Comprehensive Overview of Sleep Disorders in Patients with Chronic Liver Disease
Abstract
1. Introduction
2. Classification of SDs
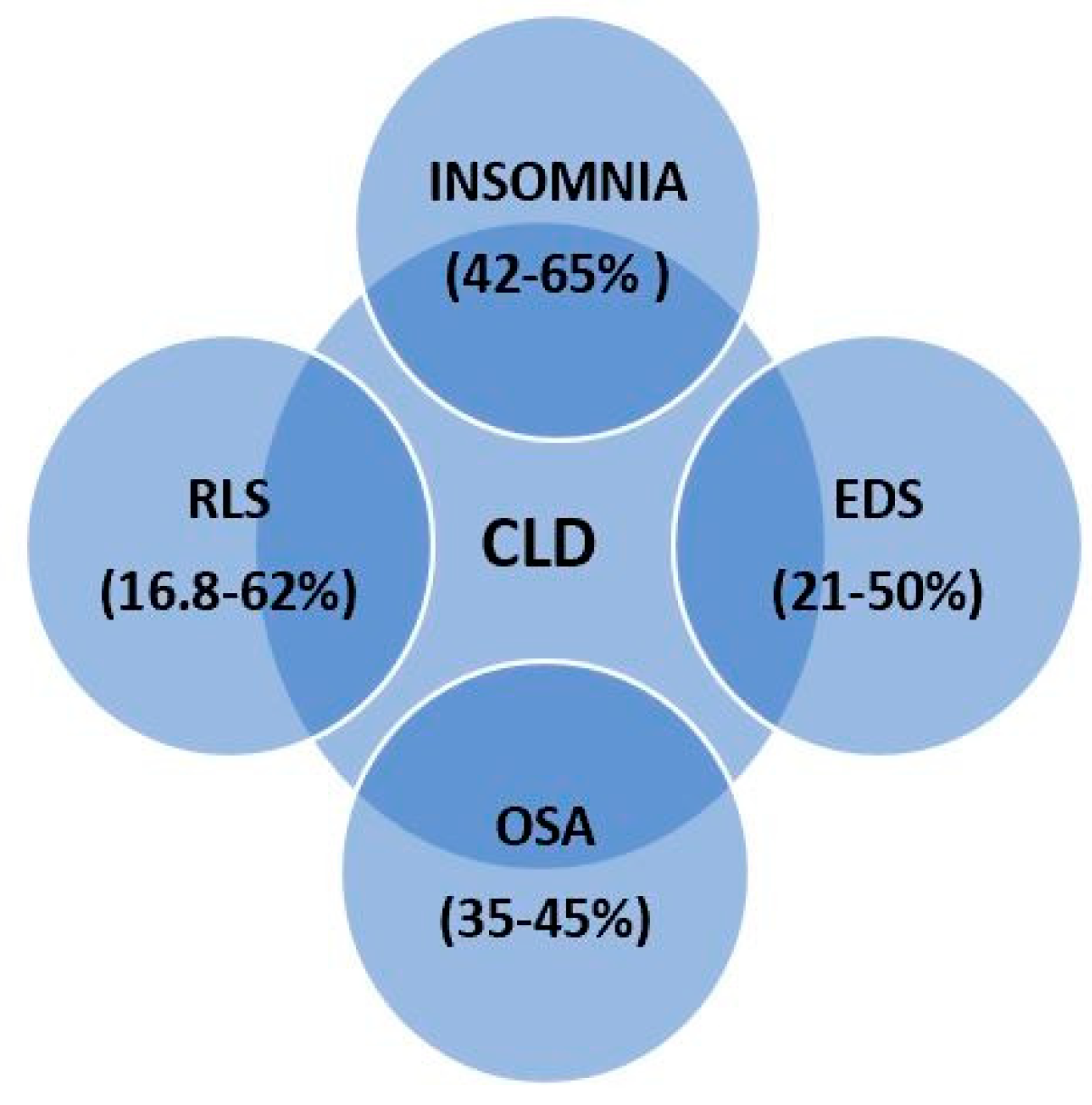
3. Epidemiology and Phenotypes of SDs in CLD
4. Discussion
4.1. Etiology of CLD
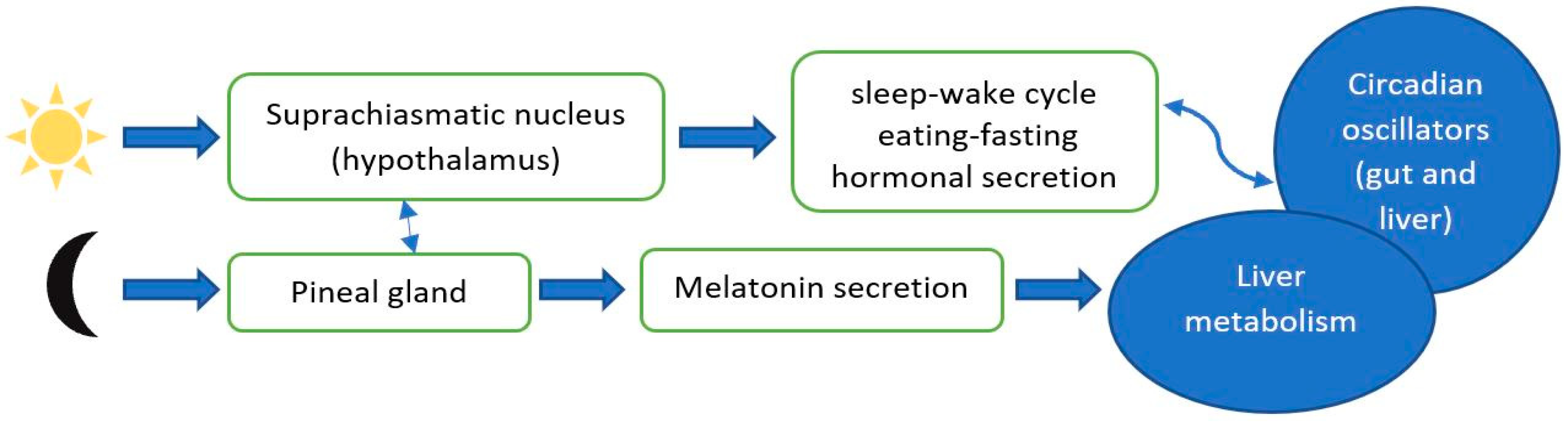
4.2. Circadian Clock Misalignment in CLD
4.3. SDs Attributed to the Etiology or Treatment of CLD
- −
- NAFLD and OSA are epidemiologically linked and pathophysiologically overlapped [69]. OSA produces chronic intermittent hypoxia which promotes systemic inflammation, oxidative stress, insulin resistance, and adipose tissue dysfunction with serum lipid peroxidation. OSA is responsible, irrespective of other comorbidities, for NAFLD development and progression to cirrhosis [70]. Moreover, a strong association between severe OSA and NAFLD has been reported [71]. The bidirectional relation between OSA and liver is supported by high levels of HIF-1α (hypoxia-inducing factor), a serum protein and crucial transcription factor responsible for oxygen metabolism homeostasis [72]. Chronic intermittent hypoxia, seen in patients with OSA, increases the levels of HIF-1α in organs like brain and liver, aggravating the progression of NAFLD. HIF-1α promotes liver fibrosis in NAFLD by activating phosphatase and tensin homolog (PTEN)/p65 signaling pathway, which may be targeted for therapy [73]. The main symptom of OSA is excessive daytime sleepiness (EDS), being also the reason for which patients seek medical advice. The mechanisms implied in appearance of EDS are related to the consequences of OSA such as chronic sleep deprivation, intermittent hypoxia, and oxidative injuries in wake-promoting brain regions [74]. Gabryelska et al. suggested in a recent study that patients with OSA are also at risk for developing clock disruption, a process which might be mediated by HIF-1α, since its increased level was associated with the overexpression of circadian clock proteins [75]. Previously to these findings, a group of researchers investigated the expression level of mRNA coding for clock genes. They reported that this level was altered in OSA patients compared to healthy controls and did not decrease after one month of continuous positive airway pressure (CPAP) treatment [76]. Consistent with the aforementioned results, Yang et al. showed that the transcripts of all the investigated circadian clock genes displayed daily oscillation patterns in peripheral blood of controls, while three of them were arrhythmic in patients with OSA [77]. Studies also demonstrated dysregulation of 24-h melatonin secretion in patients with OSA, explained by correlations between OSA severity and low urinary 6-HMS, with elevated serum levels of melatonin in the afternoon [78,79].
