Associations between Sex Hormones and Circulating Growth Differentiation Factor-15 in Male Patients with Major Depressive Disorder
Abstract
:1. Introduction
2. Methods
2.1. Research Objects
2.2. Sample Preparation
2.3. Laboratory Analyses
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Correlation between Sex Hormone Levels and GDF-15
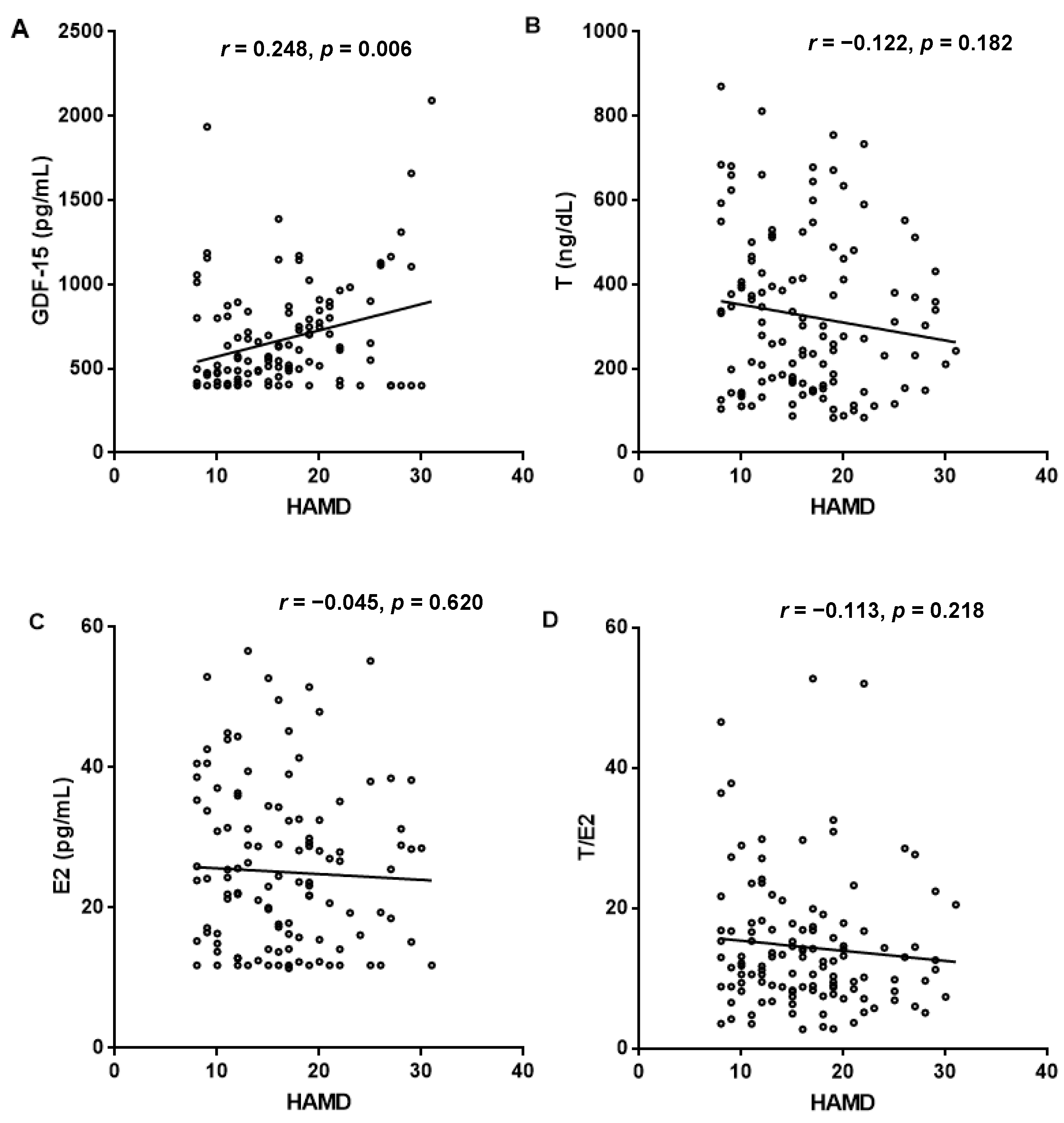
3.3. Association of HAMD-24 Scores with Sex Hormone and GDF-15 Levels
3.4. Correlation between GDF-15 and Risk Factors of Depression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Yao, Q.; Li, Y. Increased serum levels of complement C1q in major depressive disorder. J. Psychosom. Res. 2020, 133, 110105. [Google Scholar] [CrossRef] [PubMed]
- Toenders, Y.J.; Schmaal, L.; Harrison, B.J.; Dinga, R.; Berk, M.; Davey, C.G. Neurovegetative symptom subtypes in young people with major depressive disorder and their structural brain correlates. Transl. Psychiatry 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocrinol. 2014, 35, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Albert, K.M.; Newhouse, P.A. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu. Rev. Clin. Psychol. 2019, 15, 399–423. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Dai, W.; Li, Y. Neuroprotective effect of a physiological ratio of testosterone and estradiol on corticosterone-induced apoptosis in PC12 cells via Traf6/TAK1 pathway. Toxicol. Vitr. 2018, 50, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Alderson, L.M.; Baum, M.J. Differential effects of gonadal steroids on dopamine metabolism in mesolimbic and nigro-striatal pathways of male rat brain. Brain Res. 1981, 218, 189–206. [Google Scholar] [CrossRef]
- Rodgers, S.; Grosse Holtforth, M.; Hengartner, M.P.; Müller, M.; Aleksandrowicz, A.A.; Rössler, W.; Ajdacic-Gross, V. Serum testosterone levels and symptom-based depression subtypes in men. Front. Psychiatry 2015, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Zarrouf, F.A.; Artz, S.; Griffith, J.; Sirbu, C.; Kommor, M. Testosterone and depression: Systematic review and meta-analysis. J. Psychiatr. Pract. 2009, 15, 289–305. [Google Scholar] [CrossRef]
- Pope, H.G., Jr.; Cohane, G.H.; Kanayama, G.; Siegel, A.J.; Hudson, J.I. Testosterone gel supplementation for men with refractory depression: A randomized, placebo-controlled trial. Am. J. Psychiatry 2003, 160, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Kanayama, G.; Amiaz, R.; Seidman, S.; Pope, H.G., Jr. Testosterone supplementation for depressed men: Current research and suggested treatment guidelines. Exp. Clin. Psychopharmacol. 2007, 15, 529–538. [Google Scholar] [CrossRef]
