Effects of Mind–Body Exercise on Brain Structure and Function: A Systematic Review on MRI Studies
Abstract
1. Introduction
2. Methods
2.1. Literature Search and Study Selection
2.2. Inclusion and Exclusion Criteria
- (i)
- Participants: study populations consisted of healthy adults or older adults, regardless of sex, racial, and ethnic groups. We excluded studies with subjects who had cognitive impairment or suffering from organic diseases such as diabetes, fibromyalgia, knee osteoarthritis, or tinnitus to avoid interference of the results by these factors.
- (ii)
- Type of exercise: the experimental group engaged in mind–body exercises, including Tai Chi Chuan (TCC), Qigong, and yoga. Studies with multimodal interventions comprising mind–body exercises were also included to increase the number of enrolled articles. However, we excluded studies that examined the sole effects of mindfulness or meditation since the aim of this study was to examine the effect of holistic mind–body exercises which involve structured movements, breath control, and attention modulation on brain health.
- (iii)
- Study design: to gain an overall understanding of mind–body exercise-related changes in the brain plasticity of healthy adults, there were no restrictions on the study design. Therefore, randomized and non-randomized controlled interventions and within-subjects intervention studies as well as cross-sectional studies comparing experts to novices were all included. With reference to previous studies [25,26], for the intervention studies, the experimental group must have exercised for at least 4 weeks with more than one session per week, and for the cross-sectional studies, the regular exercise duration must have been not less than 3 years to provide sufficient time for changes in the brain structure and function to occur.
- (iv)
- Outcomes: the imaging technique was restricted to MRI, including sMRI, task fMRI, and resting-state fMRI. The outcome measures included changes in structure (i.e., gray matter volume, density, and cortical thickness) and task or resting-state (de)activation, functional connectivity for pre- to post-mind–body intervention, or mind–body expert–novice comparison.
- (v)
- Literature type: peer-reviewed articles published either in English or Chinese language.
2.3. Data Extraction
2.4. Quality Assessment
3. Results
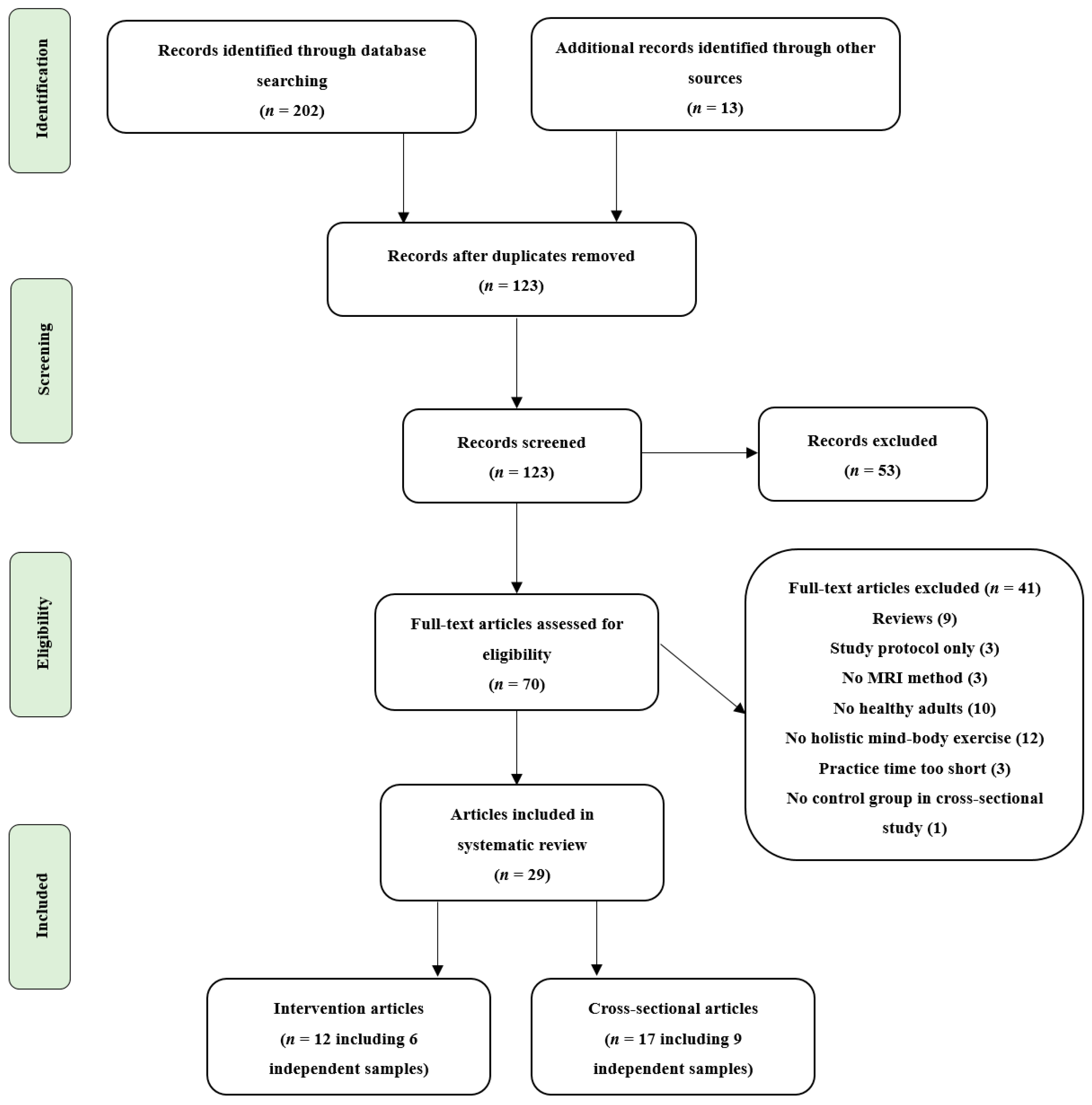
3.1. Study Search and Characteristics
3.2. Quality Assessment
3.3. Changes in Brain Regions
3.3.1. Prefrontal Cortex
3.3.2. Hippocampus/Medial Temporal Lobe
3.3.3. Lateral Temporal Lobe
3.3.4. Insula
3.3.5. Cingulate Cortex
3.3.6. Other Regions
3.4. Changes in Brain Functional Connectivity and Network
4. Discussion
4.1. Brain Regions
4.1.1. Prefrontal Cortex
4.1.2. Hippocampus/Medial Temporal Lobe
4.1.3. Lateral Temporal Lobe
4.1.4. Insula and Cingulate Cortex
4.2. Brain Networks
4.2.1. Cognitive Control Network
4.2.2. Default Mode Network
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bo, A.; Mao, W.; Lindsey, M.A. Effects of mind–body interventions on depressive symptoms among older Chinese adults: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2017, 32, 509–521. [Google Scholar] [CrossRef]
- Schmalzl, L.; Kerr, C.E. Editorial: Neural mechanisms underlying movement-based embodied contemplative practices. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef]
- Russell, T.A.; Arcuri, S.M. A Neurophysiological and Neuropsychological Consideration of Mindful Movement: Clinical and Research Implications. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Wayne, P.M.; Walsh, J.N.; Taylor-Piliae, R.E.; Wells, R.E.; Papp, K.V.; Donovan, N.J.; Yeh, G.Y. Effect of Tai Chi on Cognitive Performance in Older Adults: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. JAGS 2014, 62, 25–39. [Google Scholar] [CrossRef]
- Chan, J.S.Y.; Deng, K.; Wu, J.; Yan, J.H. Effects of Meditation and Mind–Body Exercises on Older Adults’ Cognitive Performance: A Meta-analysis. Gerontologist 2019, 59, e782–e790. [Google Scholar] [CrossRef] [PubMed]
- Büssing, A.; Michalsen, A.; Khalsa, S.B.S.; Telles, S.; Sherman, K.J. Effects of Yoga on Mental and Physical Health: A Short Summary of Reviews. Evid. Based Complement. Altern. Med. 2012, 2012, 165410–165417. [Google Scholar] [CrossRef]
- Zhu, W.; Guan, S.; Yang, Y. Clinical Implications of Tai Chi Interventions: A Review. Am. J. Lifestyle Med. 2010, 4, 418–432. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, X. Improvement of balance control ability and flexibility in the elderly Tai Chi Chuan (TCC) practitioners: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 60, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Roland, K.P.; Jakobi, J.M.; Jones, G.R. Does yoga engender fitness in older adults? A critical review. J. Aging Phys. Act. 2011, 19, 62–79. [Google Scholar] [CrossRef]