- −
- Chronic hepatitis C. Sleep disorders were estimated in 65% of patients with chronic hepatitis C, independent of the antiviral treatment and before advanced stages of the disease [80]. Several researchers presented evidence which suggests that hepatitis C virus may cause brain dysfunction, even in the absence of severe hepatic disease or other risk factors [81,82,83]. It is unclear whether cerebral effects are connected to the pathogenesis of sleep complaints, but an association between sleep quality and immunological and virological biomarkers has been recorded [84,85]. The treatment of hepatitis C with interferon α (IFNα) brings additional risk of developing sleep symptoms, associated with depression. The underlying mechanisms may be related to the changes induced by IFN in serotonin metabolism and elevations in interleukin-6 (IL-6) and interleukin-1 (IL-1), known as sleep modulation cytokines [86].
- −
- Autoimmune cholestatic liver disease, primary biliary cirrhosis in particular, manifests with sleep symptoms (EDS, RLS) which are strongly associated with fatigue and pruritus [87,88]. Disorders like OSA and RLS are the main causes for EDS and fatigue encountered among these patients. EDS in these patients is neither correlated with the severity of the disease, nor with the presence of HE [87,89]. A possible incriminated mechanism is the elevated level of IL-6 which is responsible for mediating other sleep regulating cytokines (IL-1 and tumor necrosis factor—TNF) [90]. Furthermore, it is worth mentioning that administration of IL-1, TNF or IFNα in the cerebral ventricle of rabbits has been proven to induce NREM sleep. Increases in TNF levels are associated with shorter duration of sleep, while longer sleep time is associated with high levels of C-reactive protein and IL-6. Sochal et al. showed that sleep quality is affected in patients with inflammatory bowel disease, confirming that inflammation can lead to sleep disturbances which vice versa may affect the immune system [91].
- −
- Wilson’s disease and sleep disorders. The scarce publications reported a very wide frequency interval of SDs among these patients: 42–80% [92,93]. The main SDs are insomnia and RLS. Patients with Wilson’s disease complain of frequent nocturnal awakenings, sleep fragmentations, delayed wakeups in the morning, and EDS. These sleep abnormalities are caused by nocturia, associated psychiatric and behavioral comorbidities (such as depression and anxiety) or treatment with various drugs (such as dopaminergic therapy in high doses). Trindade et al. [94] reported another SD among patients with Wilson’s disease. RLS was present in 31% of the patients, in the absence of well-known associated factors (iron deficiency, neuropathy, chronic kidney disease) [94]. RLS in Wilson’s disease might be caused by accumulation of copper in thalamus, impairments in dopaminergic transmission and iron metabolism [95].
- −
- OSA was reported as a new and underdiagnosed complication of cirrhosis with ascites for the first time in 2003 by Crespo et al. [96]. After excluding subclinical HE, researchers conducted a prospective study which included 24 patients with alcohol- and viral-induced cirrhosis and ascites. The results showed that OSA could be a complication of high-volume ascites caused mainly by mechanical factors: diaphragmatic elevation led to decreased residual volume and obstruction of upper airways. Interestingly, the removal of ascitic fluid caused remission of OSA [96]. These findings are in line with those of Ogata et al. [97], who performed a larger study on 48 cirrhotic patients. They reported strong correlations between apnea-hypopnea index (AHI), as an objective measure of OSA, and volume of ascites. AHI was significantly higher in severe cirrhosis [97].
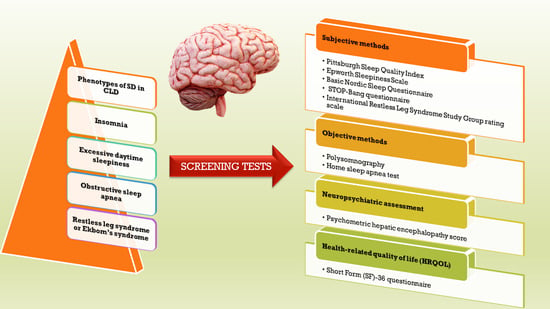
5. Assessment of Sleep Disorders in Patients with CLD
5.1. Sleep Assessment
5.1.1. Subjective Methods
- (a) Sleep Diaries
- (b) The Pittsburgh Sleep Quality Index (PSQI)
- (c) Sleep Timing and Sleep Quality Screening Questionnaire
- (d) The Epworth Sleepiness Scale (ESS)
- (e) The Basic Nordic Sleep Questionnaire (BNSQ)
- (f) STOP-Bang questionnaire
- (g) Berlin questionnaire (BQ)
- (h) The International Restless Leg Syndrome Study Group rating scale (IRLSS)
5.1.2. Objective Methods
- (a)
- Polysomnography (PSG) encompasses electroencephalogram, electrooculogram, electromyogram, and measurements of nasal and oral airflow. PSG is currently the “gold standard” diagnostic test for OSA and other sleep disorders, but may have limited access, being an expensive, time-consuming, in-laboratory test [1,38,122]. In 1972, Kurtz et al. opened the area of electroencephalography and neurophysiology in cirrhosis by investigating the EEG recordings of patients with different stages of encephalopathy [123]. Later, Teodoro and colleagues [115] showed that patients with cirrhosis experience an increased REM latency and reduced REM sleep. Recently, a group of researchers [117] assessed the prevalence of sleep-disordered breathing, OSA in particular, among cirrhotic patients of viral etiology. The evaluation was done through subjective tools and a full-night PSG sleep study. It resulted that cirrhotics had a significantly higher percentage of OSA (56.2%) compared to healthy controls (12.5%), while there were no significant differences between groups in terms of sleep efficiency and base SpO2 [117].
- (b)
- Home sleep apnea test (HSAT), also known as unattended sleep testing or portable monitoring, is an alternative less expensive and more convenient than polysomnography but has some disadvantages: it does not typically include electroencephalography, electrooculography, or electromyography sensors and might underestimate the severity of OSA [124]. Portable devices should measure peripheral arterial tonometry (PAT), oximetry, heart rate, snoring, wrist activity (actigraphy), and body position section [125].
- (c)
- Actigraphy is considered a semi-quantitative method which records the patient’s locomotor activity by means of an accelerometer. The recorded data are further analyzed via a software which estimates sleep parameters [1]. Actigraphs have been used together with sleep diaries and questionnaires in several studies in order to assess the prevalence of SDs in patients with CLD before and after therapeutic management [119,121,126].