- Seidman, S.N.; Spatz, E.; Rizzo, C.; Roose, S.P. Testosterone replacement therapy for hypogonadal men with major depressive disorder: A randomized, placebo-controlled clinical trial. J. Clin. Psychiatry 2001, 62, 406–412. [Google Scholar] [CrossRef]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends. Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Dai, W.; Zhu, C.; Liu, H.; Li, Y.; Zhang, P. Circulating levels of growth differentiation factor 15 and sex hormones in male patients with HBV-associated hepatocellular carcinoma. Biomed. Pharm. 2020, 121, 109574. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Ormstad, H.; Aass, H.C.; Malt, U.F.; Bendz, L.T.; Sandvik, L.; Brundin, L.; Andreassen, O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014, 45, 77–86. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ho, R.C.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Van den Biggelaar, A.H.; Gussekloo, J.; de Craen, A.J.; Frölich, M.; Stek, M.L.; van der Mast, R.C.; Westendorp, R.G. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp. Gerontol. 2007, 42, 693–701. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, T.; Trollor, J.N.; Crawford, J.; Brown, D.A.; Baune, B.T.; Samaras, K.; Campbell, L.; Breit, S.N.; Brodaty, H.; Sachdev, P.; et al. Macrophage inhibitory cytokine-1 is associated with cognitive impairment and predicts cognitive decline—The Sydney Memory and Aging Study. Aging Cell 2013, 12, 882–889. [Google Scholar] [CrossRef]
- Jiang, J.; Wen, W.; Sachdev, P.S. Macrophage inhibitory cytokine-1/growth differentiation factor 15 as a marker of cognitive ageing and dementia. Curr. Opin. Psychiatry 2016, 29, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Che, B.; Zhu, Z.; Bu, X.; Yin, J.; Han, L.; Xu, T.; Ju, Z.; Liu, J.; Zhang, J.; Chen, J.; et al. Multiple biomarkers covering several pathways for the prediction of depression after ischemic stroke. J. Affect. Disord. 2021, 280, 442–449. [Google Scholar] [CrossRef]
- Lu, X.; Duan, J.; Cheng, Q.; Lu, J. The association between serum growth differentiation factor-15 and 3-month depression after acute ischemic stroke. J. Affect. Disord. 2020, 260, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Kakehi, Y.; Segawa, T.; Wu, X.X.; Kulkarni, P.; Dhir, R.; Getzenberg, R.H. Down-regulation of macrophage inhibitory cytokine-1/prostate derived factor in benign prostatic hyperplasia. Prostate 2004, 59, 351–356. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Peng, R.; Dai, W.; Li, Y. Low serum free thyroxine level is correlated with lipid profile in depressive patients with suicide attempt. Psychiatry Res. 2018, 266, 111–115. [Google Scholar] [CrossRef]
- Kempf, T.; Wollert, K.C. Risk stratification in critically ill patients: GDF-15 scores in adult respiratory distress syndrome. Crit. Care 2013, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Ago, T.; Sadoshima, J. GDF15, a cardioprotective TGF-beta superfamily protein. Circ. Res. 2006, 98, 294–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Altena, R.; Fehrmann, R.S.; Boer, H.; de Vries, E.G.; Meijer, C.; Gietema, J.A. Growth differentiation factor 15 (GDF-15) plasma levels increase during bleomycin- and cisplatin-based treatment of testicular cancer patients and relate to endothelial damage. PLoS ONE 2015, 10, e0115372. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Wallentin, L.; Kempf, T.; Tapken, H.; Quint, A.; Lindahl, B.; Olofsson, S.; Venge, P.; Larsson, A.; Hulthe, J.; et al. Growth-differentiation factor-15 is an independent marker of cardiovascular dysfunction and disease in the elderly: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur. Heart J. 2009, 30, 2346–2353. [Google Scholar] [CrossRef] [Green Version]
