. Chronic stage schizophrenia: n.s. |
| 15 | Parčiauskaitė et al. 2019 [71] | Healthy subjects: 28; 28/0; 25.8 (3.3) | Psychology Experiment Building Language based task battery: Choice response time task (CRT), Stroop test (SOO), Tower of London test (TOL), Lexical decision task (LDT) and Semantic categorization task (SCT) | 40 Hz; clicks; 500 ms; 150 trials each containing 20 clicks | EEG PLI and ERSP; the left (F3, F1, FC1, C1, FC3, C3), central (Fz, FCz, Cz), and right (F4, F2, FC2, C2, FC4, C4) regions | Healthy subjects: Tower of London task (number of moves) and 40-Hz PLI and ERSP: left (PLI: r = 0.55, p < 0.01; ERSP: r = 0.57, p < 0.01), center (PLI: r = 0.37, p = 0.05; ERSP: r = 0.42, p = 0.03) and right (PLI: r = 0.43, p = 0.02; ERSP: r = 0.46, p = 0.01) regions |
| 16 | Puvvada et al. 2018 [72] | Healthy controls: 108; 71/37; 37.9 (13.8) Schizophrenia: 128; 86/42; 37.8 (13.1) First-degree relatives of schizophrenia patients: 55; 17/38; 46.6 (13.6) | Digit sequencing task (digit span) | 40, 80 Hz; clicks; 375 ms and 187.5 ms; 75 trials, each containing 15 clicks; | EEG Power and PLI; at frontocentral electrodes (AF3, AFZ, AF4, F3, F1, FZ, F2, F4, FC3, FC1, FCZ, FC2, FC4, C1, CZ, C2) | Healthy controls: n.s. Schizophrenia: Digit span and power at 40-Hz (r = 0.20, p = 0.033); First-degree relatives of schizophrenia patients: Digit span and power at 40-Hz (r = 0.42, p = 0.003) |
| 17 | Rass et al. 2010 [73] | Healthy controls: 77; 40/47; 41.0 (10.3) Euthymic bipolar disorder: 22; 43.6 (10.5) Acute bipolar disorder: 43; 42.6 (10.3) | Subtests from The Wechsler Abbreviated Scale of Intelligence (WAIS-III): Picture Completion, Digit Symbol Coding, Digit Span, Similarities | 30, 40, 50 Hz; clicks; 467–480 ms; 80 trials | EEG MTP and PLF; at FCz | Healthy controls: n.s. Bipolar disorder: n.s. |
| 18 | Rass et al. 2012 [74] | Healthy controls: 56; 26/30; 38.75 (10.4) Schizophrenia and schizoaffective disorder: 42; 23/19; 36.86 (12.8) First-degree relatives of schizophrenia patients: 35; 13/22; 36.03 (12.5) Schizotypal personality disorder: 34; 20/14; 37.35 (9.2) | Subtests from The Wechsler Abbreviated Scale of Intelligence (WAIS-III): Picture Completion, Digit Symbol Coding, Digit Span, Similarities | 30, 40, 50 Hz; clicks; 467–480 ms; 80 trials | EEG MTP and PLF; at FCz | Healthy controls: Similarities and 40-Hz PLF (r = 0.38, p < 0.01); Symbol Coding and 50-Hz MTP (r = 0.26, p = 0.03) Schizophrenia and schizoaffective disorder: Similarities and 40-Hz MTP (r = 0.34, p = 0.04), 40-Hz PLF (r = 0.34, p = 0.04); Digit span and 50-Hz PLF (r = 0.38, p = 0.02) First-degree relatives of schizophrenia patients: Similarities and 40-Hz PLF (r = 0.39, p = 0.03); Similarities and 50-Hz PLF (r = 0.45, p = 0.01) Schizotypal personality disorder: Similarities and 40-Hz MTP (r = 0.34, p = 0.04); Similarities and 50-Hz PLF (r = 0.40, p = 0.02) |
| 19 | Rojas et al. 2011 [75] | Healthy controls: 20; 7/13; 43.84 (6.86) Parents of children with ASD: 21; 6/15; 43.67 (7.33) | The Wechsler Abbreviated Scale of Intelligence (WAIS): Verbal IQ, Performance IQ, Full scale IQ | 32, 40, 48 Hz; AM tone; 500 ms; 150 trials | MEG PLF, evoked, induced and total power: left and right hemispheres | Healthy controls: n.s. Parents of children with ASD: n.s. |
| 20 | Sun et al. 2018 [76] | Healthy controls: 30; 16/14; 34.2 (10.3) Schizophrenia: 24; 13/11; 33.0 (11.0) | MATRICS Consensus Cognitive Battery (MCCB) Chinese version: Trail Making Test: Part A, Symbol Coding Test, Animal Naming Test, Continuous Performance Test (Identical Pairs), Wechsler Memory Scale Third Edition (Spatial Span test and Letter-Number Span test), Hopkins Verbal Learning Test, Simple Visuospatial Memory Test, Mazes test, Mayer–Salovey–Caruso Emotional Intelligence Test (Managing Emotions) | 40 Hz; clicks; 500 ms; 150 trains | EEG Power, PLF, ITPC; 128 electrodes | Healthy controls: Mazes test and PLF (r = 0.66); Mazes test and ITPC (r = 0.69); Trail Making Test: Part A and PLF (r = 0.56, p < 0.05); Trail Making Test: Part A and ERSP (r = 0.62, p < 0.05); Cognitive assessment total score and PLF (r = 0.48, p < 0.05); Cognitive assessment total score and ERSP (r = 0.59, p < 0.05) Schizophrenia: Mazes test and 40-Hz PLF (r = 0.55, p < 0.05); Mazes test and ITPC (r = 0.54, p < 0.05) |
