Effect of Neck Muscle Fatigue on Hand Muscle Motor Performance and Early Somatosensory Evoked Potentials
Abstract
:1. Introduction
2. Methods
2.1. Ethical Approval
2.2. Participants
2.3. Inclusion and Exclusion Criteria
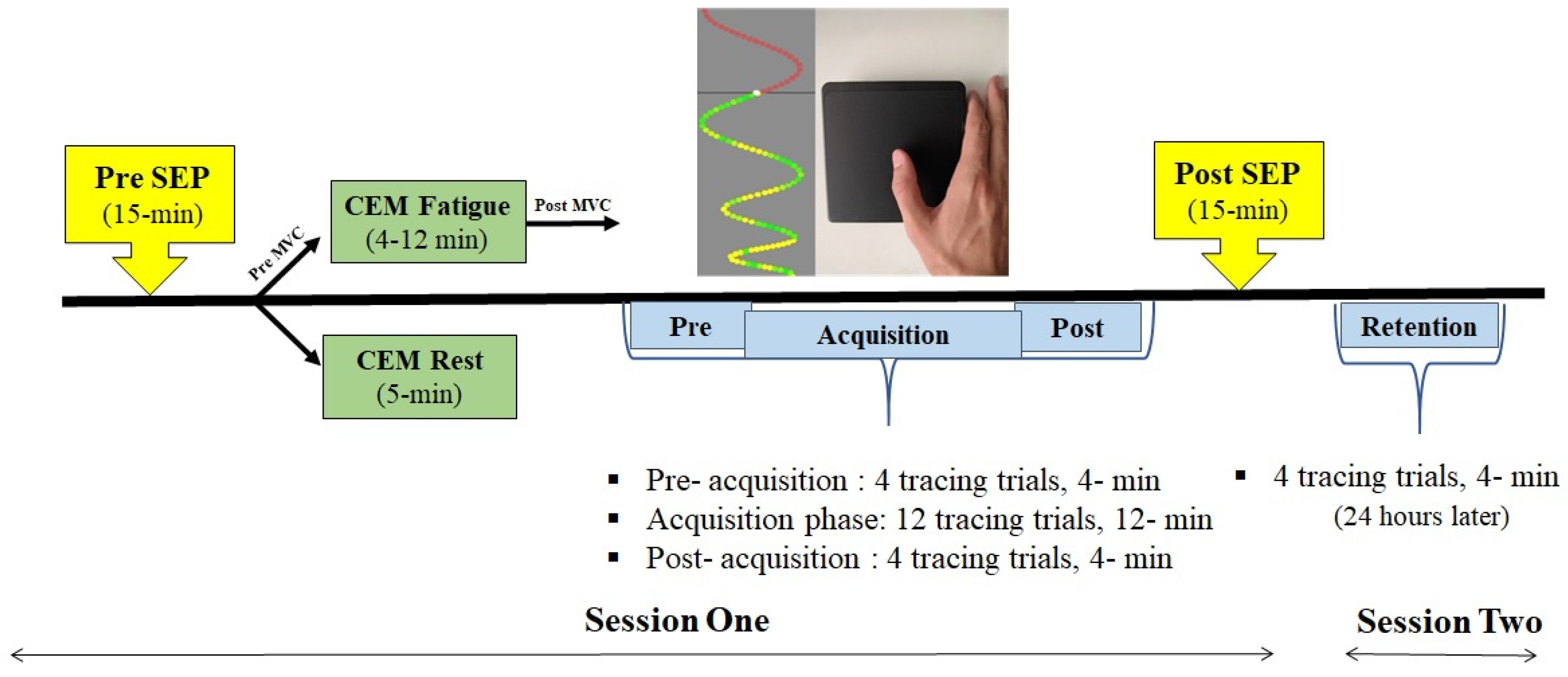
2.4. Experimental Protocol
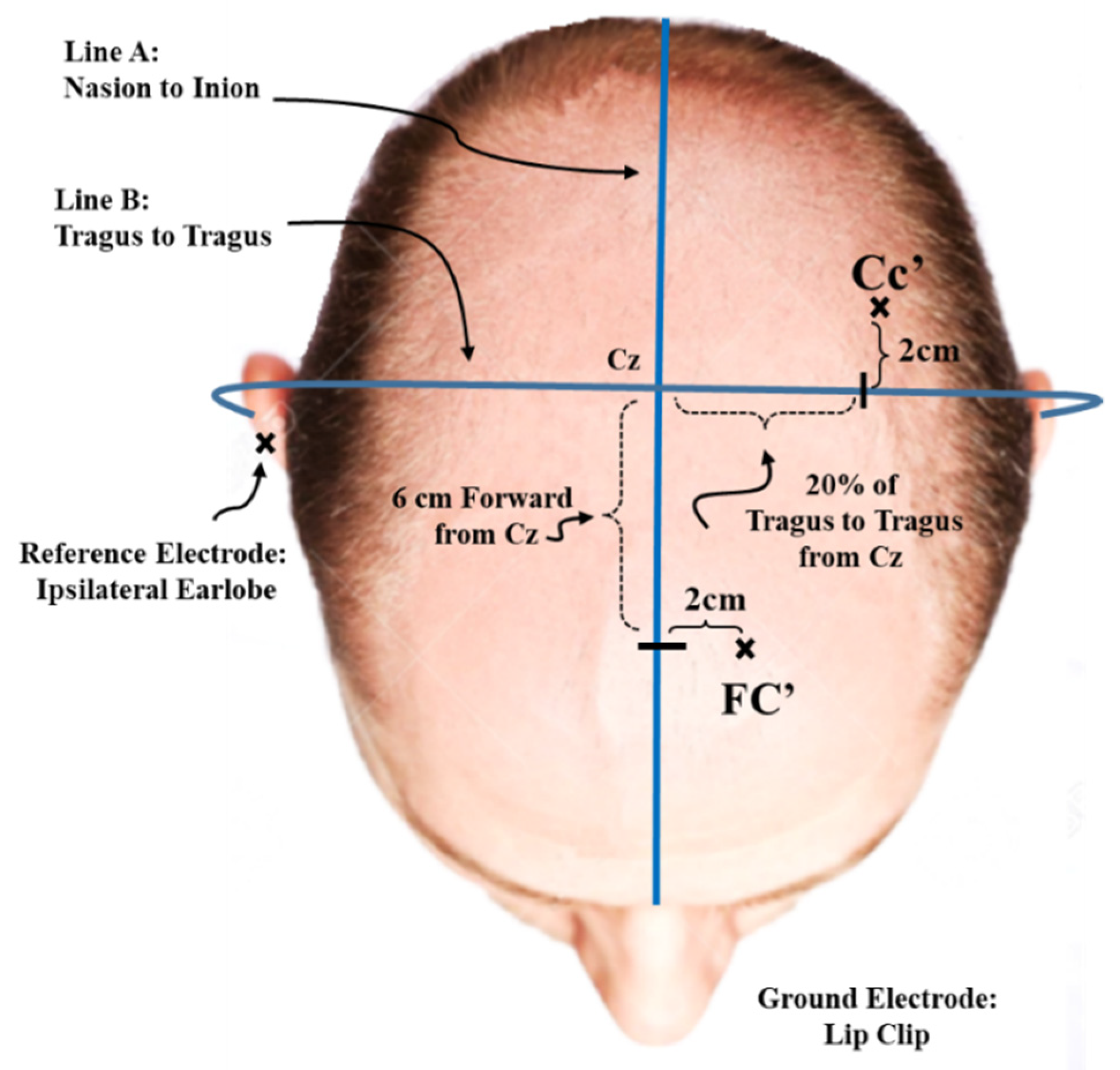
2.5. SEP Recording Parameters
2.6. Stimulation Parameters
2.7. Motor Skill Acquisition Tracing Task Parameters
2.8. CEM Surface EMG
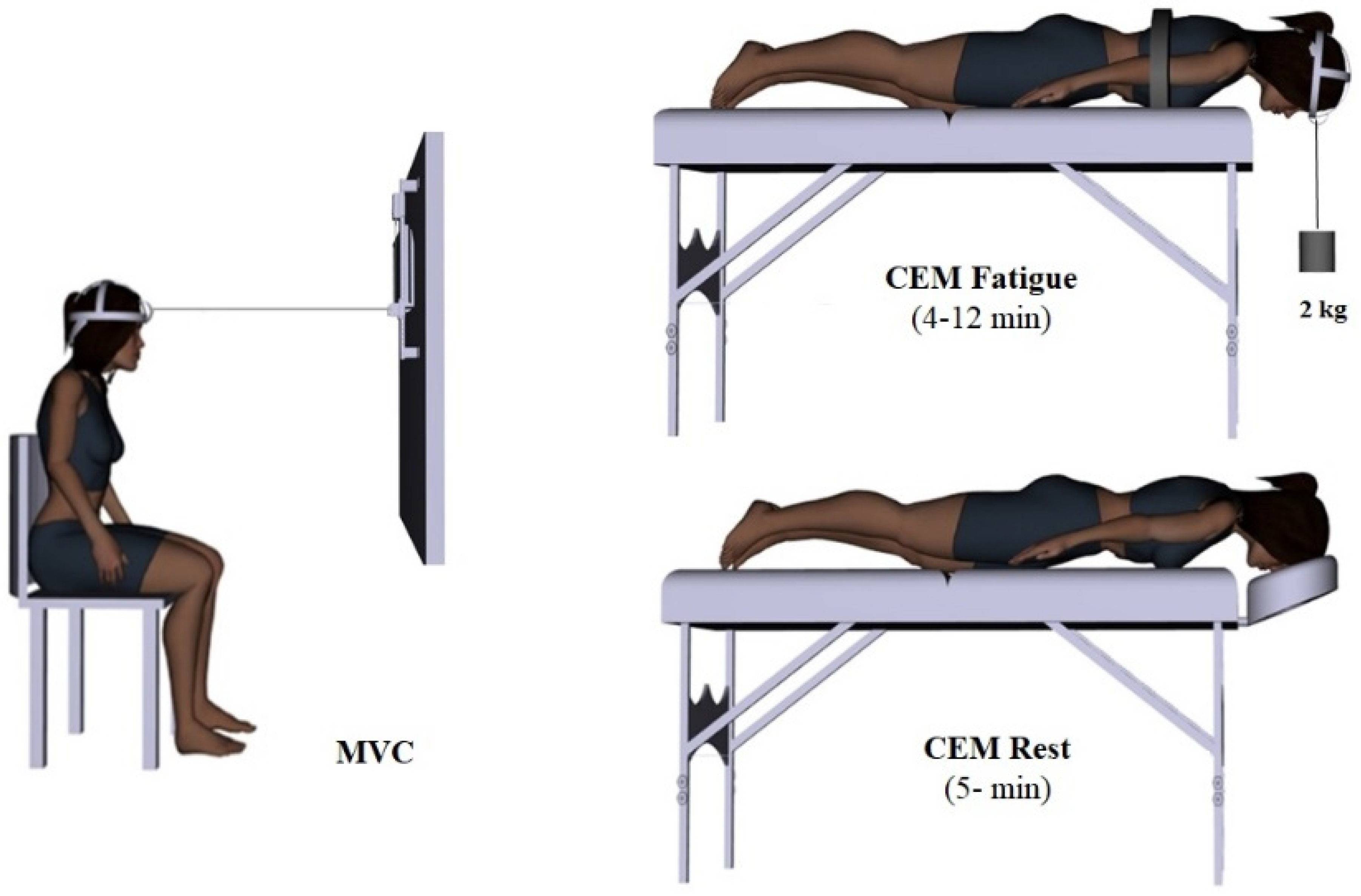
2.9. CEM Fatigue Protocol
2.10. CEM Rest Protocol
3. Data Processing
4. Statistical Analysis
4.1. Baseline Differences between Groups
4.2. Behavioral Data
4.3. Neurophysiological Data
5. Results
5.1. Participants
5.2. Fatigue Data
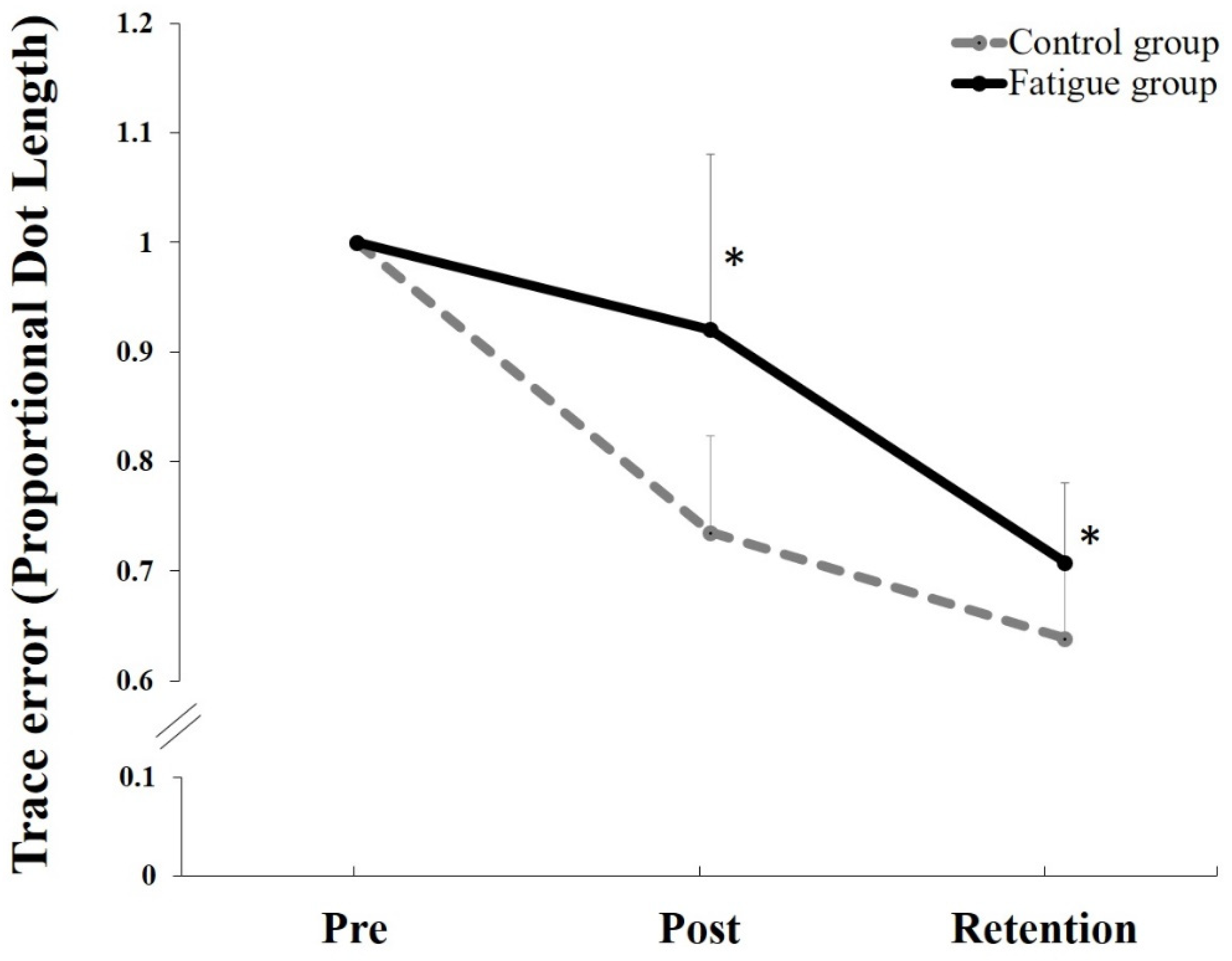
5.3. Behavioral Data
5.4. Neurophysiological SEPs Data
6. Discussion
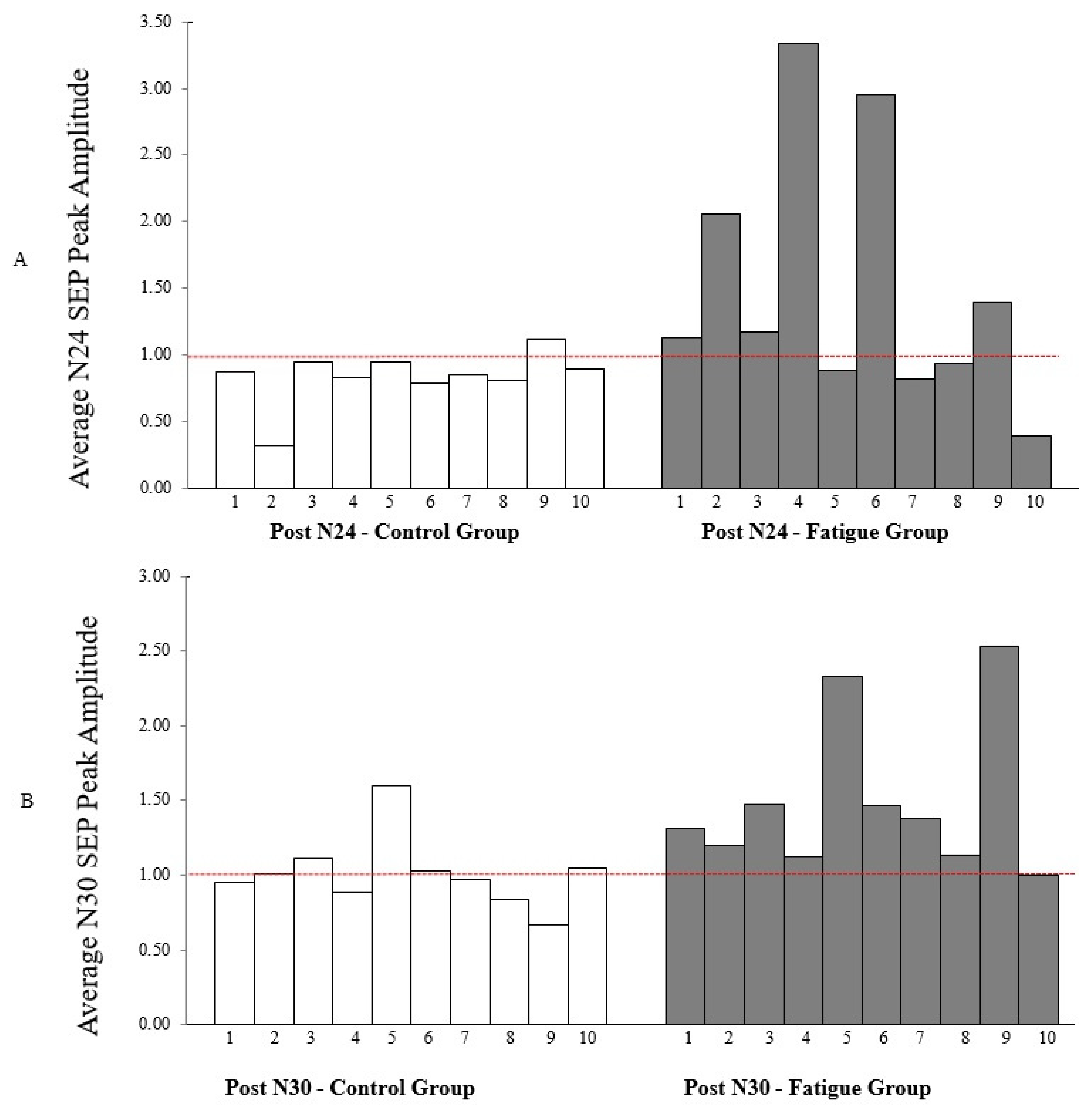
6.1. N24 SEP Peak Changes
6.2. N30 SEP Peak Changes
6.3. Behavioral Data
6.4. Effect of Fatigue
7. Limitations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbruzzese, G.; Berardelli, A. Sensorimotor integration in movement disorders. Mov. Disord. 2003, 18, 231–240. [Google Scholar] [CrossRef]
- Andrew, D.; Yielder, P.; Haavik, H.; Murphy, B. The effects of subclinical neck pain on sensorimotor integration following a complex motor pursuit task. Exp. Brain Res. 2018, 236, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Leone, A.; Nguyet, D.; Cohen, L.G.; Brasil-Neto, J.P.; Cammarota, A.; Hallett, M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 1995, 74, 1037–1045. [Google Scholar] [CrossRef]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Taylor, H.H.; Wilson, S.; Oliphant, G.; Mathers, K. Rapid reversible changes to multiple levels of the human somatosensory system following the cessation of repetitive contractions: A somatosensory evoked potential study. Clin. Neurophysiol. 2003, 114, 1531–1537. [Google Scholar] [CrossRef]
- Middleton, A.F.; Strick, P.L. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res. Rev. 2000, 31, 236–250. [Google Scholar] [CrossRef]
- Doyon, J.; Song, A.W.; Karni, A.; Lalonde, F.; Adams, M.M.; Ungerleider, L.G. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. USA 2002, 99, 1017–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, D.P.; Passingham, R.E. Predicting sensory events. Exp. Brain Res. 2001, 138, 251–257. [Google Scholar] [CrossRef]
- Dancey, E.; Murphy, B.; Andrew, D.; Yielder, P. Interactive effect of acute pain and motor learning acquisition on sensorimotor integration and motor learning outcomes. J. Neurophysiol. 2016, 116, 2210–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treleaven, J. Sensorimotor disturbances in neck disorders affecting postural stability, head and eye movement control. Man. Ther. 2008, 13, 2–11. [Google Scholar] [CrossRef]