5.2. Neuropsychiatric Assessment
5.3. Health-Related Quality of Life (HRQOL)
6. Management of Sleep Disorders in Patients with Chronic Liver Disease
6.1. Therapeutic Options for HE
6.2. Therapeutic Options for Sleep Disorders
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Formentin, C.; Garrido, M.; Montagnese, S. Assessment and Management of Sleep Disturbance in Cirrhosis. Curr. Hepatol. Rep. 2018, 17, 52–69. [Google Scholar] [CrossRef]
- Montagnese, S.; Middleton, B.; Skene, D.J.; Morgan, M.Y. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 2009, 29, 1372–1382. [Google Scholar] [CrossRef]
- Bueno, C.G.R.; Andrechuk, C.R.S.; Ceolim, M.F. Is Sleep Quality Impaired and Is There Increased Risk of Obstructive Sleep Apnea Syndrome in Patients with Compensated Liver Cirrhosis? Gastroenterol. Nurs. 2020, 43, 126–134. [Google Scholar] [CrossRef]
- Bruyneel, M.; Sersté, T. Sleep disturbances in patients with liver cirrhosis: Prevalence, impact, and management challenges. Nat. Sci Sleep. 2018, 10, 369–375. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Thacker, L.R.; Leszczyszyn, D.; Taylor, S.A.; Heuman, D.M.; Raman, S.; Sterling, R.K.; Siddiqui, M.S.; Stravitz, R.T.; Sanyal, A.J.; et al. Effects of obstructive sleep apnea on sleep quality, cognition, and driving performance in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2015, 13, 390–397.e1. [Google Scholar] [CrossRef]
- Abdullah, A.E.; Al-Jahdali, F.; Ahmed, A.E.; Shirbini, N.; Salim, B.; Ali, Y.Z.; Abdulrahman, A.; Khan, M.; Khaleid, A.; Hamdan, A.-J. Symptoms of Daytime Sleepiness and Sleep Apnea in Liver Cirrhosis Patients. Ann. Hepatol. 2017, 16, 591–598. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep. 2017, 19, 151–161. [Google Scholar] [CrossRef]
- Darien, I.L. American Academy of Sleep Medicine. In International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014. [Google Scholar]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Drake, C.L.; Harvey, A.G.; Krystal, A.D.; Manber, R.; Riemann, D.; Spiegelhalder, K. Insomnia disorder. Nat. Rev. Dis Primers. 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed]
- Foldvary-Schaefer, N.R.; Waters, T.E. Sleep-Disordered Breathing. Sleep Neurol. 2017, 23, 1093–1116. [Google Scholar] [CrossRef] [PubMed]
- Mohammadieh, A.; Sutherland, K.; Cistulli, P.A. Sleep disordered breathing: Management update. Intern. Med. J. 2017, 47, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Culnan, E.; McCullough, L.M.; Wyatt, J.K. Circadian Rhythm Sleep-Wake Phase Disorders. Neurol. Clin. 2019, 37, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalder, K.; Nissen, C.; Riemann, D. Clinical Sleep-Wake Disorders II: Focus on Insomnia and Circadian Rhythm Sleep Disorders. Handb. Exp. Pharmacol. 2019, 253, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Bollu, P.C.; Goyal, M.K.; Thakkar, M.M.; Sahota, P. Sleep Medicine: Parasomnias. Mol. Med. 2018, 115, 169–175. [Google Scholar]
- Trotti, L.M. Restless Legs Syndrome and Sleep-Related Movement Disorders. Sleep Neurol. 2017, 23, 1005–1016. [Google Scholar] [CrossRef]
- Khan, Z.; Trotti, L.M. Central Disorders of Hypersomnolence: Focus on the Narcolepsies and Idiopathic Hypersomnia. Chest 2015, 148, 262–273. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Barateau, L. Narcolepsy and Other Central Hypersomnias. Sleep Neurol. 2017, 23, 989–1004. [Google Scholar] [CrossRef]
- Rains, J.C. Sleep and Migraine: Assessment and Treatment of Comorbid Sleep Disorders. Headache. 2018, 58, 1074–1091. [Google Scholar] [CrossRef]
- Grandner, M.A. Sleep, Health, and Society. Sleep Med. Clin. 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Grandner, M.A. Addressing sleep disturbances: An opportunity to prevent cardiometabolic disease? Int. Rev. Psychiatry 2014, 26, 155–176. [Google Scholar] [CrossRef]
- Van Ryswyk, E.; Mukherjee, S.; Chai-Coetzer, C.L.; Vakulin, A.; McEvoy, R.D. Sleep Disorders, Including Sleep Apnea and Hypertension. Am. J. Hypertens. 2018, 31, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Yuan, Q.; Wang, G.; Tang, F. Association between sleep quality and metabolic syndrome: A systematic review and meta-analysis. Psychiatry Res. 2019, 274, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Simonelli, G.; Marshall, N.S.; Grillakis, A.; Miller, C.B.; Hoyos, C.M.; Glozier, N. Sleep health epidemiology in low and middle-income countries: A systematic review and meta-analysis of the prevalence of poor sleep quality and sleep duration. Sleep Health 2018, 4, 239–250. [Google Scholar] [CrossRef]
- Mathias, J.L.; Cant, M.L.; Burke, A.L.J. Sleep disturbances and sleep disorders in adults living with chronic pain: A meta-analysis. Sleep Med. 2018, 52, 198–210. [Google Scholar] [CrossRef]
- Córdoba, J.; Cabrera, J.; Lataif, L.; Penev, P.; Zee, P.; Blei, A.T. High prevalence of sleep disturbance in cirrhosis. Hepatology 1998, 27, 339–345. [Google Scholar] [CrossRef]
- Bianchi, G.; Marchesini, G.; Nicolino, F.; Graziani, R.; Sgarbi, D.; Loguercio, C.; Abbiati, R.; Zoli, M. Psychological status and depression in patients with liver cirrhosis. Dig. Liver Dis. 2005, 37, 593–600. [Google Scholar] [CrossRef]
- Ghabril, M.; Jackson, M.; Gotur, R.; Weber, R.; Orman, E.; Vuppalanchi, R.; Chalasani, N. Most Individuals with Advanced Cirrhosis Have Sleep Disturbances, which are Associated with Poor Quality of Life. Clin. Gastroenterol. Hepatol. 2017, 15, 1271–1278.e6. [Google Scholar] [CrossRef]
- Sherlock, S.; Summerskill, W.H.; White, L.P.; Phear, E.A. Portal-systemic encephalopathy; neurological complications of liver disease. Lancet 1954, 267, 454–457. [Google Scholar] [CrossRef]
- Mostacci, B.; Ferlisi, M.; Baldi Antognini, A.; Sama, C.; Morelli, C.; Mondini, S.; Cirignotta, F. Sleep disturbance and daytime sleepiness in patients with cirrhosis: A case control study. Neurol. Sci. 2008, 29, 237–240. [Google Scholar] [CrossRef]
- Iwasa, M.; Karino, Y.; Kawaguchi, T.; Nakanishi, H.; Miyaaki, H.; Shiraki, M.; Nakajima, T.; Sawada, Y.; Yoshiji, H.; Okita, K.; et al. Relationship of muscle cramps to quality of life and sleep disturbance in patients with chronic liver diseases: A nationwide study. Liver Int. 2018, 38, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; De Pittà, C.; De Rui, M.; Corrias, M.; Turco, M.; Merkel, C.; Amodio, P.; Costa, R.; Skene, D.J.; Gatta, A. Sleep-wake abnormalities in patients with cirrhosis. Hepatology 2014, 59, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Samanta, J.; Dhiman, R.K.; Khatri, A.; Thumburu, K.K.; Grover, S.; Duseja, A.; Chawla, Y. Correlation between degree and quality of sleep disturbance and the level of neuropsychiatric impairment in patients with liver cirrhosis. Metab. Brain Dis. 2013, 28, 249–259. [Google Scholar] [CrossRef] [PubMed]