| 21 | Tada et al. 2016 [44] | Healthy controls: 21; 11/10; 22.4 (3.3) First-episode schizophrenia: 13; 8/5; 24.5 (5.9) Ultra-high-risk individuals: 15; 9/6; 22.1 (4.0) | BACS-J: Verbal memory, Digit sequencing task (digit span), Token motor task, Category fluency, Letter fluency, Symbol coding, Tower of London | 30, 40 Hz; clicks; 500 ms; 200 trials | EEG ITPC and ERSP; late latency at FCz | Healthy controls: n/a. First-episode schizophrenia: Symbol coding and the 40-Hz ITPC (r = 0.75, p = 0.003) and ERSP (r = 0.76, p = 0.003) Ultra-high-risk individuals: n.s. |
| 22 | van Deursen et al. 2011 [55] | Healthy controls: 20; 12/8; 69.5 (6.1) Mild Alzheimer’s disease: 15; 11/4; 75.2 (6.9) Mild cognitive impairment: 20; 12/8; 70.6 (7.2) | The cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-cog) | 40 Hz; clicks; 450 ms; 80 trials | EEG Power; at T5, T6, O2, Fz, Pz, Cz | Combined sample: ADAS-cog and 40-Hz power at T5 (r = 0.43, p = 0.019) and T6 (r = 0.38, p = 0.028) |
| Domain | Assessment | |
|---|---|---|
| 1 | Global cognition/ functioning or intellectual ability (g) | MCCB (Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery), WAIS (Wechsler Adult Intelligence Scale), ADAS-cog (The Alzheimer’s Disease Assessment Scale–Cognitive Subscale), BACS (Brief Assessment of Cognition in Schizophrenia) |
| 2 | Attentional control and executive functions | Choice response task, Stroop test, Continuous Performance Test, Identical pairs, Serial addition task, Paced auditory serial addition task, Category fluency, Semantic categorization task, Semantic word list generation |
| 3 | Processing speed | Symbol search, Trial making test, Symbol coding, Symbol Digit Modalities Test, Rapid naming, Verbal fluency (letter, category), Picture completion |
| 4 | Short-term and working memory | List learning, Digit span, Spatial span, Letter–Number span, Letter–Number sequencing, Visuospatial Memory Test, Verbal memory, Hopkins Verbal Learning Test, Buschke Selective Reminding Test, Spatial recall test, California Verbal Learning Test (CVLT) |
| 5 | Cognitive flexibility and reasoning | Wisconsin Card Sorting Test, Similarities, Mazes test, Tower of London, Mayer–Salovey–Caruso Emotional Intelligence |
| 6 | Language abilities | Verbal IQ, Vocabulary, Reading, Non-word repetition, Auditory verbal learning, Spoonerism, Literacy, Random automatized naming, The Wide Range Achievement Test 3 Reading subtest, Lexical decision test, Alouette test |
| 7 | Motor abilities | Token task |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parciauskaite, V.; Bjekic, J.; Griskova-Bulanova, I. Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain Sci. 2021, 11, 217. https://doi.org/10.3390/brainsci11020217
Parciauskaite V, Bjekic J, Griskova-Bulanova I. Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain Sciences. 2021; 11(2):217. https://doi.org/10.3390/brainsci11020217
Chicago/Turabian StyleParciauskaite, Vykinta, Jovana Bjekic, and Inga Griskova-Bulanova. 2021. "Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review" Brain Sciences 11, no. 2: 217. https://doi.org/10.3390/brainsci11020217
APA StyleParciauskaite, V., Bjekic, J., & Griskova-Bulanova, I. (2021). Gamma-Range Auditory Steady-State Responses and Cognitive Performance: A Systematic Review. Brain Sciences, 11(2), 217. https://doi.org/10.3390/brainsci11020217