- Zou, L.; SasaKi, J.E.; Wang, H.; Xiao, Z.; Fang, Q.; Zhang, M. A Systematic Review and Meta-Analysis Baduanjin Qigong for Health Benefits: Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2017, 2017, 4548706. [Google Scholar] [CrossRef]
- Wang, S.; Yin, H.; Jia, Y.; Zhao, L.; Wang, L.; Chen, L. Effects of Mind-Body Exercise on Cognitive Function in Older Adults With Cognitive Impairment: A Systematic Review and Meta-analysis. J. Nerv. Ment. Dis. 2018, 206, 913–924. [Google Scholar] [CrossRef]
- Wu, C.; Yi, Q.; Zheng, X.; Cui, S.; Chen, B.; Lu, L.; Tang, C. Effects of Mind-Body Exercises on Cognitive Function in Older Adults: A Meta-Analysis. J. Am. Geriatr. Soc. 2019, 67, 749–758. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zou, L.; Liu, X.; Song, W. The Effects of Mind-Body Exercise on Cognitive Performance in Elderly: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2791. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.S.; Tsunaka, M.; Tsang, H.W.; Chan, E.P.; Cheung, W.M. Effects of yoga on stress management in healthy adults: A systematic review. Altern. Ther. Health Med. 2011, 17, 32–38. [Google Scholar] [PubMed]
- Rogers, C.E.; Larkey, L.K.; Keller, C. A review of clinical trials of tai chi and qigong in older adults. West. J. Nurs. Res. 2009, 31, 245–279. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Ho, R.T.; Chung, K.F.; Wang, C.W.; Yao, T.J.; Ng, S.M.; Chan, C.L. Qigong exercise alleviates fatigue, anxiety, and depressive symptoms, improves sleep quality, and shortens sleep latency in persons with chronic fatigue syndrome-like illness. Evid. Based Complement. Altern. Med. 2014, 2014, 106048. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Langhorst, J.; Dobos, G. Yoga for depression: A systematic review and meta-analysis. Depress. Anxiety 2013, 30, 1068–1083. [Google Scholar] [CrossRef]
- Wang, F.; Lee, E.K.; Wu, T.; Benson, H.; Fricchione, G.; Wang, W.; Yeung, A.S. The effects of tai chi on depression, anxiety, and psychological well-being: A systematic review and meta-analysis. Int. J. Behav. Med. 2014, 21, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.; Tailor, A.; Bhatt, T. Effects of yoga on brain waves and structural activation: A review. Complement. Ther. Clin. Pract. 2015, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.; Rüdiger, S.; Tony, S.; Theodor, R. Reliability of quantitative transverse relaxation time mapping with T2-prepared whole brain pCASL. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Giedd, J.N.; Raznahan, A.; Alexander-Bloch, A.; Schmitt, E.; Gogtay, N.; Rapoport, J.L. Child psychiatry branch of the national institute of mental health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology 2015, 40, 43–49. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Intrinsic Fluctuations within Cortical Systems Account for Intertrial Variability in Human Behavior. Neuron 2007, 56, 171–184. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ Clin. Res. Ed. 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Su, X.; Fang, Q.; Hou, L.; Lee, Y.; Chen, C.C.; Lamberth, J.; Kim, M.L. The Effects of Tai Chi Intervention on Healthy Elderly by Means of Neuroimaging and EEG: A Systematic Review. Front. Aging Neurosci. 2018, 10, 110. [Google Scholar] [CrossRef]
- Gothe, N.P.; Khan, I.; Hayes, J.; Erlenbach, E.; Damoiseaux, J.S. Yoga Effects on Brain Health: A Systematic Review of the Current Literature. Brain Plast. 2019, 5, 105–122. [Google Scholar] [CrossRef]
- Chung, S.-C.; Brooks, M.M.; Rai, M.; Balk, J.L.; Rai, S. Effect of Sahaja Yoga Meditation on Quality of Life, Anxiety, and Blood Pressure Control. J. Altern. Complement. Med. 2012, 18, 589–596. [Google Scholar] [CrossRef]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E.M.Z., Ed.; The Joanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Goplen, C.M.; Verbeek, W.; Kang, S.H.; Jones, C.A.; Voaklander, D.C.; Churchill, T.A.; Beaupre, L.A. Preoperative opioid use is associated with worse patient outcomes after Total joint arthroplasty: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2019, 20, 234. [Google Scholar] [CrossRef]
- Melo, G.; Dutra, K.L.; Rodrigues Filho, R.; Ortega, A.O.L.; Porporatti, A.L.; Dick, B.; Flores-Mir, C.; De Luca Canto, G. Association between psychotropic medications and presence of sleep bruxism: A systematic review. J. Oral Rehabil. 2018, 45, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Froeliger, B.; Garland, E.L.; Kozink, R.V.; Modlin, L.A.; Chen, N.K.; McClernon, F.J.; Greeson, J.M.; Sobin, P. Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond. Evid. Based Complement. Altern. Med. 2012, 2012, 680407. [Google Scholar] [CrossRef] [PubMed]