- Van Dooren, F.E.; Schram, M.T.; Schalkwijk, C.G.; Stehouwer, C.D.; Henry, R.M.; Dagnelie, P.C.; Schaper, N.C.; van der Kallen, C.J.; Koster, A.; Sep, S.J.; et al. Associations of low grade inflammation and endothelial dysfunction with depression—The Maastricht Study. Brain Behav. Immun. 2016, 56, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Teunissen, C.E.; Durieux-Lu, S.; Blankenstein, M.A.; Oude Voshaar, R.C.; Comijs, H.C. The inflammatory marker GDF-15 is not independently associated with late-life depression. J. Psychosom. Res. 2016, 83, 46–49. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.; Wang, L.; Lingappan, K. Sex-specific differences in the modulation of Growth Differentiation Factor 15 (GDF15) by hyperoxia in vivo and in vitro: Role of Hif-1. Toxicol. Appl. Pharm. 2017, 332, 8–14. [Google Scholar] [CrossRef]
- Campbell, R.A.; Bhat-Nakshatri, P.; Patel, N.M.; Constantinidou, D.; Ali, S.; Nakshatri, H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: A new model for anti-estrogen resistance. J. Biol. Chem. 2001, 276, 9817–9824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaue, N.; Yabe, H.; Nagai, M. Serum growth differentiation factor 15, but not lactate, is elevated in patients with Parkinson’s disease. J. Neurol. Sci. 2020, 409, 116616. [Google Scholar] [CrossRef]
- Liu, T.; Bauskin, A.R.; Zaunders, J.; Brown, D.A.; Pankhurst, S.; Russell, P.J.; Breit, S.N. Macrophage inhibitory cytokine 1 reduces cell adhesion and induces apoptosis in prostate cancer cells. Cancer Res. 2003, 63, 5034–5040. [Google Scholar]
- Liu, H.; Dai, W.; Cui, Y.; Lyu, Y.; Li, Y. Potential associations of circulating growth differentiation factor-15 with sex hormones in male patients with coronary artery disease. Biomed. Pharm. 2019, 114, 108792. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lyu, Y.; Li, D.; Cui, Y.; Huang, Y.; Dai, W.; Li, Y. Potential relation between soluble growth differentiation factor-15 and testosterone deficiency in male patients with coronary artery disease. Cardiovasc. Diabetol. 2019, 18, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Controls (n = 105) | MDD Patients (n = 121) | Statistics | p |
|---|---|---|---|---|
| Clinical variables | ||||
| Age (years) | 32.00 (26.00–35.00) | 28.00 (18.00–41.50) | Z = −1.600 | 0.109 |
| Smoking n (%) | 12 (11.43%) | 17 (14.05%) | χ2 = 0.345 | 0.557 |
| Alcohol consumption n (%) | 7 (6.67%) | 11 (9.09%) | χ2 = 0.451 | 0.502 |
| Traumatic life events n (%) | - | 6 (4.96%) | - | - |
| Family history of depression n (%) | - | 17 (14.05%) | - | - |
| HAMD-24 score | - | 16 (11.50–20.00) | - | - |