- Berthoz, A.; Llinás, R. Afferent neck projection to the cat cerebellar cortex. Exp. Brain Res. 1974, 20, 385–401. [Google Scholar] [CrossRef]
- Anderson, M.; Yoshida, M.; Wilson, V. Influence of superior colliculus on cat neck motoneurons. J. Neurophysiol. 1971, 34, 898–907. [Google Scholar] [CrossRef]
- Wilson, V.J.; Yoshida, M. Monosynaptic inhibition of neck motoneurons by the medial vestibular nucleus. Exp. Brain Res. 1969, 9, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Gdowski, M.J.; Newlands, S.D.; Gdowski, G.T. Convergence of Vestibular and Neck Proprioceptive Sensory Signals in the Cerebellar Interpositus. J. Neurosci. 2013, 33, 1198. [Google Scholar] [CrossRef]
- Bolton, P.S. The somatosensory system of the neck and its effects on the central nervous system. J. Manip. Physiol. Ther. 1998, 21, 553–563. [Google Scholar]
- Zabihhosseinian, M.; Holmes, M.W.; Ferguson, B.; Murphy, B. Neck muscle fatigue alters the cervical flexion relaxation ratio in sub-clinical neck pain patients. Clin. Biomech. 2015, 30, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Stapley, P.J.; Beretta, M.V.; Toffola, E.D.; Schieppati, M. Neck muscle fatigue and postural control in patients with whiplash injury. Clin. Neurophysiol. 2006, 117, 610–622. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B. Subclinical neck pain and the effects of cervical manipulation on elbow joint position sense. J. Manip. Physiol. Ther. 2011, 34, 88–97. [Google Scholar] [CrossRef]
- Popper, K.R.; Eccles, J.C. The Self and Its Brain; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Mehta, B.; Schaal, S. Forward models in visuomotor control. J. Neurophysiol. 2002, 88, 942–953. [Google Scholar] [CrossRef]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef]
- Penhune, V.B.; Steele, C.J. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 2012, 226, 579–591. [Google Scholar] [CrossRef]
- Daligadu, J.; Haavik, H.; Yielder, P.C.; Baarbe, J.; Murphy, B. Alterations in cortical and cerebellar motor processing in subclinical neck pain patients following spinal manipulation. J. Manip. Physiol. Ther. 2013, 36, 527–537. [Google Scholar] [CrossRef]
- Zabihhosseinian, M.; Yielder, P.; Holmes, M.W.; Murphy, B. Neck muscle fatigue affects performance of an eye-hand tracking task. J. Neurophysiol. 2020, 124, 844–855. [Google Scholar] [CrossRef]