- Froeliger, B.; Garland, E.L.; McClernon, F.J. Yoga meditation practitioners exhibit greater gray matter volume and fewer reported cognitive failures: Results of a preliminary voxel-based morphometric analysis. Evid. Based Complement. Altern. Med. 2012, 2012, 821307. [Google Scholar] [CrossRef]
- Froeliger, B.E.; Garland, E.L.; Modlin, L.A.; McClernon, F.J. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: A pilot study. Front. Integr. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.X.; Dong, H.M.; Yang, Z.; Luo, J.; Zuo, X.N. Tai Chi Chuan optimizes the functional organization of the intrinsic human brain architecture in older adults. Front. Aging Neurosci. 2014, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-X.; Gong, Z.-Q.; Yang, Z.; Zuo, X.-N. Mind-Body practice changes fractional amplitude of low frequency fluctuations in intrinsic control networks. Front. Psychol. 2017, 8, 1049. [Google Scholar] [CrossRef]
- Wei, G.-X.; Xu, T.; Fan, F.-M.; Dong, H.-M.; Jiang, L.-L.; Li, H.-J.; Yang, Z.; Luo, J.; Zuo, X.-N. Can Taichi Reshape the Brain? A Brain Morphometry Study. PLoS ONE 2013, 8, e61038. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Yuan, X.; Zhang, Y.; Zhang, S.; Zou, L.; Yang, L.; Chang, Y.K.; Xia, Q.; Wang, Y.; Wei, G.X. Brain Functional Specialization is Enhanced among Tai Chi Chuan Practitioners. Arch. Phys. Med. Rehabil. 2020. [Google Scholar] [CrossRef] [PubMed]
- Villemure, C.; Ceko, M.; Cotton, V.A.; Bushnell, M.C. Insular cortex mediates increased pain tolerance in yoga practitioners. Cereb Cortex 2014, 24, 2732–2740. [Google Scholar] [CrossRef]
- Villemure, C.; Čeko, M.; Cotton, V.A.; Catherine Bushnell, M. Neuroprotective effects of yoga practice: Age-, experience-, and frequency-dependent plasticity. Front. Hum. Neurosci. 2015, 9. [Google Scholar] [CrossRef]
- Gard, T.; Taquet, M.; Dixit, R.; Hölzel, B.K.; Dickerson, B.C.; Lazar, S.W. Greater widespread functional connectivity of the caudate in older adults who practice kripalu yoga and vipassana meditation than in controls. Front. Hum. Neurosci. 2015, 9, 137. [Google Scholar] [CrossRef]
- Afonso, R.F.; Balardin, J.B.; Lazar, S.; Sato, J.R.; Igarashi, N.; Santaella, D.F.; Lacerda, S.S.; Amaro, E., Jr.; Kozasa, E.H. Greater Cortical Thickness in Elderly Female Yoga Practitioners-A Cross-Sectional Study. Front. Aging Neurosci. 2017, 9, 201. [Google Scholar] [CrossRef]
- Santaella, D.F.; Balardin, J.B.; Afonso, R.F.; Giorjiani, G.M.; Sato, J.R.; Lacerda, S.S.; Amaro, E., Jr.; Lazar, S.; Kozasa, E.H. Greater Anteroposterior Default Mode Network Functional Connectivity in Long-Term Elderly Yoga Practitioners. Front. Aging Neurosci. 2019, 11, 158. [Google Scholar] [CrossRef]
- Gothe, N.P.; Hayes, J.M.; Temali, C.; Damoiseaux, J.S. Differences in Brain Structure and Function Among Yoga Practitioners and Controls. Front. Integr. Neurosci. 2018, 12, 26. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Li, L.; Guo, X. Functional Connectivity Within the Executive Control Network Mediates the Effects of Long-Term Tai Chi Exercise on Elders’ Emotion Regulation. Front. Aging Neurosci. 2018, 10, 315. [Google Scholar] [CrossRef]
- Port, A.P.; Santaella, D.F.; Lacerda, S.S.; Speciali, D.S.; Balardin, J.B.; Lopes, P.B.; Afonso, R.F.; Radvany, J.; Amaro, E., Jr.; Kozasa, E.H. Cognition and brain function in elderly Tai Chi practitioners: A case-control study. Explore 2018, 14, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Yang, Z.; Chen, S.; Pan, Y.; Yuan, X.; Wang, E.; Yue, C. The Effect of Tai Chi Chuan Exercise on the Brain’s Fractional Amplitude of Low Frequency Fluctuation of the Elderly in the Resting State. Chin. J. Sports Med. 2019, 38, 449–453. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, Y.; Jian, M.; Herold, F.; Yu, Q.; Mueller, P.; Lin, J.; Wang, G.; Tao, Y.; Zhang, Z.; et al. Differential Effects of Tai Chi Chuan (Motor-Cognitive Training) and Walking on Brain Networks: A Resting-State fMRI Study in Chinese Women Aged 60. Healthcare 2020, 8, 67. [Google Scholar] [CrossRef]
- Hariprasad, V.R.; Varambally, S.; Shivakumar, V.; Kalmady, S.V.; Venkatasubramanian, G.; Gangadhar, B.N. Yoga increases the volume of the hippocampus in elderly subjects. Indian J. Psychiatry 2013, 55, S394–S396. [Google Scholar] [CrossRef]