- AL-Jahdali, H.; Al Enezi, A.; Anwar, A.E.; AL-Harbi, A.; Baharoon, S.; Aljumah, A.; Shimemeri, A.; Abudallah, K. Prevalence of insomnia and sleep patterns among liver cirrhosis patients. J. Circadian Rhythms. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- AL-Jahdali, H.; Al Enezi, A.; BaHammam, A.; Aljumah, A.; Baharoon, S.; Abdo, A. Patients With Liver Cirrhosis Are At High Risk Of Obstructive Sleep Apnea And Excessive Daytime Sleepiness. C72. Sleep and sleep disordered breathing in other medical disorders. Am. J. Respirat. Crit. Care Med. 2012, 185, A5027. [Google Scholar] [CrossRef]
- De Rui, M.; Schiff, S.; Aprile, D.; Angeli, P.; Bombonato, G.; Bolognesi, M.; Sacerdoti, D.; Gatta, A.; Merkel, C.; Amodio, P.; et al. Excessive daytime sleepiness and hepatic encephalopathy: It is worth asking. Metab. Brain Dis. 2013, 28, 245–248. [Google Scholar] [CrossRef]
- Parikh, M.P.; Gupta, N.M.; McCullough, A.J. Obstructive Sleep Apnea and the Liver. Clin. Liver Dis. 2019, 23, 363–382. [Google Scholar] [CrossRef]
- Marin-Alejandre, B.A.; Abete, I.; Cantero, I.; Riezu-Boj, J.I.; Milagro, F.I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Quiroga, J.; et al. Association between Sleep Disturbances and Liver Status in Obese Subjects with Nonalcoholic Fatty Liver Disease: A Comparison with Healthy Controls. Nutrients 2019, 11, 322. [Google Scholar] [CrossRef]
- Chou, T.C.; Liang, W.M.; Wang, C.B.; Wu, T.N.; Hang, L.W. Obstructive sleep apnea is associated with liver disease: A population-based cohort study. Sleep Med. 2015, 16, 955–960. [Google Scholar] [CrossRef]
- Klingelhoefer, L.; Bhattacharya, K.; Reichmann, H. Restless legs syndrome. Clin. Med. 2016, 16, 379–382. [Google Scholar] [CrossRef]
- Franco, R.A.; Ashwathnarayan, R.; Deshpandee, A.; Knox, J.; Daniel, J.; Eastwood, D.; Franco, J.; Saeian, K. The high prevalence of restless legs syndrome symptoms in liver disease in an academic-based hepatology practice. J. Clin. Sleep Med. 2008, 4, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Ichikawa, T.; Kondo, H.; Taura, N.; Miyaaki, H.; Isomoto, H.; Takeshima, F.; Nakao, K. Prevalence of restless legs syndrome in Japanese patients with chronic liver disease. Hepatol. Res. 2012, 42, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Rajender, A.; Mathur, S.; Choudhary, P.; Upadhyay, S.; Rajender, G.; Bhargava, R.; Nepalia, S. Restless leg syndrome a common undiagnosed comorbidity of clinical significance in cirrhosis. Gastroenterol. Hepatol. Bed Bench. 2019, 12, 13–16. [Google Scholar] [PubMed]
- Halkurike-Jayadevappa, V.K.; Goel, A.; Paliwal, V.K.; Rai, P.; Aggarwal, R. Liver disease severity is poorly related to the presence of restless leg syndrome in patients with cirrhosis. Neurol. India 2019, 67, 732–737. [Google Scholar] [CrossRef]
- Swain, M.G.; Jones, D.E.J. Fatigue in chronic liver disease: New insights and therapeutic approaches. Liver Int. 2019, 39, 6–19. [Google Scholar] [CrossRef]
- Carskadon, M.A.; Dement, W.C. Normal Human Sleep: An Overview. In Principles and Practice of Sleep Medicine, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 2; Volume 2, pp. 15–24. ISBN 9780323242882. [Google Scholar]
- Tahara, Y.; Shibata, S. Circadian rhythms of liver physiology and disease: Experimental and clinical evidence. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 217–226. [Google Scholar] [CrossRef]
- Montagnese, S.; Middleton, B.; Mani, A.R.; Skene, D.J.; Morgan, M.Y. On the origin and the consequences of circadian abnormalities in patients with cirrhosis. Am. J. Gastroenterol. 2010, 105, 1773–1781. [Google Scholar] [CrossRef]
- Szmyd, B.; Rogut, M.; Białasiewicz, P.; Gabryelska, A. The impact of glucocorticoids and statins on sleep quality. Sleep Med. Rev. 2020, 55, 101380. [Google Scholar] [CrossRef]
- Chojnacki, C.; Wachowska-Kelly, P.; Błasiak, J.; Reiter, R.J.; Chojnacki, J. Melatonin secretion and metabolism in patients with hepatic encephalopathy. J. Gastroenterol. Hepatol. 2013, 28, 342–347. [Google Scholar] [CrossRef]
- Velissaris, D.; Karanikolas, M.; Kalogeropoulos, A.; Solomou, E.; Polychronopoulos, P.; Thomopoulos, K.; Labropoulou-Karatza, C. Pituitary hormone circadian rhythm alterations in cirrhosis patients with subclinical hepatic encephalopathy. World J. Gastroenterol. 2008, 14, 4190–4195. [Google Scholar] [CrossRef]
- Blei, A.T.; Zee, P. Abnormalities of circadian rhythmicity in liver disease. J. Hepatol. 1998, 29, 832–835. [Google Scholar] [CrossRef]
- Steindl, P.E.; Finn, B.; Bendok, B.; Rothke, S.; Zee, P.; Blei, A.T. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann. Intern. Med. 1995, 123, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, C.; Romanowski, M.; Winczyk, K.; Błasiak, J.; Chojnacki, J. Melatonin levels in serum and ascitic fluid of patients with hepatic encephalopathy. Gastroenterol. Res. Pract. 2012, 2012, 510764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Birkeland, A.J. Plasma melatonin levels and nocturnal transitions between sleep and wakefulness. Neuroendocrinology 1982, 34, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Brun, J.; Garry, P.; Roussel, B.; Sassolas, G. A once-repeated study of nocturnal plasma melatonin patterns and sleep recordings in six normal young men. J. Pineal Res. 1986, 3, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Zee, P.C.; Mehta, R.; Turek, F.W.; Blei, A.T. Portacaval anastomosis disrupts circadian locomotor activity and pineal melatonin rhythms in rats. Brain Res. 1991, 560, 17–22. [Google Scholar] [CrossRef]
- Llansola, M.; Cantero, J.L.; Hita-Yañez, E.; Mirones-Maldonado, M.J.; Piedrafita, B.; Ahabrach, H.; Errami, M.; Agusti, A.; Felipo, V. Progressive reduction of sleep time and quality in rats with hepatic encephalopathy caused by portacaval shunts. Neuroscience 2012, 201, 199–208. [Google Scholar] [CrossRef]
- Ahabrach, H.; Piedrafita, B.; Ayad, A.; El Mlili, N.; Errami, M.; Felipo, V.; Llansola, M. Chronic hyperammonemia alters the circadian rhythms of corticosteroid hormone levels and of motor activity in rats. J. Neurosci. Res. 2010, 88, 1605–1614. [Google Scholar] [CrossRef]
