Aberrant Auditory and Visual Memory Development of Children with Upper Limb Motor Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Cognitive Functions
2.3. Statistical Analysis
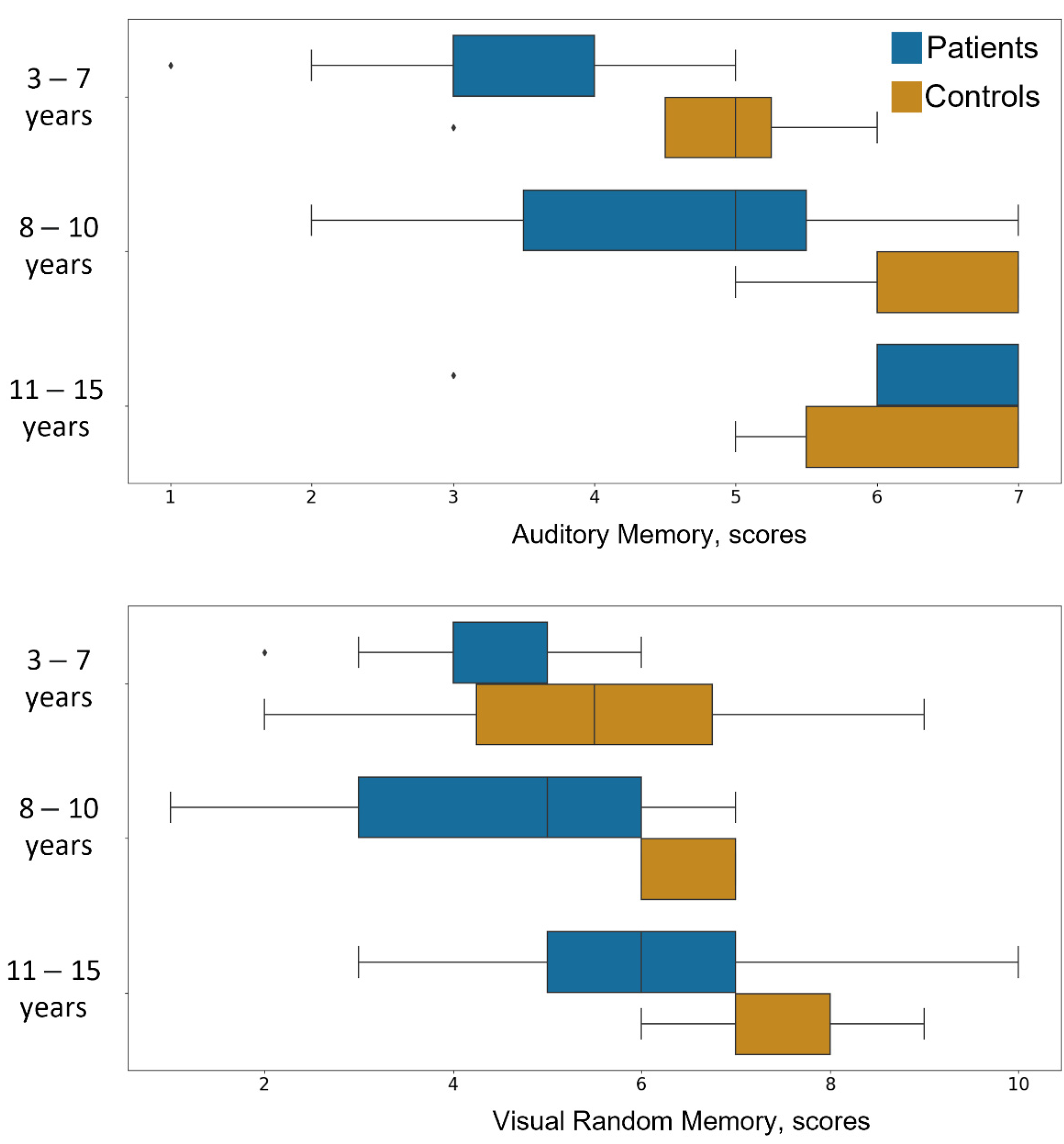
3. Results
4. Discussion
5. Conclusions
- Motor dysfunction of the upper limb does impair cognitive functions, especially auditory and visual memory.
- The link between cognitive skills and motor impairment is especially manifested between the ages of 8 and 10 years old.