- Li, R.; Zhu, X.; Yin, S.; Niu, Y.; Zheng, Z.; Huang, X.; Wang, B.; Li, J. Multimodal intervention in older adults improves resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Front. Aging Neurosci. 2014, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhu, X.; Li, R.; Niu, Y.; Wang, B.; Zheng, Z.; Huang, X.; Huo, L.; Li, J. Intervention-induced enhancement in intrinsic brain activity in healthy older adults. Sci. Rep. 2014, 4, 7309. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhu, X.; Yin, S.; Wang, B.; Niu, Y.; Huang, X.; Li, R.; Li, J. Combined cognitive-psychological-physical intervention induces reorganization of intrinsic functional brain architecture in older adults. Neural Plast 2015, 2015, 713104. [Google Scholar] [CrossRef]
- Tao, J.; Chen, X.; Egorova, N.; Liu, J.; Xue, X.; Wang, Q.; Zheng, G.; Li, M.; Hong, W.; Sun, S.; et al. Tai Chi Chuan and Baduanjin practice modulates functional connectivity of the cognitive control network in older adults. Sci. Rep. 2017, 7, 41581. [Google Scholar] [CrossRef]
- Tao, J.; Chen, X.L.; Liu, J.; Egorova, N.; Xue, X.H.; Liu, W.L.; Zheng, G.H.; Li, M.; Wu, J.S.; Hu, K.; et al. Tai Chi Chuan and Baduanjin Mind-Body Training Changes Resting-State Low-Frequency Fluctuations in the Frontal Lobe of Older Adults: A Resting-State fMRI Study. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Liu, J.; Egorova, N.; Chen, X.; Sun, S.; Xue, X.; Huang, J.; Zheng, G.; Wang, Q.; Chen, L.; et al. Increased Hippocampus-Medial Prefrontal Cortex Resting-State Functional Connectivity and Memory Function after Tai Chi Chuan Practice in Elder Adults. Front. Aging Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Liu, J.; Liu, W.; Huang, J.; Xue, X.; Chen, X.; Wu, J.; Zheng, G.; Chen, B.; Li, M.; et al. Tai Chi Chuan and Baduanjin Increase Grey Matter Volume in Older Adults: A Brain Imaging Study. J. Alzheimer’s Dis. 2017, 60, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tao, J.; Liu, W.; Huang, J.; Xue, X.; Li, M.; Yang, M.; Zhu, J.; Lang, C.; Park, J.; et al. Different modulation effects of Tai Chi Chuan and Baduanjin on resting-state functional connectivity of the default mode network in older adults. Soc. Cogn. Affect. Neurosci. 2019, 14, 217–224. [Google Scholar] [CrossRef]
- Wu, M.T.; Tang, P.F.; Goh, J.O.S.; Chou, T.L.; Chang, Y.K.; Hsu, Y.C.; Chen, Y.J.; Chen, N.C.; Tseng, W.I.; Gau, S.S.; et al. Task-Switching Performance Improvements After Tai Chi Chuan Training Are Associated With Greater Prefrontal Activation in Older Adults. Front. Aging Neurosci. 2018, 10, 280. [Google Scholar] [CrossRef]
- Cui, L.; Yin, H.; Lyu, S.; Shen, Q.; Wang, Y.; Li, X.; Li, J.; Li, Y.; Zhu, L. Tai Chi Chuan vs General Aerobic Exercise in Brain Plasticity: A Multimodal MRI Study. Sci. Rep. 2019, 9, 17264. [Google Scholar] [CrossRef]
- Garner, M.; Reith, W.; Krick, C. 10-Week Hatha Yoga Increases Right Hippocampal Density Compared to Active and Passive Control Groups: A Controlled Structural cMRI Study. J. Neuroimaging Psychiatry Neurol. 2019, 4. [Google Scholar] [CrossRef]
- Young, K.S.; van der Velden, A.M.; Craske, M.G.; Pallesen, K.J.; Fjorback, L.; Roepstorff, A.; Parsons, C.E. The impact of mindfulness-based interventions on brain activity: A systematic review of functional magnetic resonance imaging studies. Neurosci. Biobehav. Rev. 2018, 84, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wang, P.; Li, S.; Zhang, Y.; Li, X. Effect of Baduanjin exercise for hypertension: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2015, 80, 370–378. [Google Scholar] [CrossRef]
- Kim, S.; Dede, A.J.; Hopkins, R.O.; Squire, L.R. Memory, scene construction, and the human hippocampus. Proc. Natl. Acad. Sci. USA 2015, 112, 4767–4772. [Google Scholar] [CrossRef]
- Lech, R.K.; Suchan, B. The medial temporal lobe: Memory and beyond. Behav. Brain Res. 2013, 254, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, B.K.; Carmody, J.; Vangel, M.; Congleton, C.; Yerramsetti, S.M.; Gard, T.; Lazar, S.W. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011, 191, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Sander, D.; Grandjean, D.; Pourtois, G.; Schwartz, S.; Seghier, M.L.; Scherer, K.R.; Vuilleumier, P. Emotion and attention interactions in social cognition: Brain regions involved in processing anger prosody. NeuroImage 2005, 28, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Liang, X.; He, Y.; Zang, Y.; Han, Z.; Caramazza, A.; Bi, Y. Predicting conceptual processing capacity from spontaneous neuronal activity of the left middle temporal gyrus. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 481–489. [Google Scholar] [CrossRef]
- Zheng, G.; Ye, B.; Zheng, Y.; Xiong, Z.; Xia, R.; Qiu, P.; Tao, J.; Chen, L. The effects of exercise on the structure of cognitive related brain regions: A meta-analysis of functional neuroimaging data. Int. J. Neurosci. 2019, 129, 406–415. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. An insular view of anxiety. Biol. Psychiatry 2006, 60, 383–387. [Google Scholar] [CrossRef]