- Knox, J.J.; Hodges, P.W. Changes in head and neck position affect elbow joint position sense. Exp. Brain Res. 2005, 165, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Cordo, P.; Skoss, R.; Durrant, S.; Hodges, P. Illusory changes in head position induced by neck muscle vibration can alter the perception of elbow position. Behav. Neurosci. 2006, 120, 1211. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Zabihhosseinian, M.; Yielder, P.; Berkers, V.; Ambalavanar, U.; Holmes, M.; Murphy, B. Neck muscle fatigue impacts plasticity and sensorimotor integration in cerebellum and motor cortex in response to novel motor skill acquisition. J. Neurophysiol. 2020, 124, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Valeriani, M.; Barba, C.; le Pera, D.; Capecci, M.; Filippini, V.; Molinari, M. Functional changes of the primary somatosensory cortex in patients with unilateral cerebellar lesions. Brain 2001, 124, 757–768. [Google Scholar] [CrossRef]
- Edmondston, S.; Björnsdóttir, G.; Pálsson, T.; Solgård, H.; Ussing, K.; Allison, G. Endurance and fatigue characteristics of the neck flexor and extensor muscles during isometric tests in patients with postural neck pain. Man. Ther. 2011, 16, 332–338. [Google Scholar] [CrossRef]
- Kaňovský, P.; Bareš, M.; Rektor, I. The selective gating of the N30 cortical component of the somatosensory evoked potentials of median nerve is different in the mesial and dorsolateral frontal cortex: Evidence from intracerebral recordings. Clin. Neurophysiol. 2003, 114, 981–991. [Google Scholar] [CrossRef]
- Rossini, P. Methodological and physiological aspects of motor evoked potentials. Electroencephalogr. Clin. Neurophysiol. 1989, 41, 124–133. [Google Scholar]
- Rossi, S.; della Volpe, R.; Ginanneschi, F.; Ulivelli, M.; Bartalini, S.; Spidalieri, R.; Rossi, A. Early somatosensory processing during tonic muscle path in humans: Relation to loss of proprioception and motor ‘defensive’ strategies. Clin. Neurophysiol. 2003, 114, 1351–1358. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E. GPOWER: A Priori-, Post hoc-, and Compromise Power Analyses for MS-DOS. Ph. D. Thesis, Bonn University, Bonn, Germany, 1992. [Google Scholar]
- Hunter, S.K. Sex differences in human fatigability: Mechanisms and insight to physiological responses. Acta Physiol. 2014, 210, 768–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, L.; Murphy, B.; Passmore, S.; Yielder, P. Differences in corticomotor excitability between hemispheres following performance of a novel motor training task. Neurosci. Biomed. Eng. 2017, 5, 116–125. [Google Scholar] [CrossRef]