- These findings must be considered in the development of rehabilitation programs for individuals with motor disorders.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van der Fels, I.M.J.; Te Wierike, S.C.M.; Hartman, E.; Elferink-Gemser, M.T.; Smith, J.; Visscher, C. The relationship between motor skills and cognitive skills in 4–16 year old typically developing children: A systematic review. J. Sci. Med. Sport 2015, 18, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Hauert, C.A. The relationship between motor function and cognition in the developmental perspective. Ital. J. Neurol. Sci. 1986, 5, 101–107. [Google Scholar] [PubMed]
- Iverson, J.M. Developing language in a developing body: The relationship between motor development and language development. J. Child Lang. 2010, 37, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Martzog, P.; Stoeger, H.; Suggate, S. Relations between Preschool Children’s Fine Motor Skills and General Cognitive Abilities. J. Cogn. Dev. 2019, 20, 443–465. [Google Scholar] [CrossRef]
- Stöckel, T.; Hughes, C.M.L. The relation between measures of cognitive and motor functioning in 5- to 6-year-old children. Psychol. Res. 2016, 80, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, O.; Ammar, A.; Chtourou, H.; Wagner, M.; Knisel, E.; Hökelmann, A.; Bös, K. Relationship between motor and cognitive learning abilities among primary school-aged children. Alex. J. Med. 2019, 53, 325–331. [Google Scholar] [CrossRef]
- Salman, M.S.; Tsai, P. The Role of the Pediatric Cerebellum in Motor Functions, Cognition, and Behavior: A Clinical Perspective. Neuroimaging Clin. N. Am. 2016, 26, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.E.; Pitchford, N.J.; Jaspan, T.; McArthur, D.; Walker, D. Development of cognitive and motor function following cerebellar tumour injury sustained in early childhood. Cortex 2010, 46, 919–932. [Google Scholar] [CrossRef]
- Bugalho, P.; Correa, B.; Viana-Baptista, M. Papel do cerebelo nas funções cognitivas e comportamentais: Bases científicas e modelos de estudo. Acta Med. Port. 2006, 19, 257–267. [Google Scholar] [PubMed]
- Kojima, M.; Nagano, A. Assessment of physical activity and cognitive function and their potential correlation in convalescent patients of cerebrovascular disease. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anderson, V.A.; Anderson, P.; Northam, E.; Jacobs, R.; Catroppa, C. Development of executive functions through late childhood and adolescence in an Australian sample. Dev. Neuropsychol. 2001, 20, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Roebers, C.M.; Kauer, M. Motor and cognitive control in a normative sample of 7-year-olds. Dev. Sci. 2009, 12, 175–181. [Google Scholar] [CrossRef]
- Walle, T.; Hartikainen-Sorri, A.-L. Obstetric shoulder injury: Associated risk factors, prediction and prognosis. Acta Obstet. Gynecol. Scand. 1993, 72, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Pondaag, W.; Malessy, M.J.A.; Van Dijk, J.G.; Thomeer, R.T.W.M. Natural history of obstetric brachial plexus palsy: A systematic review. Dev. Med. Child Neurol. 2004, 46, 138–144. [Google Scholar] [CrossRef]
- Bellew, M.; Kay, S.P.J.; Webb, F.; Ward, A. Developmental and behavioural outcome in obstetric brachial plexus palsy. J. Hand Surg. Am. 2000, 25, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Anguelova, G.V.; Malessy, M.J.A.; Buitenhuis, S.M.; Van Zwet, E.W.; Gert Van Dijk, J. Impaired Automatic Arm Movements in Obstetric Brachial Plexus Palsy Suggest a Central Disorder. J. Child Neurol. 2016, 31, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.; Cupido, C.; Scarfone, H.; Pape, K.; Galea, V.; McComas, A.J. Developmental apraxia arising from neonatal brachial plexus palsy. Neurology 2000, 55, 24–30. [Google Scholar] [CrossRef]
- Lin, Y.J.; Kao, T.W.; Chen, W.L. Relationship between peripheral neuropathy and cognitive performance in the elderly population. Medicine 2021, 100, e26071. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.G.; Holdnack, J.A.; Saklofske, D.H.; Prifitera, A. Wechsler Intelligence Scale for Children—Fifth Edition, WISC-V.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Raiford, S.E.; Coalson, D.L. Essentials of WPPSI-IV Assessment; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Colman, A.M. A Dictionary of Psychology; Oxford Quick Reference: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Shipitsyna, L.M. Psychological Diagnosis of Deviations in the Development of Children of Primary School Age: Book, Methodological Guide; Rech: Sankt Petersburg, Russia, 2008; 48, ISBN 5-9268-0682-8. [Google Scholar]