- Butterworth, R.F.; Norenberg, M.D.; Felipo, V.; Ferenci, P.; Albrecht, J.; Blei, A.T. Members of the ISHEN Commission on Experimental Models of HE. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009, 29, 783–788. [Google Scholar] [CrossRef]
- Bersagliere, A.; Raduazzo, I.D.; Nardi, M.; Schiff, S.; Gatta, A.; Amodio, P.; Achermann, P.; Montagnese, S. Induced hyperammonemia may compromise the ability to generate restful sleep in patients with cirrhosis. Hepatology 2012, 55, 869–878. [Google Scholar] [CrossRef]
- Marini, S.; Santangeli, O.; Saarelainen, P.; Middleton, B.; Chowdhury, N.; Skene, D.; Costa, R.; Porkka-Heiskanen, T.; Montagnese, S. Abnormalities in the Polysomnographic, Adenosine and Metabolic Response to Sleep Deprivation in an Animal Model of Hyperammonemia. Front. Physiol. 2017, 8, 636. [Google Scholar] [CrossRef] [PubMed]
- Boy, C.; Meyer, P.T.; Kircheis, G.; Holschbach, M.H.; Herzog, H.; Elmenhorst, D.; Kaiser, H.J.; Coenen, H.H.; Haussinger, D.; Zilles, K.; et al. Cerebral A1 adenosine receptors (A1AR) in liver cirrhosis. Eur. J. Nucl. Med. Mol. Imag. 2008, 35, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Krauchi, K.; Deboer, T. The interrelationship between sleep regulation and thermoregulation. Front. Biosci. 2010, 15, 604–625, (Landmark Ed). [Google Scholar] [CrossRef] [PubMed]
- Raymann, R.J.; Swaab, D.F.; Van Someren, E.J. Cutaneous warming promotes sleep onset. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1589–R1597. [Google Scholar] [CrossRef]
- Garrido, M.; Saccardo, D.; De Rui, M.; Vettore, E.; Verardo, A.; Carraro, P.; Di Vito Francesco, N.; Mani, A.R.; Angeli, P.; Bolognesi, M.; et al. Abnormalities in the 24-hour rhythm of skin temperature in cirrhosis: Sleep-wake and general clinical implications. Liver Int. 2017, 37, 1833–1842. [Google Scholar] [CrossRef]
- Bolognesi, M.; Di Pascoli, M.; Verardo, A.; Gatta, A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J. Gastroenterol. 2014, 14, 2555–2563. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Olivetti, C.; Rosina, F.; Carbone, G.; Gambino, R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes. Rev. 2013, 14, 417–431. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am. J. Respir Crit Care Med. 2019, 199, 830–841. [Google Scholar] [CrossRef]
- Labarca, G.; Horta, G. Obstructive sleep apnea and nonalcoholic fatty liver disease: Do we need to consider this association in current clinical practice? Sleep Med. 2020, 20, S1389–S9457. [Google Scholar] [CrossRef]
- Gabryelska, A.; Szmyd, B.; Szemraj, J.; Stawski, R.; Sochal, M.; Białasiewicz, P. Patients with obstructive sleep apnea present with chronic up-regulation of serum HIF-1α protein. J. Clin. Sleep Med. 2020, 16, 1761–1768. [Google Scholar] [CrossRef]
- Han, J.; He, Y.; Zhao, H.; Xu, X. Hypoxia inducible factor-1 promotes liver fibrosis in nonalcoholic fatty liver disease by activating PTEN/p65 signaling pathway. J. Cell Biochem. 2019, 120, 14735–14744. [Google Scholar] [CrossRef]
- Javaheri, S. Update on Persistent Excessive Daytime Sleepiness in OSA. Chest 2020, 158, 776–786. [Google Scholar] [CrossRef]
- Gabryelska, A.; Sochal, M.; Turkiewicz, S.; Białasiewicz, P. Relationship between HIF-1 and Circadian Clock Proteins in Obstructive Sleep Apnea Patients-Preliminary Study. J. Clin. Med. 2020, 9, 1599. [Google Scholar] [CrossRef]
- Moreira, S.; Rodrigues, R.; Barros, A.B.; Pejanovic, N.; Neves-Costa, A.; Pedroso, D.; Pereira, C.; Fernandes, D.; Rodrigues, J.V.; Barbara, C.; et al. Changes in Expression of the CLOCK Gene in Obstructive Sleep Apnea Syndrome Patients Are Not Reverted by Continuous Positive Airway Pressure Treatment. Front. Med. (Lausanne) 2017, 4, 187. [Google Scholar] [CrossRef]
- Yang, M.Y.; Lin, P.W.; Lin, H.C.; Lin, P.M.; Chen, I.Y.; Friedman, M.; Hung, C.F.; Salapatas, A.M.; Lin, M.C.; Lin, S.F. Alternations of Circadian Clock Genes Expression and Oscillation in Obstructive Sleep Apnea. J. Clin. Med. 2019, 8, 1634. [Google Scholar] [CrossRef]
- Reutrakul, S.; Siwasaranond, N.; Nimitphong, H.; Saetung, S.; Chirakalwasan, N.; Chailurkit, L.-O.; Srijaruskul, K.; Ongphiphadhanakul, B.; Thakkinstian, A. Associations between nocturnal urinary 6-sulfatoxymelatonin, obstructive sleep apnea severity and glycemic control in type 2 diabetes. Chronobiol. Int. 2017, 34, 382–392. [Google Scholar] [CrossRef]
- Ulfberg, J.; Micic, S.; Strøm, J. Afternoon serum-melatonin in sleep disordered breathing. J. Intern. Med. 1998, 244, 163–168. [Google Scholar] [CrossRef]
- Carlson, M.D.; Hilsabeck, R.C.; Barakat, F.; Perry, W. Role of Sleep Disturbance in Chronic Hepatitis C Infection. Curr. Hepat. Rep. 2010, 9, 25–29. [Google Scholar] [CrossRef]
- Forton, D.M.; Taylor-Robinson, S.D.; Thomas, H.C. Central nervous system changes in hepatitis C virus infection. Eur. J. Gastroenterol. Hepatol. 2006, 18, 333–338. [Google Scholar] [CrossRef]
- Weissenborn, K.; Krause, J.; Bokemeyer, M.; Hecker, H.; Schüler, A.; Ennen, J.C.; Ahl, B.; Manns, M.P.; Böker, K.W. Hepatitis C virus infection affects the brain—Evidence from psychometric studies and magnetic resonance spectroscopy. J. Hepatol. 2004, 41, 845–851. [Google Scholar] [CrossRef]
- McAndrews, M.P.; Farcnik, K.; Carlen, P.; Damyanovich, A.; Mrkonjic, M.; Jones, S.; Heathcote, E.J. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology 2005, 41, 801–808. [Google Scholar] [CrossRef]
- Wilkinson, J.; Radkowski, M.; Eschbacher, J.M.; Laskus, T. Activation of brain macrophages/microglia cells in hepatitis C infection. Gut 2010, 59, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.M.O.; De Lima, T.A.; Braga, J.U.; Braga, W.D.S.; Peruhype-Magalhães, V.; Teixeira-Carvalho, A.; Martins-Filho, O.A.; Torres, K.L.; Malheiro, A. Immunological/virological peripheral bloodbiomarkers and distinct patterns of sleeping quality in chronic hepatitis C patients. Scand. J. Immunol. 2011, 73, 486–495. [Google Scholar] [CrossRef]
- Sockalingam, S.; Abbey, S.E.; Alosaimi, F.; Novak, M. A review of sleep disturbance in hepatitis C. J. Clin. Gastroenterol. 2010, 44, 38–45. [Google Scholar] [CrossRef]
- Newton, J.L.; Gibson, G.J.; Tomlinson, M.; Wilton, K.; Jones, D. Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology 2006, 44, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, S.; Nsemi, L.M.; Cazzagon, N.; Facchini, S.; Costa, L.; Bergasa, N.V.; Amodio, P.; Floreani, A. Sleep-Wake profiles in patients with primary biliary cirrhosis. Liver Int. 2013, 33, 203–209. [Google Scholar] [CrossRef]
- Anderson, K.; Jones, D.E.; Wilton, K.; Newton, J.L. Restless leg syndrome is a treatable cause of sleep disturbance and fatigue in primary biliary cirrhosis. Liver Int. 2013, 33, 239–243. [Google Scholar] [CrossRef]