- Craig, A.D. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003, 26, 303–307. [Google Scholar] [CrossRef]
- Craig, A.D. Significance of the insula for the evolution of human awareness of feelings from the body. Ann. N. Y. Acad. Sci. 2011, 1225, 72–82. [Google Scholar] [CrossRef]
- Taylor, K.S.; Seminowicz, D.A.; Davis, K.D. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 2009, 30, 2731–2745. [Google Scholar] [CrossRef]
- van Veen, V.; Carter, C.S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 2002, 77, 477–482. [Google Scholar] [CrossRef]
- Nakata, H.; Sakamoto, K.; Kakigi, R. Meditation reduces pain-related neural activity in the anterior cingulate cortex, insula, secondary somatosensory cortex, and thalamus. Front. Psychol. 2014, 5, 1489. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Egorova, N.; Yang, X.Q.; Zhang, W.Y.; Chen, J.; Yang, X.Y.; Hu, L.J.; Sun, S.; Tu, Y.; Kong, J. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl. Psychiatry 2015, 5, e683. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef] [PubMed]
- Gutchess, A.H.; Hebrank, A.; Sutton, B.P.; Leshikar, E.; Chee, M.W.; Tan, J.C.; Goh, J.O.; Park, D.C. Contextual interference in recognition memory with age. NeuroImage 2007, 35, 1338–1347. [Google Scholar] [CrossRef]
- Bakker, A.; Krauss, G.L.; Albert, M.S.; Speck, C.L.; Jones, L.R.; Stark, C.E.; Yassa, M.A.; Bassett, S.S.; Shelton, A.L.; Gallagher, M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 2012, 74, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. NeuroImage 2010, 50, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef]
- Eyre, H.A.; Acevedo, B.; Yang, H.; Siddarth, P.; Van Dyk, K.; Ercoli, L.; Leaver, A.M.; Cyr, N.S.; Narr, K.; Baune, B.T.; et al. Changes in Neural Connectivity and Memory Following a Yoga Intervention for Older Adults: A Pilot Study. J. Alzheimer’s Dis. 2016, 52, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Meister, K.; Juckel, G. A Systematic Review of Mechanisms of Change in Body-Oriented Yoga in Major Depressive Disorders. Pharmacopsychiatry 2018, 51, 73–81. [Google Scholar] [CrossRef] [PubMed]
| Study | Sample Size (Female) | Group Age in Years (SD) | Group Description | Main Outcome Measures | Primary MRI Results | Association with Behavior Results |
|---|---|---|---|---|---|---|
| Froeliger, 2012a, 2012b, 2012c [31,32,33] | Yoga: 7 (6) CON: 7 (6) n = 14 (healthy adults) | Yoga: 36.4 (11.9) CON: 35.5 (7.1) | Yoga participants reported maintaining an active and ongoing modern Hatha yoga practice (>45-min per day, 3–4 times per week, >3 (9.4 ± 2.4) years, and had 5.6 ± 4.2 years of meditation. The age-, sex-, years-of-education-matched CG reported no current or past dedicated meditation or yoga practice. | Cognitive Failures Questionnaire, Affective Stroop task with fMRI, sMRI, R-fMRI | Compared to CG, the yoga group exhibited greater GMV in frontal (bilateral orbital-frontal, right middle frontal, and left precentral gyri), limbic (hippocampus, insula), temporal (left STG), occipital (right lingual gyrus), and cerebellar; had less Stroop BOLD response in left SFG; as well as exhibited greater rsFC within the DAN (ROI). | GMV in regions identified significant group differences were positively correlated with the duration of yoga practice and fewer cognitive failures. |
| Wei, 2013, 2014, 2017 [34,35,36], Chen, 2020 [37] | TCC: 22 (15) CON: 18 (10) n = 40 (healthy old adults) | TCC: 52.36 (6.88) CON: 54.83 (6.77) | TCC group had 14 ± 8 years of TCC experience and 11 ± 3 h per week (TCC styles mainly included Yang, Wu, Sun, and modified Chen). CG had no physical exercise, yoga, or meditation experience. Two groups matched for sex, age, race, and years of education. | ANT, sMRI, R-fMRI | Compared to CG, the TCC group showed significantly thicker cortex in precentral gyrus, insula sulcus, middle frontal sulcus (part of dlPFC) in the right hemisphere and STG, medial occipito-temporal and lingual sulcus in the left hemisphere; had greater ReHo in the right PosCG and less ReHo in left ACC and the right dlPFC; showed lower MFG VMHC; and revealed significantly decreased fALFF in the bilateral frontoparietal network, DMN, and dorsal prefrontal-angular gyri network. | Thicker cortex in left medial occipito-temporal and lingual sulcus was associated with greater intensity of TCC practice, and the reaction time of ANT was positively correlated with cortical thickness of left STG. Increased ReHo in PosCG was correlated with TCC experience, decreases in ReHo in left ACC and increases in ReHo in right PosCG both predicted performance gains on ANT. For TCC practitioners, the longer they practice, the lower their VMHC is in precentral and precuneus. Significant association between TCC practice experience and fALFF in the DMN, as well as an association between cognitive control performance and fALFF of the frontoparietal network. |