- Cohen, L.A. Role of eye and neck proprioceptive mechanisms in body orientation and motor coordination. J. Neurophysiol. 1961, 24, 1–11. [Google Scholar] [CrossRef]
- Vernon, H. The Neck Disability Index: State-of-the-art, 1991-2008. J. Manip. Physiol. Ther. 2008, 31, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Holland, L.; Murphy, B.; Passmore, S.; Yielder, P. Time course of corticospinal excitability changes following a novel motor training task. Neurosci. Lett. 2015, 591, 81–85. [Google Scholar] [CrossRef]
- Nuwer, M.R.; Aminoff, M.; Desmedt, J.; Eisen, A.A.; Goodin, D.; Matsuoka, S.; Mauguière, F.; Shibasaki, H.; Sutherling, W.; Vibert, J.-F. IFCN recommended standards for short latency somatosensory evoked potentials. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 6–11. [Google Scholar] [CrossRef]
- Wagner, W. SEP testing in deeply comatose and brain dead patients: The role of nasopharyngeal, scalp and earlobe derivations in recording the P14 potential. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1991, 80, 352–363. [Google Scholar] [CrossRef]
- Sonoo, M.; Sakuta, M.; Shimpo, T.; Genba, K.; Mannen, T. Widespread N18 in median nerve SEP is preserved in a pontine lesion. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1991, 80, 238–240. [Google Scholar] [CrossRef]
- Noël, P.; Ozaki, I.; Desmedt, J.E. Origin of N18 and P14 far-fields of median nerve somatosensory evoked potentials studied in patients with a brain-stem lesion. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 167–170. [Google Scholar] [CrossRef]
- Mauguiere, F. Somatosensory evoked potentials: Normal responses, abnormal waveforms and clinical applications in neurological diseases. In Electrocephalography: Basic Principles, Clinical Applications and Related Fields; Niedermeyer, E., Lopes Da Silva, F., Eds.; Williams and Wilkins: Baltimore, MD, USA, 1999; pp. 1014–1058. [Google Scholar]
- Uesaka, Y.; Terao, Y.; Ugawa, Y.; Yumoto, M.; Hanajima, R.; Kanazawa, I. Magnetoencephalographic analysis of cortical myoclonic jerks. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 141–148. [Google Scholar] [CrossRef]
- Waberski, T.D.; Buchner, H.; Perkuhn, M.; Gobbelé, R.; Wagner, M.; Kücker, W.; Silny, J. N30 and the effect of explorative finger movements: A model of the contribution of the motor cortex to early somatosensory potentials. Clin. Neurophysiol. 1999, 110, 1589–1600. [Google Scholar] [CrossRef]
- Cebolla, A.-M.; Palmero-Soler, E.; Dan, B.; Cheron, G. Frontal phasic and oscillatory generators of the N30 somatosensory evoked potential. NeuroImage 2011, 54, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Larrea, L.G.; Bastuji, H.; Mauguière, F. Unmasking of cortical SEP components by changes in stimulus rate: A topographic study. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1992, 84, 71–83. [Google Scholar] [CrossRef]