- De Cruz, S.; Espiritu, J.; Zeidler, M.; Wang, T. Sleep Disorders in Chronic Liver Disease. Semin. Respirat. Crit. Care Med. 2012, 33, 26–35. [Google Scholar] [CrossRef]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Talar-Wojnarowska, R.; Szmyd, B.; Krzywdzińska, M.; Białasiewicz, P. Determinants of Sleep Quality in Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 2921. [Google Scholar] [CrossRef]
- Portala, K.; Westermark, K.; Ekselius, L.; Broman, J.-E. Sleep in patients with treated Wilson’s disease. A questionnaire study. Nord. J. Psychiatry 2002, 56, 291–297. [Google Scholar] [CrossRef]
- Nevsimalova, S.; Bušková, J.; Bruha, R.; Kemlink, D.; Sonka, K.; Vítek, L.; Marecek, Z. Sleep disorders in Wilson’s disease. Eur. J. Neurol. 2011, 18, 184–190. [Google Scholar] [CrossRef]
- Trindade, M.C.; Bittencourt, T.; Lorenzi-Filho, G.; Alves, R.C.; de Andrade, D.C.; Fonoff, E.T.; Bor-Seng-Shu, E.; Machado, A.A.; Teixeira, M.J.; Barbosa, E.R.; et al. Restless legs syndrome in Wilson’s disease: Frequency, characteristics, and mimics. Acta Neurol. Scand. 2017, 135, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Earley, C.J.; Connor, J.; Garcia-Borreguero, D.; Jenner, P.; Winkelman, J.; Zee, P.C.; Allen, R. Altered brain iron homeostasis and dopaminergic function in restless legs syndrome (Willis-Ekbom Disease). Sleep Med. 2014, 15, 1288–1301. [Google Scholar] [CrossRef]
- Crespo, J.; Cifrián, J.; Pinto, J.A.; Jiménez-Gómez, A.; Pons-Romero, F. Sleep apnea obstructive syndrome: A new complication previously undescribed in cirrhotic patients with ascites. Am. J. Gastroenterol. 2003, 98, 2815–2816. [Google Scholar] [CrossRef]
- Ogata, T.; Nomura, M.; Nakaya, Y.; Ito, S. Evaluation of episodes of sleep apnea in patients with liver cirrhosis. J. Med. Invest. 2006, 53, 159–166. [Google Scholar] [CrossRef]
- Elaghori, A.; Salem, P.E.S.; Azzam, E.; Abu Elfotoh, N. Ghrelin Level In Patients With Liver Cirrhosis. Acta Endocrinol. 2019, 5, 62–68. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Saeian, K.; Schubert, C.M.; Franco, R.; Franco, J.; Heuman, D.M. Disruption of sleep architecture in minimal hepatic encephalopathy and ghrelin secretion. Aliment. Pharmacol. Ther. 2011, 34, 103–105. [Google Scholar] [CrossRef]
- Carney, C.E.; Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Krystal, A.D.; Lichstein, K.L.; Morin, C.M. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep 2012, 35, 287–302. [Google Scholar] [CrossRef]
- Heeren, M.; Sojref, F.; Schuppner, R.; Worthmann, H.; Pflugrad, H.; Tryc, A.B.; Pasedag, T.; Weissenborn, K. Active at night, sleepy all day--sleep disturbances in patients with hepatitis C virus infection. J. Hepatol. 2014, 60, 732–740. [Google Scholar] [CrossRef]
- Montagnese, S.; Middleton, B.; Skene, D.J.; Morgan, M.Y. Sleep-wake patterns in patients with cirrhosis: All you need to know on a single sheet. A simple sleep questionnaire for clinical use. J. Hepatol. 2009, 51, 690–695. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Gencdal, G.; Gunsar, F.; Meral, C.E.; Salman, E.; Gürsel, B.; Oruc, N.; Karasu, Z.; Ersoz, G.; Akarca, U.S. Sleep disorders in cirrhotics; how can we detect? Liver Int. 2014, 34, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Yoshimura, E.; Ichikawa, T.; Miyaaki, H.; Taura, N.; Miuma, S.; Shibata, H.; Honda, T.; Takeshima, F.; Nakao, K. Screening for minimal hepatic encephalopathy in patients with cirrhosis by cirrhosis-related symptoms and a history of overt hepatic encephalopathy. Biomed. Rep. 2016, 5, 193–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marques, D.M.; Teixeira, H.R.; Lopes, A.R.; Martins-Pedersoli, T.A.; Ziviani, L.C.; Mente, Ê.D.; Castro-e-Silva, O.; Galvão, C.M.; Mendes, K.S. Sleep Quality Assessment and Daytime Sleepiness of Liver Transplantation Candidates. Transplant. Proc. 2016, 48, 2356–2360. [Google Scholar] [CrossRef]
- Partinen, M.; Gislason, T. Basic Nordic Sleep Questionnaire (BNSQ): A quantitated measure of subjective sleep complaints. J. Sleep Res. 1995, 4, 150–155. [Google Scholar] [CrossRef]
- Bhat, M.; Wyse, J.M.; Moodie, E.; Ghali, P.; Hilzenrat, N.; Wong, P.; Deschenes, M. Prevalence and predictors of sleep disturbance among liver diseases in long-term transplant survivors. Can. J. Gastroenterol. Hepatol. 2015, 29, 440–444. [Google Scholar] [CrossRef][Green Version]
- Velissaris, D.; Karamouzos, V.; Polychronopoulos, P.; Karanikolas, M. Chronotypology and melatonin alterations in minimal hepatic encephalopathy. J. Circadian Rhythms. 2009, 7, 6. [Google Scholar] [CrossRef]
- Nagappa, M.; Liao, P.; Wong, J.; Auckley, D.; Ramachandran, S.K.; Memtsoudis, S.G.; Mokhlesi, B.; Chung, F. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS ONE. 2015, 10, e0143697. [Google Scholar] [CrossRef]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Chen, P.Y.; Chuang, L.P.; Chen, N.H.; Tu, Y.K.; Hsieh, Y.J.; Wang, Y.C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Pollock, R.; Uhanova, J.; Kryger, M.; Hawkins, K.; Minuk, G.Y. Symptoms of obstructive sleep apnea in patients with nonalcoholic fatty liver disease. Dig. Dis Sci. 2005, 50, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, W.R.; Júnior, M.A.B.; Lucchesi, L.M.; Cavignolli, D.; De Mello, M.T.; Kondo, M.; Tufik, S. Polysomnographic sleep aspects in liver cirrhosis: A case control study. World J. Gastroenterol. 2013, 19, 3433–3438. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Nishikawa, H.; Enomoto, H.; Iwata, Y.; Kishino, K.; Shimono, Y.; Hasegawa, K.; Nakano, C.; Takata, R.; Nishimura, T.; et al. Comparison of sleep disorders in chronic hepatitis C patients treated with interferon-based therapy and direct acting antivirals using actigraphy. Hepatol Res. 2016, 46, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Elgammal, N.; Zaher, T.; Elkomy, H.; Abdelmoaty, A.; Abdallah, M.; Emara, M. How frequent is sleep-disordered breathing among Egyptian cirrhotic adults? Clin. Exp. Hepatol. 2020, 6, 150–157. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, B.C.; Puri, V.; Sachdeva, S.; Srivastava, S. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab. Brain Dis. 2017, 32, 595–605. [Google Scholar] [CrossRef]
- Bruyneel, M.; Sersté, T.; Libert, W.; van den Broecke, S.; Ameye, L.; Dachy, B.; Mulkay, J.P.; Moreno, C.; Gustot, T. Improvement of sleep architecture parameters in cirrhotic patients with recurrent hepatic encephalopathy with the use of rifaximin. Eur. J. Gastroenterol. Hepatol. 2017, 29, 302–308. [Google Scholar] [CrossRef]