- Raven, J.C.; Court, J.H. Ravens Educational; Pearson Assessment: San Antonio, TX, USA, 2008. [Google Scholar]
- Khamis, H. Measures of association: How to choose? J. Diagn. Med Sonogr. 2008, 24, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Sirotyuk, A.L. Attention Deficit Hyperactivity Disorder. Diagnosis, Correction and Practical Recommendations for Parents and Teachers. TC Sphere: Moscow, Russia, 2003; 128, ISBN 978-5-9949-0083-3. [Google Scholar]
- Piek, J.P.; Dawson, L.; Smith, L.M.; Gasson, N. The role of early fine and gross motor development on later motor and cognitive ability. Hum. Mov. Sci. 2008, 27, 668–681. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bo, J.; Anguera, J.A. Neurocognitive contributions to motor skill learning: The role of working memory. J. Mot. Behav. 2012, 44, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hady, S.S.; Abd El-Azim, F.H.; El-Talawy, H.A.E.A.M. Correlation between cognitive function, gross motor skills and health—Related quality of life in children with Down syndrome. Egypt. J. Med. Hum. Genet. 2018, 19, 97–101. [Google Scholar] [CrossRef]
- Diamond, A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000, 71, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Rigoli, D.; Piek, J.P.; Kane, R.; Oosterlaan, J. An examination of the relationship between motor coordination and executive functions in adolescents. Dev. Med. Child Neurol. 2012, 54, 1025–1031. [Google Scholar] [CrossRef] [Green Version]
- Nourbakhsh, P. Perceptual-motor abilities and their relationships with academic performance of fifth grade pupils in comparison with Oseretsky Scale. Kinesiology 2006, 38, 40–48. [Google Scholar]
- Chun, M.M.; Turk-Browne, N.B. Interactions between attention and memory. Curr. Opin. Neurobiol. 2007, 17, 177–184. [Google Scholar] [CrossRef]
- Davis, E.E.; Pitchford, N.J.; Limback, E. The interrelation between cognitive and motor development in typically developing children aged 4-11 years is underpinned by visual processing and fine manual control. Br. J. Psychol. 2011, 102, 569–584. [Google Scholar] [CrossRef]
- Brown, R.M.; Palmer, C. Auditory-motor learning influences auditory memory for music. Mem. Cogn. 2012, 40, 567–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenni, O.G.; Chaouch, A.; Caflisch, J.; Rousson, V. Correlations between motor and intellectual functions in normally developing children between 7 and 18 years. Dev. Neuropsychol. 2013, 38, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Vygotsky, L.S.; Luria, A.; Van Der Veer, R. Tool and Symbol in Child Development; Van Der Veer, R., Valsiner, J., Eds.; The Vygotsky Reader; Blackwell Publishers: Oxford, UK, 1994; ISBN 0631188975. [Google Scholar]
- Zeng, N.; Ayyub, M.; Sun, H.; Wen, X.; Xiang, P.; Gao, Z. Effects of physical activity on motor skills and cognitive development in early childhood: A systematic review. Biomed Res. Int. 2017, 2017, 2760716. [Google Scholar] [CrossRef]
- Asonitou, K.; Koutsouki, D.; Kourtessis, T.; Charitou, S. Motor and cognitive performance differences between children with and without developmental coordination disorder (DCD). Res. Dev. Disabil. 2012, 33, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Piaget, J. A Construção do Espaço, Segundo Jean Piaget; The Mendeley Support Team: London, UK, 2005. [Google Scholar]
- Gazzaniga, M.S. On neural circuits and cognition. Neural Comput. 1995, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Spring, K.E.; Johnson, J.L.; Carroll, A.V.; Sassi, J.M.; Pangelinan, M.P.; Rudisill, M.E.; Wadsworth, D.D. The impact of a fundamental motor skill intervention on body composition outcomes in preschool children. Med. Sci. Sport. Exerc. 2021, 53, 293. [Google Scholar] [CrossRef]