- Haavik, H.; Murphy, B. Selective changes in cerebellar-cortical processing following motor training. Exp. Brain Res. 2013, 231, 397–403. [Google Scholar] [CrossRef] [PubMed]
- MauguieÁre, F.; Allison, T.; Babilon, C. Somatosensory evoked potentials. In Recommendations for the Practice of Clinical Neurophysiology: Guidelines of the IFCN; IFCN: St. Petersburg, FL, USA, 1996; pp. 79–90. [Google Scholar]
- Gandevia, S.; Burke, D.; Keon, B.M. The projection of muscle afferents from the hand to cerebral cortex in man. Brain 1984, 107, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Andrew, D.; Haavik, H.; Dancey, E.; Yielder, P.; Murphy, B. Somatosensory evoked potentials show plastic changes following a novel motor training task with the thumb. Clin. Neurophysiol. 2015, 126, 575–580. [Google Scholar] [CrossRef]
- Holland, L. Cortical adaption influences excitability in the dominant and non-dominant hands following complex novel motor training, in Faculty of Health Sciences. In Fulfilment of Master of Health Sciences; University of Ontario Institute of Technology: Oshawa, ON, Canada, 2014; p. 104. [Google Scholar]
- Tanaka, H.; Kazui, T.; Sato, H.; Inoue, N.; Yamada, O.; Komatsu, S. Experimental study on the optimum flow rate and pressure for selective cerebral perfusion. Ann. Thorac. Surg. 1995, 59, 651–657. [Google Scholar] [CrossRef]
- Cruccu, G.; Aminoff, M.; Curio, G.; Guerit, J.; Kakigi, R.; Mauguiere, F.; Rossini, P.; Treede, R.-D.; Garcia-Larrea, L. Recommendations for the clinical use of somatosensory-evoked potentials. Clin. Neurophysiol. 2008, 119, 1705–1719. [Google Scholar] [CrossRef]
- Valeriani, M.; Restuccia, D.; di Lazzaro, V.; le Pera, D.; Barba, C.; Tonali, P. The scalp to earlobe montage as standard in routine SEP recording. Comparison with the non-cephalic reference in patients with lesions of the upper cervical cord. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1998, 108, 414–422. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Pedersen, J.; Lönn, J.; Hellström, F.; Djupsjöbacka, M.; Johansson, H. Localized muscle fatigue decreases the acuity of the movement sense in the human shoulder. Med. Sci. Sports Exerc. 1999, 31, 1047–1052. [Google Scholar] [CrossRef]
- Hayward, L.; Wesselmann, U.; Rymer, W. Effects of muscle fatigue on mechanically sensitive afferents of slow conduction velocity in the cat triceps surae. J. Neurophysiol. 1991, 65, 360–370. [Google Scholar] [CrossRef]