- Montagnese, S.; Middleton, B.; Mani, A.R.; Skene, D.J.; Morgan, M.Y. Sleep and circadian abnormalities in patients with cirrhosis: Features of delayed sleep phase syndrome? Metab. Brain Dis. 2009, 24, 427–439. [Google Scholar] [CrossRef]
- Spahr, L.; Coeytaux, A.; Giostra, E.; Hadengue, A.; Annoni, J.M. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: A randomized controlled pilot trial. Am. J. Gastroenterol. 2007, 102, 744–753. [Google Scholar] [CrossRef]
- Rosen, I.M.; Kirsch, D.B.; Chervin, R.D.; Carden, K.A.; Ramar, K.; Aurora, R.N.; Kristo, D.A.; Malhotra, R.K.; Martin, J.L.; Olson, E.J.; et al. Clinical use of a home sleep apnea test: An American Academy of Sleep Medicine Position Statement. J. Clin. Sleep Med. 2017, 13, 1205–1207. [Google Scholar] [CrossRef]
- Kurtz, D.; Zenglein, J.; Imler, M.; Girardel, M.; Grinspan, G.; Peter, B.; Rohmer, F. Night sleep in porto-caval encephalopathy. Electroencephalogr. Clin. Neurophysiol. 1972, 33, 167–178. [Google Scholar] [CrossRef]
- Rosen, I.M.; Kirsch, D.B.; Carden, K.A.; Malhotra, R.K.; Ramar, K.; Aurora, R.N.; Kristo, D.A.; Martin, J.L.; Olson, E.J.; Rosen, C.L.; et al. Clinical use of a home sleep apnea test: An updated American Academy of Sleep Medicine Position Statement. J. Clin. Sleep Med. 2018, 14, 2075–2077. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, E.J.; Kim, Y.S.; Choi, J.; Kim, T.H.; Kwon, S.Y.; Lee, H.M.; Lee, S.H.; Shin, C.; Lee, S.H. Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol. 2010, 130, 838–843. [Google Scholar] [CrossRef] [PubMed]
- De Rui, M.; Middleton, B.; Sticca, A.; Gatta, A.; Amodio, P.; Skene, D.; Montagnese, S. Sleep and circadian rhythms in hospitalized patients with decompensated cirrhosis: Effect of light therapy. Neurochem. Res. 2015, 40, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.P.; Shah, N.J. Clinical and Neurologic Manifestation of Minimal Hepatic Encephalopathy and Overt Hepatic Encephalopathy. Clin. Liver Dis. 2015, 19, 461–472. [Google Scholar] [CrossRef]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Patidar, K.R.; Bajaj, J.S. Covert and overt hepatic encephalopathy: Diagnosis and management. Clin. Gastroenterol. Hepatol. 2015, 13, 2048–2061. [Google Scholar] [CrossRef]
- Luo, M.; Yu, X.B.; Hu, S.J.; Bai, F.H. EncephalApp Stroop App predicts poor sleep quality in patients with minimal hepatic encephalopathy due to hepatitis B-induced liver cirrhosis. Saudi J. Gastroenterol. 2020, 26, 120–128. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Thacker, L.R.; Heuman, D.M.; Fuchs, M.; Sterling, R.K.; Sanyal, A.J.; Puri, P.; Siddiqui, M.S.; Stravitz, R.T.; Bouneva, I.; et al. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology 2013, 58, 1122–1132. [Google Scholar] [CrossRef]
- Allampati, S.; Duarte-Rojo, A.; Thacker, L.R.; Patidar, K.R.; White, M.B.; Klair, J.S.; John, B.; Heuman, D.M.; Wade, J.B.; Flud, C.; et al. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop Encephal App: A Multicenter US-Based, Norm-Based Study. Am. J. Gastroenterol. 2016, 111, 78–86. [Google Scholar] [CrossRef]
- Hourmand-Ollivier, I.; Piquet, M.A.; Toudic, J.P.; Denise, P.; Dao, T. Actigraphy: A new diagnostic tool for hepatic encephalopathy. World J. Gastroenterol. 2006, 12, 2243–2244. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ming, Y.; Liu, J.; Liu, L.; Cheng, K.; Mao, P. Sleep Quality and Psychosocial Factors in Liver Transplant Recipients at an Outpatient Follow-Up Clinic in China. Ann. Transplant. 2020, 25, e920984. [Google Scholar] [CrossRef]
- Stewart, C.A.; Auger, R.; Enders, F.T.B.; Felmlee-Devine, D.; Smith, G.E. The effects of poor sleep quality on cognitive function of patients with cirrhosis. J. Clin. Sleep Med. 2014, 10, 21–26. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Boparai, N.; Price, L.L.; Kiwi, M.L.; McCormick, M.; Guyatt, G. Health-related quality of life in chronic liver disease: The impact of type and severity of disease. Am. J. Gastroenterol. 2001, 96, 2199–2205. [Google Scholar] [CrossRef]
- Agrawal, S.; Umapathy, S.; Dhiman, R.K. Minimal hepatic encephalopathy impairs quality of life. J. Clin. Exp. Hepatol. 2015, 5, S42–S48. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- Prasad, S.; Dhiman, R.K.; Duseja, A.; Chawla, Y.K.; Sharma, A.; Agarwal, R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 2007, 45, 549–559. [Google Scholar] [CrossRef]
- Auger, R.R.; Burgess, H.J.; Emens, J.S.; Deriy, L.V.; Thomas, S.M.; Sharkey, K.M. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2015, 11, 1199–1236. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc-Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Ramar, K.; Olson, E.J. Management of common sleep disorders. Am. Fam Phys. 2013, 88, 231–238. [Google Scholar]
- Gerrard, P.; Malcolm, R. Mechanisms of modafinil: A review of current research. Neuropsychiatr. Dis. Treat. 2007, 3, 349–364. [Google Scholar] [PubMed]
- Hardy, T.; MacDonald, C.; Jones, D.E.; Newton, J.L. A follow-up study of modafinil for the treatment of daytime somnolence and fatigue in primary biliary cirrhosis. Liver Int. 2010, 30, 1551–1552. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Newton, J.L. An open study of modafinil for the treatment of daytime somnolence and fatigue in primary biliary cirrhosis. Aliment. Pharmacol. Ther. 2007, 25, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.R.; Lucchesi, L.M.; Rueda, A.D.; Garbuio, S.A.; Palombini, L.O.; Guilleminault, C.; Tufik, S. Placebo and modafinil effect on sleepiness in obstructive sleep apnea. Prog. Neuropsychol. Pharmacol. Biol. Psychiatry 2008, 32, 552–559. [Google Scholar] [CrossRef]
- Sharma, M.K.; Kainth, S.; Kumar, S.; Bhardwaj, A.; Agarwal, H.K.; Maiwall, R.; Jamwal, K.D.; Shasthry, S.M.; Jindal, A.; Choudhary, A.; et al. Effects of zolpidem on sleep parameters in patients with cirrhosis and sleep disturbances: A randomized, placebo-controlled trial. Clin. Mol. Hepatol. 2019, 25, 199–209. [Google Scholar] [CrossRef]
- Gonciarz, M.; Gonciarz, Z.; Bielanski, W.; Mularczyk, A.; Konturek, P.C.; Brzozowski, T.; Konturek, S.J. The effects of long-term melatonin treatment on plasma liver enzymes levels and plasma concentrations of lipids and melatonin in patients with nonalcoholic steatohepatitis: A pilot study. J. Physiol. Pharmacol. 2012, 63, 35–40. [Google Scholar]