| Villemure, 2014, 2015 [38,39] | Yoga: 14 (9) CON: 14 (9) n = 28 (healthy adults) | Yoga: 37.0 (6.6) CON: 36.7 (7.3) | Yoga group had 9.6 ± 2.8 (range 6–16) years of regular yoga practice and 8.6 ± 4.1 h per week (included different yoga styles that integrated postures, breath, and meditation). CG was matched to yogis for sex, age, BMI, education, and exercise level other than yoga postures. | Cold pain tolerance task, sMRI | Compared to CG, the GMV in yogis increased in right cingulate, left dorsal mPFC and insula, and the CT in yogis increased in right insula, right cingulate cortex. | Years of yoga experience correlated with GMV in the insula, frontal operculum and OFC in the left hemisphere, and the number of hours of weekly practice correlated with GMV in SPL, PCC/precuneus, and hippocampus. Combination of postures and meditation contributed the most to the size of the hippocampus, precuneus/PCC, and S1/SPL, and combination of meditation and breathing exercises contributed the most to V1 volume. The insular GMV positively correlated with yoga experience and uniquely correlated with pain tolerance. |
| Gard, 2015 [40] | Yoga: 16 (69%)Meditators: 16 (63%) CON: 15 (60%) n = 47 (healthy adults) | Yoga: 49.38 (7.79) Meditators: 54.06 (8.15) CON: 52.93 (9.84) | Yoga practitioners were primarily trained in Kripalu yoga and had an average of 13,534 ± 9950 h of yoga experience. Meditators were primarily trained in Vipassana meditation and had a 7458 ± 5734 h of meditation experience. CON had less than 4 yoga or meditation classes in the last year and less than 10 classes in their lifetime. | R-fMRI | Compared to CG, yoga practitioners and meditators had greater widespread rsFC (increased degree centrality) of the caudate. | Not reported |
| Afonso, 2017 [41], Santaella, 2019 [42] | Yoga: 21 (21) CON: 21 (21) n = 42 (healthy old women) | Yoga: 66.2 (0.98) CON: 67.9 (1.004) | Yoga group practiced at least twice a week for a minimum of 8 years (yoga style was Hatha). CG was naive to yoga, meditation, or any mind–body intervention and were matched to the yoga group in age, years of formal education, and level of physical activity. | sMRI, R-fMRI | Compared to CG, yoga practitioners showed significantly greater CT in a left prefrontal lobe cluster, which included portions of the lateral MFG and anterior and dorsal SFG; and had a higher rsFC between mPFC (ROI) and the right AGr. | Not reported |
| Gothe, 2018 [43] | Yoga: 13 (12) CON: 13 (12) n = 26 (healthy adults) | Yoga: 35.77 (15.43) CON: 35.69 (14.57) | Yoga group had 9.31 ± 6.25 (range 5–24) years of yoga experience (yoga style was mainly Hatha). CG had no current or past experience with yoga or any other type of mind–body practice. No group differences in demographic measures of income and education and in estimated VO2max or physical activity levels. | sMRI, Sternberg Working Memory Task with fMRI | Compared to CG, yoga practitioners had greater GMV in the left hippocampus and revealed less activation in the dlPFC during the encoding phase of the Sternberg task. | Not reported |
| Liu, 2018 [44] | TCC: 26 (18) CON: 25 (16) n = 51 (healthy old adults) | TCC: 65.19 (2.30) CON: 63.92 (2.87) | TCC group had engaged in TCC for an average of 10.44 ± 5.48 years (TCC style was not reported). CG was active in other types of physical exercise without a meditation component, such as jogging and square dancing.Two groups matched for age, gender, years of education, and physical exercise per day. | Five-Facet Mindfulness Questionnaire, Sequential decision task, R-fMRI | Compared to CG, the TCC group showed a weaker rsFC between the dlPFC (ROI) and MFG. | FC between the dlPFC and the MFG in the TCC group fully mediated the impact of non-judgment of inner experience on their emotion regulation ability. |
| Port, 2018 [45] | TCC: 8 (5) WA: 8 (5) n = 16 (healthy old adults) | TCC: 66.4 (7.0) WA: 66.4 (4.9) | TCC (TCC style was not reported) or WA group matched by age, gender, and years of education, had at least 3 years experience and two times a week of practice in TCC or WA. | Stroop Word Color Task and N-back Task with fMRI | Compared to WA group, the TCC group had less brain activation in the right lateral occipital cortex during the Stroop Word Color Task and presented less brain activation in the right frontal pole and SFG during the N-back task. | Not reported |