- Zabihhosseinian, M.; Holmes, M.W.; Murphy, B. Neck muscle fatigue alters upper limb proprioception. Exp. Brain Res. 2015, 233, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.; Mota, J.; Oliveira, J. Effect of exercise-induced fatigue on position sense of the knee in the elderly. Eur. J. Appl. Physiol. 2007, 99, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Baarbé, J.; Yielder, P.; Daligadu, J.; Behbahani, H.; Haavik, H.; Murphy, B. A novel protocol to investigate motor training-induced plasticity and sensorimotor integration in the cerebellum and motor cortex. J. Neurophysiol. 2013, 111, 715–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miall, R.; Weir, D.J.; Wolpert, D.M.; Stein, J. Is the cerebellum a smith predictor? J. Mot. Behav. 1993, 25, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, D. The cerebellum and sensorimotor coupling: Looking at the problem from the perspective of vestibular reflexes. Cerebellum 2007, 6, 24–37. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z. Computational principles of movement neuroscience. Nat. Neurosci. 2000, 3, 1212. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.-J.; Sirigu, A. Action prediction in the cerebellum and in the parietal lobe. Exp. Brain Res. 2003, 153, 239–245. [Google Scholar] [CrossRef]
- Restuccia, D.; Marca, G.D.; Valeriani, M.; Leggio, M.G.; Molinari, M. Cerebellar damage impairs detection of somatosensory input changes. A somatosensory mismatch-negativity study. Brain 2007, 130, 276–287. [Google Scholar] [CrossRef] [Green Version]
- Manzoni, D. The cerebellum may implement the appropriate coupling of sensory inputs and motor responses: Evidence from vestibular physiology. Cerebellum 2005, 4, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.G.; Latash, M.L. Testing hypotheses and the advancement of science: Recent attempts to falsify the equilibrium point hypothesis. Exp. Brain Res. 2005, 161, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M. Principles of Neural Science; McGraw-Hill: New York, NY, USA, 2000; Volume 4. [Google Scholar]
- Popa, L.S.; Hewitt, A.L.; Ebner, T.J. Purkinje cell simple spike discharge encodes error signals consistent with a forward internal model. Cerebellum 2013, 12, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Tracy, J.I.; Faro, S.S.; Mohammed, F.; Pinus, A.; Christensen, H.; Burkland, D. A comparison of ‘Early’and ‘Late’stage brain activation during brief practice of a simple motor task. Cogn. Brain Res. 2001, 10, 303–316. [Google Scholar] [CrossRef]