- Higashionna, T.; Iwanaga, R.; Tokunaga, A.; Nakai, A.; Tanaka, K.; Nakane, H.; Tanaka, G. Relationship between motor coordination, cognitive abilities, and academic achievement in Japanese children with neurodevelopmental disorders. Hong Kong, J. Occup. Ther. 2017, 30, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Koziol, L.F.; Lutz, J.T. From movement to thought: The development of executive function. Appl. Neuropsychol. Child 2013, 2, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kersten, A.W. Bridging the gap between perception and higher cognition. Appl. Cogn. Psychol. 2006, 20. [Google Scholar] [CrossRef] [Green Version]
- Gallese, V.; Cuccio, V. The neural exploitation hypothesis and its implications for an embodied approach to language and cognition: Insights from the study of action verbs processing and motor disorders in Parkinson’s disease. Cortex 2018, 100, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.L.; Englund, J.A.; Carboni, J.A.; Brooks, J.H. Cognitive and Developmental Influences in Visual-Motor Integration Skills in Young Children. Psychol. Assess. 2011, 23, 1010. [Google Scholar] [CrossRef] [PubMed]
- Stahlschmidt, L.; Zernikow, B.; Wager, J. Specialized Rehabilitation Programs for Children and Adolescents with Severe Disabling Chronic Pain: Indications, Treatment and Outcomes. Children 2016, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Pesce, C.; Crova, C.; Cereatti, L.; Casella, R.; Bellucci, M. Physical activity and mental performance in preadolescents: Effects of acute exercise on free-recall memory. Ment. Health Phys. Act. 2009, 2, 16–22. [Google Scholar] [CrossRef]
- Westendorp, M.; Houwen, S.; Hartman, E.; Mombarg, R.; Smith, J.; Visscher, C. Effect of a ball skill intervention on children’s ball skills and cognitive functions. Med. Sci. Sports Exerc. 2014, 46, 414. [Google Scholar] [CrossRef] [PubMed]
| Group | Age | Interaction | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F (1,64) | p | q | F (2,64) | p | q | F (2.64) | p | q | ||||
| Attention | 2.64 | 0.11 | 0.04 | 0.15 | 15.63 | < 0.001 *** | 0.32 | < 0.001 | 0.72 | 0.49 | 0.02 | 0.57 |
| Auditory Memory | 11.72 | < 0.01 ** | 0.15 | < 0.01 | 15.01 | < 0.001 *** | 0.32 | < 0.001 | 0.94 | 0.4 | 0.03 | 0.56 |
| Visual Memory | 15.59 | < 0.001 *** | 0.19 | < 0.001 | 11.07 | < 0.001 *** | 0.25 | < 0.001 | 1.06 | 0.35 | 0.03 | 0.82 |
| Intelligence | 0.32 | 0.57 | 0 | 0.57 | 0.58 | 0.57 | 0.02 | 0.57 | 0.64 | 0.53 | 0.02 | 0.53 |
| Storytelling | 1.45 | 0.23 | 0.02 | 0.27 | 4.87 | < 0.05 * | 0.13 | < 0.05 | 1.75 | 0.18 | 0.05 | 1 |
| Thinking | 16.55 | < 0.001 *** | 0.2 | < 0.001 | 1.34 | 0.27 | 0.04 | 0.31 | 1.09 | 0.34 | 0.03 | 1 |
| ACS | 18.51 | < 0.001 *** | 0.22 | < 0.001 | 22.19 | < 0.001 *** | 0.41 | < 0.001 | 1.02 | 0.37 | 0.03 | 0.64 |
| p | q | ||
|---|---|---|---|
| Attention | 0.24 | 0.04 * | 0.11 |
| Auditory Memory | 0.26 | 0.02 * | 0.13 |
| Visual Memory | 0.2 | 0.08 | 0.16 |
| Intelligence | −0.05 | 0.66 | 0.8 |
| Storytelling | 0.04 | 0.76 | 0.76 |
| Thinking | −0.09 | 0.42 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koriakina, M.; Agranovich, O.; Petrova, E.; Kadieva, D.; Kopytin, G.; Ermolovich, E.; Moiseenko, O.; Alekseeva, M.; Bredikhin, D.; Bermúdez-Margaretto, B.; et al. Aberrant Auditory and Visual Memory Development of Children with Upper Limb Motor Disorders. Brain Sci. 2021, 11, 1650. https://doi.org/10.3390/brainsci11121650
Koriakina M, Agranovich O, Petrova E, Kadieva D, Kopytin G, Ermolovich E, Moiseenko O, Alekseeva M, Bredikhin D, Bermúdez-Margaretto B, et al. Aberrant Auditory and Visual Memory Development of Children with Upper Limb Motor Disorders. Brain Sciences. 2021; 11(12):1650. https://doi.org/10.3390/brainsci11121650
Chicago/Turabian StyleKoriakina, Maria, Olga Agranovich, Ekaterina Petrova, Dzerassa Kadieva, Grigory Kopytin, Evgenia Ermolovich, Olesya Moiseenko, Margarita Alekseeva, Dimitri Bredikhin, Beatriz Bermúdez-Margaretto, and et al. 2021. "Aberrant Auditory and Visual Memory Development of Children with Upper Limb Motor Disorders" Brain Sciences 11, no. 12: 1650. https://doi.org/10.3390/brainsci11121650
APA StyleKoriakina, M., Agranovich, O., Petrova, E., Kadieva, D., Kopytin, G., Ermolovich, E., Moiseenko, O., Alekseeva, M., Bredikhin, D., Bermúdez-Margaretto, B., Ntoumanis, I., Shestakova, A. N., Jääskeläinen, I. P., & Blagovechtchenski, E. (2021). Aberrant Auditory and Visual Memory Development of Children with Upper Limb Motor Disorders. Brain Sciences, 11(12), 1650. https://doi.org/10.3390/brainsci11121650