| Mei, 2019 [46], Yue, 2020 [47] | TCC: 20 (20) WG: 22 (22) n = 42 (healthy old women) | TCC: 62.9 (2.38) WG: 63.27 (3.58) | The TCC group practiced Yang-style TCC 4 ± 1 times weekly for about 1.5 h each time for more than 6 years. WG mainly exercised by walking, not less than 5 times a week, with each time being no less than 1.5 h for more than 6 years. Two groups matched for age, handedness, and years of education. | 2-back task, R-fMRI | Compared to WG, The TCC group showed larger fALFF in left MFG; and had significant differences in DMN, SMN, and VN of R-fMRI. | The working memory was correlated with the fALFF of the left MFG in TCC group. |
| Study | Study Design | Sample Size (Female) | Group Age in Years (SD) | Intervention Frequency and Duration | Main Outcome Measures | Primary MRI Results | Association with Behavior Results |
|---|---|---|---|---|---|---|---|
| Hariprasad, 2013 [48] | Intervention without control | Yoga: 7 (3) n = 7 (healthy old adults) | Age range: 69–81 | Yoga: 60 min/day, 5 days/week (yoga style was mainly Hatha), lasted for 3 months + home practice for 3 months. | sMRI | Increased GMV in bilateral hippocampus (ROI) following yoga intervention. | Not reported |
| Li, 2014 [49], Yin, 2014 [50], Zheng, 2015 [51] | RCT | IG: 17 (8) CG: 17 (6) n = 34 (healthy old adults) | IG: 68.59 (5.65) CG: 71.65 (4.00) | IG: Cognitive intervention (MT and EFT): 60-min/session, 3 sessions/week, lasted for 6 weeks; TCC: 60-min/session, 3 sessions/week lasted for 6 weeks (Yang-style 24-form TCC); Group counseling: 90-min/session, 1 session/week, lasted for 6 weeks. CG: two 120-min health-related lectures on health and aging. | Cognitive function: PALT, digit span, TMT, Stroop Test, CFT Social support: SSRS Subjective well-being: SWLS, IWB, R-fMRI | For IG, after intervention, the ALFF was increased in the right MFG, left SFG, left ACL; the ReHo was increased in the left STG, left PCL, and decreased in the left MTG; and the mPFC-MTL (ROIs) FC increased dramatically. | The ALFF changes in the right MFG were positively correlated with changes in TMT and SWLS, and the baseline ALFF in the right MFG was negatively correlated with changes in TMT and SWLS. For IG, the ReHo changes in the left STG were positively correlated with changes in CFT, and changes in the right MTG were negatively correlated with changes in total PALT. For IG, changes for the mPFC-MTL FC were positively correlated with changes in CFT. |
| Tao, 2016, 2017a, 2017b, 2017c [52,53,54,55], Liu 2019 [56] | RCT | TCC: 21 (13) BDJ: 16 (10) CON: 25 (19) n = 62 (healthy old adults) | TCC: 62.38 (4.55) BDJ: 62.18 (3.79) CON: 59.76 (4.83) | TCC: 60 min/session, 5 sessions/week, lasted for 12 weeks (Yang-style 24-form), each session included warm-up and review of TCC principles (10 min), TCC exercises (30 min), breathing techniques (10 min), and relaxation (10 min). BDJ: 60 min/d, 5d/week, lasted for 12 weeks, the whole set of BDJ contains 10 postures, including the starting and ending postures; the time schedule was same as the TCC. CON: received basic health education at the beginning and kept original physical activity. | WMS-CR, sMRI, R-fMRI | Compared to CON, after 12nweeks of intervention, results showed both TCC and BDJ could significantly increase GMV in the insula, MTL/hippocampus, amygdala, and putamen, and no significant differences were observed between the two groups. TCC increased fALFF in the dlPFC and BDJ increased fALFF in the mPFC in the slow-5 and low-frequency bands; both TCC and BDJ (at a lower threshold) significantly increased the rsFC between the bilateral hippocampus (ROI) and mPFC (ROI) and no significant difference between the two groups was observed; TCC showed a significant decrease in rsFC between the dlPFC (ROI) and the left SFG, ACC, and insula/putamen (at a lower threshold); the BDJ showed a significant decrease in rsFC between the dlPFC(ROI) and the left putamen and insula and ACC (at a lower threshold), and there was no significant difference between the two groups. TCC increased rsFC between the PCC (ROI) and the right putamen/caudate, while BDJ decreased rsFC between the mPFC (ROI) and right orbital prefrontal gyrus/putamen and left ACC; compared to BDJ, TCC increased rsFC between the mPFC (ROI) and right putamen/caudate. | MQ and visual reproduction subscores were both associated with GMV increases in the putamen and hippocampus. Increased fALFF in both TCC and BDJ groups was positively associated with memory function improvement. The increases in rsFC between the bilateral hippocampus and mPFC were significantly associated with memory function improvement across all subjects. Mental control improvement was negatively associated with rsFC dlPFC-putamen changes across all subjects. Baseline visual reproduction subscore was negatively correlated with rsFC between the mPFC and right orbital prefrontal gyrus. |