- Flament, D.; Ellermann, J.M.; Kim, S.G.; Uǧurbil, K.; Ebner, T.J. Functional magnetic resonance imaging of cerebellar activation during the learning of a visuomotor dissociation task. Hum. Brain Mapp. 1996, 4, 210–226. [Google Scholar] [CrossRef]
- Imamizu, H.; Miyauchi, S.; Tamada, T.; Sasaki, Y.; Takino, R.; PuÈtz, B.; Yoshioka, T.; Kawato, M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 2000, 403, 192. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Nguyen, T.H.; Bourguet, M. Bit-mapped color imaging of human evoked potentials with reference to the N20, P22, P27 and N30 somatosensory responses. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1987, 68, 1–19. [Google Scholar] [CrossRef]
- Lelic, D.; Niazi, I.K.; Holt, K.; Jochumsen, M.; Dremstrup, K.; Yielder, P.; Murphy, B.; Drewes, A.M.; Haavik, H. Manipulation of dysfunctional spinal joints affects sensorimotor integration in the prefrontal cortex: A brain source localization study. Neural Plast. 2016, 2016, 3704964. [Google Scholar] [CrossRef] [Green Version]
- Cebolla, A.M.; de Saedeleer, C.; Bengoetxea, A.; Leurs, F.; Balestra, C.; d’Alcantara, P.; Palmero-Soler, E.; Dan, B.; Cheron, G. Movement gating of beta/gamma oscillations involved in the N30 somatosensory evoked potential. Hum. Brain Mapp. 2009, 30, 1568–1579. [Google Scholar] [CrossRef]
- Cheron, G.; Cebolla, A.M.; de Saedeleer, C.; Bengoetxea, A.; Leurs, F.; Leroy, A.; Dan, B. Pure phase-locking of beta/gamma oscillation contributes to the N30 frontal component of somatosensory evoked potentials. BMC Neurosci. 2007, 8, 75. [Google Scholar] [CrossRef]
- Dancey, E.; Murphy, B.; Srbely, J.; Yielder, P. The effect of experimental pain on motor training performance and sensorimotor integration. Exp. Brain Res. 2014, 232, 2879–2889. [Google Scholar] [CrossRef] [Green Version]
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M.I. An internal model for sensorimotor integration. Science 1995, 269, 1880–1882. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Flanagan, J.R. Motor prediction. Curr. Biol. 2001, 11, R729–R732. [Google Scholar] [CrossRef] [Green Version]
- Kawato, M.; Furukawa, K.; Suzuki, R. A hierarchical neural-network model for control and learning of voluntary movement. Biol. Cybern. 1987, 57, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Selen, L.; Beek, P.; van Dieën, J. Fatigue-induced changes of impedance and performance in target tracking. Exp. Brain Res. 2007, 181, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, D.; Yielder, P.; Murphy, B. Do pursuit movement tasks lead to differential changes in early somatosensory evoked potentials related to motor learning compared with typing tasks? J. Neurophysiol. 2015, 113, 1156–1164. [Google Scholar] [CrossRef] [Green Version]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Place, N.; Yamada, T.; Bruton, J.D.; Westerblad, H. Muscle fatigue: From observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur. J. Appl. Physiol. 2010, 110, 1–15. [Google Scholar] [CrossRef]