- Pakravan, H.; Ahmadian, M.; Fani, A.; Aghaee, D.; Brumanad, S.; Pakzad, B. The Effects of Melatonin in Patients with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. Adv. Biomed. Res. 2017, 6, 40. [Google Scholar] [CrossRef]
- Celinski, K.; Konturek, P.C.; Slomka, M.; Cichoz-Lach, H.; Brzozowski, T.; Konturek, S.J.; Korolczuk, A. Effects of treatment with melatonin and tryptophan on liver enzymes, parameters of fat metabolism and plasma levels of cytokines in patients with non-alcoholic fatty liver disease—14 months follow up. J. Physiol. Pharmacol. 2014, 65, 75–82. [Google Scholar]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [PubMed]
- Ruchała, M.; Bromińska, B.; Cyrańska-Chyrek, E.; Kuźnar-Kamińska, B.; Kostrzewska, M.; Batura-Gabryel, H. Obstructive sleep apnea and hormones—A novel insight. Arch. Med. Sci. 2017, 13, 875–884. [Google Scholar] [CrossRef]
- Vesa, C.M.; Behl, T.; Nemeth, S.; Bratu, O.G.; Diaconu, C.C.; Moleriu, R.D.; Negrut, N.; Zaha, D.C.; Bustea, C.; Ionita Radu, F.; et al. Prediction of NAFLD occurence in prediabetes patients. Exp. Ther. Med. 2020, 20, 190. [Google Scholar] [CrossRef]
- Kim, D.; Ahmed, A.; Kushida, C. Continuous positive airway pressure therapy on nonalcoholic fatty liver disease in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2018, 4, 1315–1322. [Google Scholar] [CrossRef]
- Chen, L.D.; Lin, L.; Zhang, L.J.; Zeng, H.X.; Wu, Q.Y.; Hu, M.F.; Xie, J.J.; Liu, J.N. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: A meta-analysis. Clin. Respir. J. 2018, 12, 373–381. [Google Scholar] [CrossRef]
- Pennisi, M.; Bertino, G.; Gagliano, C.; Malaguarnera, M.; Bella, R.; Borzì, A.M.; Madeddu, R.; Drago, F.; Malaguarnera, G. Resveratrol in Hepatitis C Patients Treated with Pegylated-Interferon-α-2b and Ribavirin Reduces Sleep Disturbance. Nutrients 2017, 9, 897. [Google Scholar] [CrossRef]
- Karimi-Sari, H.; Hosseini, M.A.; Nikjoo, N.; Bagheri-Baghdasht, M.S.; Alavian, S.M. Patient-reported outcomes of sleep, mood and quality of life after treatment of chronic hepatitis C infection using direct-acting antiviral agents. Clin. Microbiol. Infect. 2020, 26, 1093.e5–1093.e8. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Srivastava, P.; Bungau, S. Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 2020. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Mehta, V.; Uddin, M.S.; Bungau, S. Molecular insights into the therapeutic promise of targeting HMGB1 in depression. Pharmacol. Rep. 2020. [Google Scholar] [CrossRef]
- Turco, M.; Cazzagon, N.; Franceschet, I.; Formentin, C.; Frighetto, G.; Giordani, F.; Cellini, N.; Mazzotta, G.; Costa, R.; Middleton, B.; et al. Morning Bright Light Treatment for Sleep-Wake Disturbances in Primary Biliary Cholangitis: A Pilot Study. Front. Physiol. 2018, 9, 1530. [Google Scholar] [CrossRef]
- Liu, C.; Xie, H.; Zhang, X.; Yu, Y.; Zhang, X.; Sun, Y.; Zhou, L.; Su, B.; Wang, H. Health related management plans improve sleep disorders of patients with chronic liver disease. Int. J. Clin. Exp. Med. 2015, 8, 9883–9889. [Google Scholar]
| Groups of SDs | Classification of Main SDs | References |
|---|---|---|
| Insomnia | Chronic insomnia disorder Short-term insomnia disorder Other insomnia disorders | Sateia MJ [9] Morin et al. [10] |
| Sleep-related breathing disorders | OSA disorders CSA syndromes Sleep-related hypoventilation disorders Sleep-related hypoxemia disorder | Sateia MJ [9] Foldvary-Schaefer et al. [11] Mohammadieh et al. [12] |
| Circadian rhythm sleep-wake disorders | Sleep-wake phase disorders Sleep-wake rhythm disorders Non-24-h sleep-wake rhythm disorder Shift work disorder Jet lag disorder Circadian sleep-wake disorder not otherwise specified | Sateia MJ [8] Culnan et al. [13] Spiegelhalder et al. [14] |
| Parasomnias | NREM-related parasomnias REM-related parasomnias Other parasomnias | Sateia MJ [8] Bollu et al. [15] |
| Sleep-related movement disorders | Restless legs syndrome Sleep-related bruxism Sleep-related movement disorder due to a medical disorder Sleep-related movement disorder due to a medication or substance Sleep-related movement disorder, unspecified | Sateia MJ [8] Trotti et al. [16] |
| Central disorders of hypersomnolence | Narcolepsy Hypersomnia due to a medical disorder Hypersomnia due to a medication or substance Hypersomnia associated with a psychiatric disorder Insufficient sleep syndrome | Khan et al. [17] Dauvilliers Y et al. [18] |
| Other sleep disorders | SDs that cannot be classified elsewhere in the ICSD: (a) Sleep-related medical and neurological disorders (b) Substance-induced sleep disorders | Rains JC [19] |
| Etiology of CLD |
|---|
|
| Methods for Assessing SDs | Tools Used in Scientific Literature to Assess SDs in CLD | References |
|---|---|---|
| Subjective | Sleep diaries | [49,67,101,102,120] |
| The Pittsburgh Sleep Quality Index (PSQI) | [102,103,116,118,119] | |
| Sleep Timing and Sleep Quality Screening Questionnaire (STSQS) | [102,103,104] | |
| The Epworth Sleepiness Scale (ESS) | [6,31,34,37,62,90,105,106,107,118] | |
| The Basic Nordic Sleep Questionnaire (BNSQ) | [31,108,109,110] | |
| The Horne–Ostberg (HO) questionnaire | [27,110] | |
| STOP-Bang questionnaire | [111,112] | |
| Berlin questionnaire (BQ) | [36,114] | |
| The International Restless Leg Syndrome Study Group rating scale (IRLSS) | [89] | |
| Objective | Polysomnography | [99,115,117,118,119,123] |
| Actigraphy | [27,102,116,119,120,121,126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotogea, O.-M.; Ilie, M.; Bungau, S.; Chiotoroiu, A.L.; Stanescu, A.M.A.; Diaconu, C.C. Comprehensive Overview of Sleep Disorders in Patients with Chronic Liver Disease. Brain Sci. 2021, 11, 142. https://doi.org/10.3390/brainsci11020142
Plotogea O-M, Ilie M, Bungau S, Chiotoroiu AL, Stanescu AMA, Diaconu CC. Comprehensive Overview of Sleep Disorders in Patients with Chronic Liver Disease. Brain Sciences. 2021; 11(2):142. https://doi.org/10.3390/brainsci11020142
Chicago/Turabian StylePlotogea, Oana-Mihaela, Madalina Ilie, Simona Bungau, Alexandru Laurentiu Chiotoroiu, Ana Maria Alexandra Stanescu, and Camelia Cristina Diaconu. 2021. "Comprehensive Overview of Sleep Disorders in Patients with Chronic Liver Disease" Brain Sciences 11, no. 2: 142. https://doi.org/10.3390/brainsci11020142
APA StylePlotogea, O.-M., Ilie, M., Bungau, S., Chiotoroiu, A. L., Stanescu, A. M. A., & Diaconu, C. C. (2021). Comprehensive Overview of Sleep Disorders in Patients with Chronic Liver Disease. Brain Sciences, 11(2), 142. https://doi.org/10.3390/brainsci11020142