| Wu, 2018 [57] | RCT | TCC: 16 (13) CON: 15 (15) n = 31 (healthy old adults) | TCC: 64.9 (2.8) CON: 64.9 (3.2) | TCC: 60 min/session, 3 sessions/week, lasted for 12 weeks (24-form Yang-style TCC), each session consisting of warm-up (10 min), new TCC form learning (10 min), continuous sequential practice of learned forms (30 min), and cool-down (10 min). CON: maintained original daily routines and physical activity, only received one telephone consultation biweekly. | IED test, task-switching behavioral measures with fMRI | TCC group had an increased trend in the left SFG (ROI: PFC) activation (contrast: switch > non-switch) after intervention. | TCC group with greater PFC activation increases in the switch condition presented greater reductions in task-switching errors from pre- to post-intervention. |
| Cui, 2019 [58] | RCT | TCC: 12 (10) AE: 12 (10) CON: 12 (10) n = 36 (healthy adults) | TCC: 21.83 (2.48) AE: 21.92 (2.28) CON: 21.75 (2.45) | TCC: 60 min/session, 3 sessions/week, lasted for 8 weeks (Bafa Wubu of TCC), each session including warm-up (5 min), continuous sequential practice (50 min), cool-down (5 min). AE: 60 min/session, 3 sessions/week, lasted for 8 weeks, brisk walking. CON: maintained original daily routines and physical activity habits and did not receive any new or additional exercise interventions. | sMRI, R-fMRI | Compared to CG, GMV in the TCC group was significantly increased in the left MOG, left precuneus, left STG, and right MTG, and compared with AE group, TCC group increased the GMV in left MOG, left STG, and right MTG; significant rsFC increases between the left MFG (ROI) and left SPL in the TCC group and no significant rsFC differences were observed in the AE and control groups. | Not reported |
| Garner, 2019 [59] | NRCT | Yoga: 39 (34) SG: 32 (31) PG: 31 (21) n = 102 (healthy adults) | First cohort: 22.7 (2.3) range: 18–29 Second cohort: 22.9 (4.4) range: 18–49 | Yoga: 75 min/session, 1 session/week, lasted for 10 weeks (yoga style was Hatha), each session including breathing exercise (10 min), IRT (2 min), loosening exercise (5 min), QRT (3 min), Surya Namascar (10 min), Asanas (25 min), DRT (10 min), and meditation (10 min). SG: 45–60 min/session, 1 session/week, lasted for 10 weeks, anaerobic fitness-oriented stretching and strengthening training program. PG: maintained daily habits. | sMRI | Increased right hippocampal GMD among yoga group | Not reported |
| A | B | C | D | E | F | G | H | OQ | |
|---|---|---|---|---|---|---|---|---|---|
| Froeliger, 2012a, 2012b, 2012c [31,32,33] | + | + | ? | + | + | + | + | + | Low risk |
| Wei, 2013, 2014, 2017 [34,35,36] Chen, 2020 [37] | + | + | ? | + | + | + | + | + | Low risk |
| Villemure, 2014, 2015 [38,39] | + | + | + | + | + | + | + | + | Low risk |
| Gard, 2015 [40] | + | ? | ? | + | + | + | + | + | Low risk |
| Afonso, 2017 [41], Santaella, 2019 [42] | + | + | ? | + | + | + | + | + | Low risk |
| Gothe, 2018 [43] | + | + | + | + | + | + | + | + | Low risk |
| Liu, 2018 [44] | + | + | ? | + | + | + | + | + | Low risk |
| Port, 2018 [45] | + | ? | ? | + | + | − | + | + | Moderate risk |
| Mei, 2019 [46], Yue, 2020 [47] | + | + | ? | + | + | + | + | + | Low risk |
| EC | RA | CA | SAB | SB | IB | AB | DR | ITA | BC | PM | MQ | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hariprasad, 2013 [48] | + | NA | NA | NA | + | NA | NA | + | + | NA | + | Fair |
| Li, 2014 [49], Yin, 2014 [50], Zheng, 2015 [51] | + | + | + | + | + | ? | + | − | + | + | + | Good |
| Tao, 2016, 2017a, 2017b, 2017c [52,53,54,55], Liu, 2019 [56] | + | + | + | + | + | ? | ? | − | + | + | + | Good |
| Wu 2018 [57] | + | + | + | + | + | ? | + | + | + | + | + | Excellent |
| Cui, 2019 [58] | + | + | + | + | + | ? | ? | + | + | + | + | Good |
| Garner, 2019 [59] | + | − | − | − | + | + | ? | + | + | + | + | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zong, B.; Zhao, W.; Li, L. Effects of Mind–Body Exercise on Brain Structure and Function: A Systematic Review on MRI Studies. Brain Sci. 2021, 11, 205. https://doi.org/10.3390/brainsci11020205
Zhang X, Zong B, Zhao W, Li L. Effects of Mind–Body Exercise on Brain Structure and Function: A Systematic Review on MRI Studies. Brain Sciences. 2021; 11(2):205. https://doi.org/10.3390/brainsci11020205
Chicago/Turabian StyleZhang, Xiaoyou, Boyi Zong, Wenrui Zhao, and Lin Li. 2021. "Effects of Mind–Body Exercise on Brain Structure and Function: A Systematic Review on MRI Studies" Brain Sciences 11, no. 2: 205. https://doi.org/10.3390/brainsci11020205
APA StyleZhang, X., Zong, B., Zhao, W., & Li, L. (2021). Effects of Mind–Body Exercise on Brain Structure and Function: A Systematic Review on MRI Studies. Brain Sciences, 11(2), 205. https://doi.org/10.3390/brainsci11020205