- Boyas, S.; Guével, A. Neuromuscular fatigue in healthy muscle: Underlying factors and adaptation mechanisms. Ann. Phys. Rehabil. Med. 2011, 54, 88–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandevia, S. Neural control in human muscle fatigue: Changes in muscle afferents, moto neurones and moto cortical drive. Acta Physiol. Scand. 1998, 162, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Branscheidt, M.; Kassavetis, P.; Anaya, M.; Rogers, D.; Huang, H.D.; Lindquist, M.A.; Celnik, P. Fatigue induces long-lasting detrimental changes in motor-skill learning. ELife 2019, 8, e40578. [Google Scholar] [CrossRef] [PubMed]




| Control Group | Fatigue Group | t-Test Alpha Results | |
|---|---|---|---|
| (6 Females-6 Males) | (6 Females-6 Males) | ||
| Mean (SD) | Mean (SD) | ||
| Age | 20.91 (0.7) | 21.58 (0.9) | t (11) = −2, p = 0.071 |
| NDI score | 1.75 (1.6) | 2.16 (1.6) | t (11) = −0.613, p = 0.552 |
| EHI score | 63.33 (23.1) | 78.33 (18.5) | t (11) = −2.22, p = 0.062 |
| Handedness | 12 right handed | 12 right handed | |
| Time to task failure (min) | 5 min neck muscle rest | Average: 6.29 (2.8) Female: 5.82 (2.0) Male: 6.76 (3.6) | t (11) = 0.805, p = 0.457 No male–female difference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabihhosseinian, M.; Yielder, P.; Wise, R.; Holmes, M.; Murphy, B. Effect of Neck Muscle Fatigue on Hand Muscle Motor Performance and Early Somatosensory Evoked Potentials. Brain Sci. 2021, 11, 1481. https://doi.org/10.3390/brainsci11111481
Zabihhosseinian M, Yielder P, Wise R, Holmes M, Murphy B. Effect of Neck Muscle Fatigue on Hand Muscle Motor Performance and Early Somatosensory Evoked Potentials. Brain Sciences. 2021; 11(11):1481. https://doi.org/10.3390/brainsci11111481
Chicago/Turabian StyleZabihhosseinian, Mahboobeh, Paul Yielder, Rufeyda Wise, Michael Holmes, and Bernadette Murphy. 2021. "Effect of Neck Muscle Fatigue on Hand Muscle Motor Performance and Early Somatosensory Evoked Potentials" Brain Sciences 11, no. 11: 1481. https://doi.org/10.3390/brainsci11111481
APA StyleZabihhosseinian, M., Yielder, P., Wise, R., Holmes, M., & Murphy, B. (2021). Effect of Neck Muscle Fatigue on Hand Muscle Motor Performance and Early Somatosensory Evoked Potentials. Brain Sciences, 11(11), 1481. https://doi.org/10.3390/brainsci11111481