| Laboratory variables | ||||
| Urea(mmol/L) | 4.70 (4.12–5.50) | 4.62 (3.77–5.40) | Z = −1.416 | 0.157 |
| UA(µmol/L) | 398.70 ± 81.39 | 391.62 ± 93.57 | t = −0.603 | 0.547 |
| Glu (mmol/L) | 4.68 (4.28–5.09) | 4.18 (3.90–4.52) | Z = −6.391 | <0.001 |
| Cr(µmol/L) | 72.00 (66.00–80.00) | 66.00 (62.00–75.00) | Z = −4.077 | <0.001 |
| TC (mmol/L) | 4.48 ± 0.64 | 3.89 ± 0.76 | t = −6.386 | <0.001 |
| WBC (109/L) | 5.72 (4.86–6.40) | 6.09 (5.18–7.00) | Z = −2.759 | 0.006 |
| HDL-C (mmol/L) | 1.09 (0.98–1.29) | 1.01 (0.89–1.17) | Z = −3.442 | 0.001 |
| TG (mmol/L) | 1.15 (0.92–1.59) | 1.16 (0.82–1.66) | Z = −0.408 | 0.683 |
| hs-CRP (mg/L) | 0.11 (0.03–0.42) | 0.18 (0.04–0.91) | Z = −2.117 | 0.030 |
| LDL-C (mmol/L) | 2.66 ± 0.56 | 2.21 ± 0.70 | t = −5.404 | <0.001 |
| sdLDL-C (mmol/L) | 0.72 (0.56–0.93) | 0.62 (0.49–0.92) | Z = −1.828 | 0.068 |
| TC/HDL-C | 4.04 ± 0.84 | 3.87 ± 1.04 | t = −1.338 | 0.182 |
| T (ng/dL) | 355.59(261.63–482.73) | 279.12 (157.17–443.73) | Z = −2.891 | 0.004 |
| E2 (pg/mL) | 22.05 (15.32–28.75) | 23.61 (14.11–32.59) | Z = −0.905 | 0.365 |
| T/E2 ratio | 17.22 (12.18–21.14) | 12.44 (8.43–17.17) | Z = −4.299 | <0.001 |
| GDF-15 (pg/mL) | 478.00 (405.50–649.50) | 551.00(419.50–806.00) | Z = −2.408 | 0.016 |
| T | E2 | T/E2 Ratio | |
|---|---|---|---|
| r | −0.176 | −0.024 | −0.194 |
| p | 0.008 | 0.722 | 0.003 |
| (Constant) | GDF-15 | T | E2 | T/E2 Ratio | |
|---|---|---|---|---|---|
| Standardized β-coefficient a | 0.248 | −0.141 | −0.023 | −0.125 | |
| t | 2.646 | 2.737 | −1.507 | −0.249 | −1.331 |
| p | 0.009 | 0.007 | 0.134 | 0.804 | 0.186 |
| Urea | Cr | UA | hs-CRP | WBC | Glu | TC | TG | HDL-C | LDL-C | sdLDL-C | TC/HDL-C | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | 0.187 | 0.259 | −0.040 | 0.398 | 0.024 | 0.206 | 0.184 | 0.243 | −0.023 | 0.129 | 0.165 | 0.154 |
| p | 0.040 | 0.004 | 0.660 | <0.001 | 0.793 | 0.024 | 0.043 | 0.007 | 0.803 | 0.158 | 0.071 | 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Peng, R.; Sun, X.; Li, Y. Associations between Sex Hormones and Circulating Growth Differentiation Factor-15 in Male Patients with Major Depressive Disorder. Brain Sci. 2021, 11, 1612. https://doi.org/10.3390/brainsci11121612
Sun T, Peng R, Sun X, Li Y. Associations between Sex Hormones and Circulating Growth Differentiation Factor-15 in Male Patients with Major Depressive Disorder. Brain Sciences. 2021; 11(12):1612. https://doi.org/10.3390/brainsci11121612
Chicago/Turabian StyleSun, Ting, Rui Peng, Xiaojun Sun, and Yan Li. 2021. "Associations between Sex Hormones and Circulating Growth Differentiation Factor-15 in Male Patients with Major Depressive Disorder" Brain Sciences 11, no. 12: 1612. https://doi.org/10.3390/brainsci11121612
APA StyleSun, T., Peng, R., Sun, X., & Li, Y. (2021). Associations between Sex Hormones and Circulating Growth Differentiation Factor-15 in Male Patients with Major Depressive Disorder. Brain Sciences, 11(12), 1612. https://doi.org/10.3390/brainsci11121612






