A Novel Method to Assess Motor Cortex Connectivity and Event Related Desynchronization Based on Mass Models
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Data: Acquisition, Processing and Connectivity Estimates
2.1.1. Experimental Protocol and EEG Data Measurement
2.1.2. EEG Preprocessing and Source Reconstruction
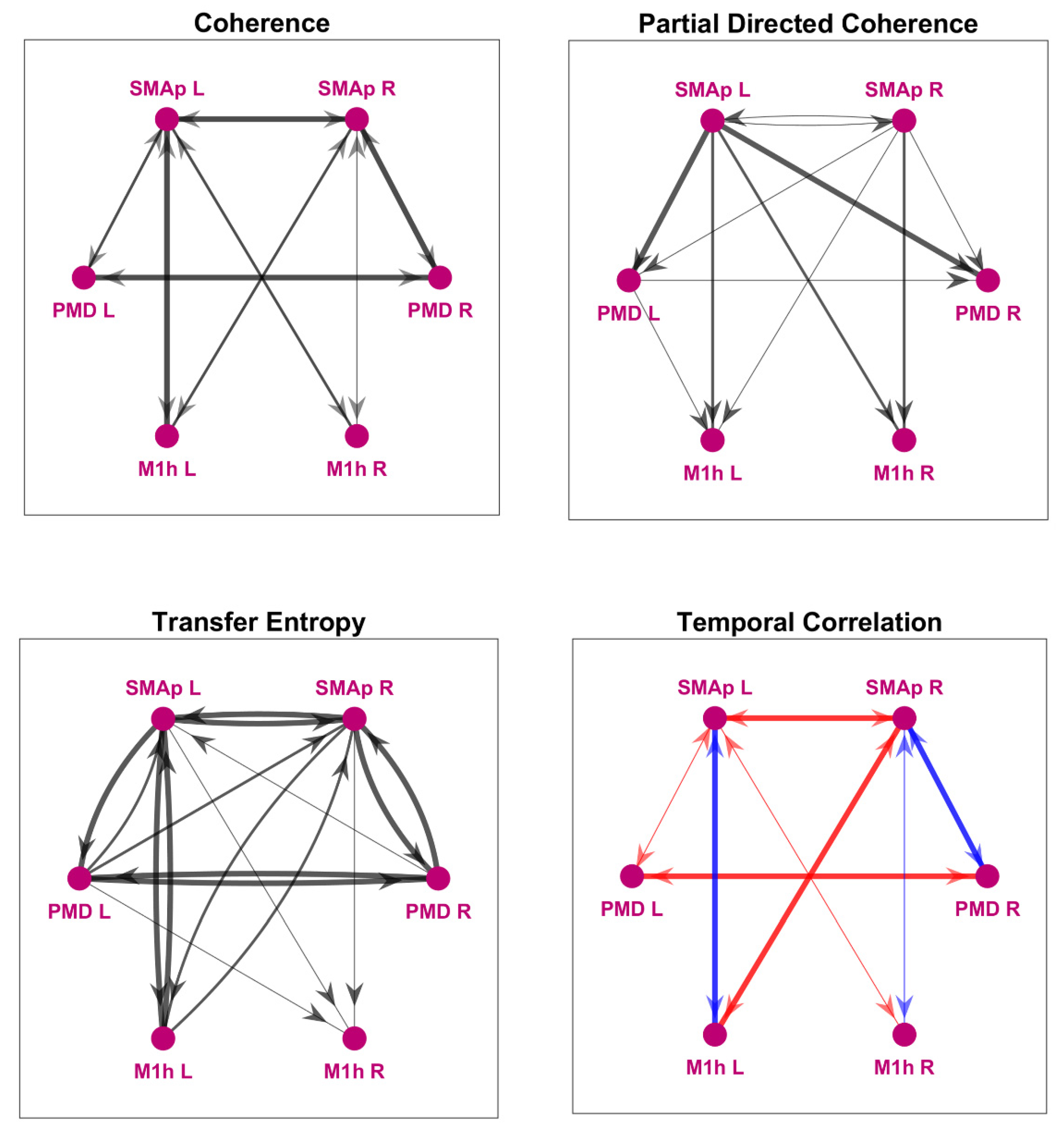
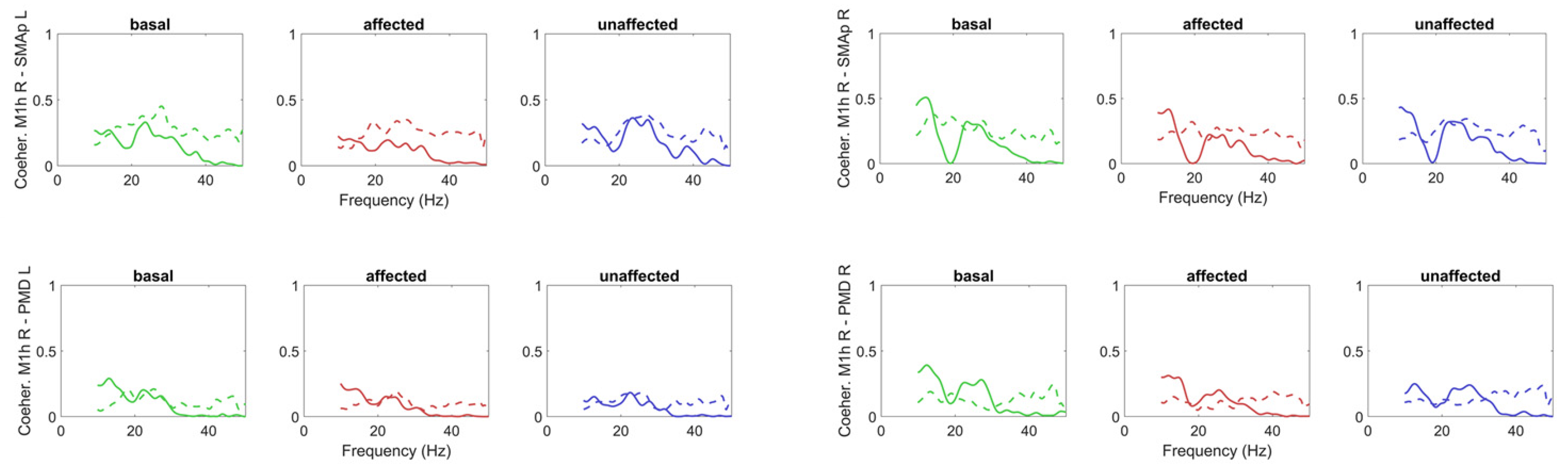
2.1.3. Model-Free Network Analysis
2.2. The Neural Mass Model and Model-Based Connectivity Estimation
2.2.1. Qualitative Description of the Neural Mass Model
2.2.2. Parameter Estimation Method
Assumptions on Parameters and Network Topology
Fitting Procedure
3. Results
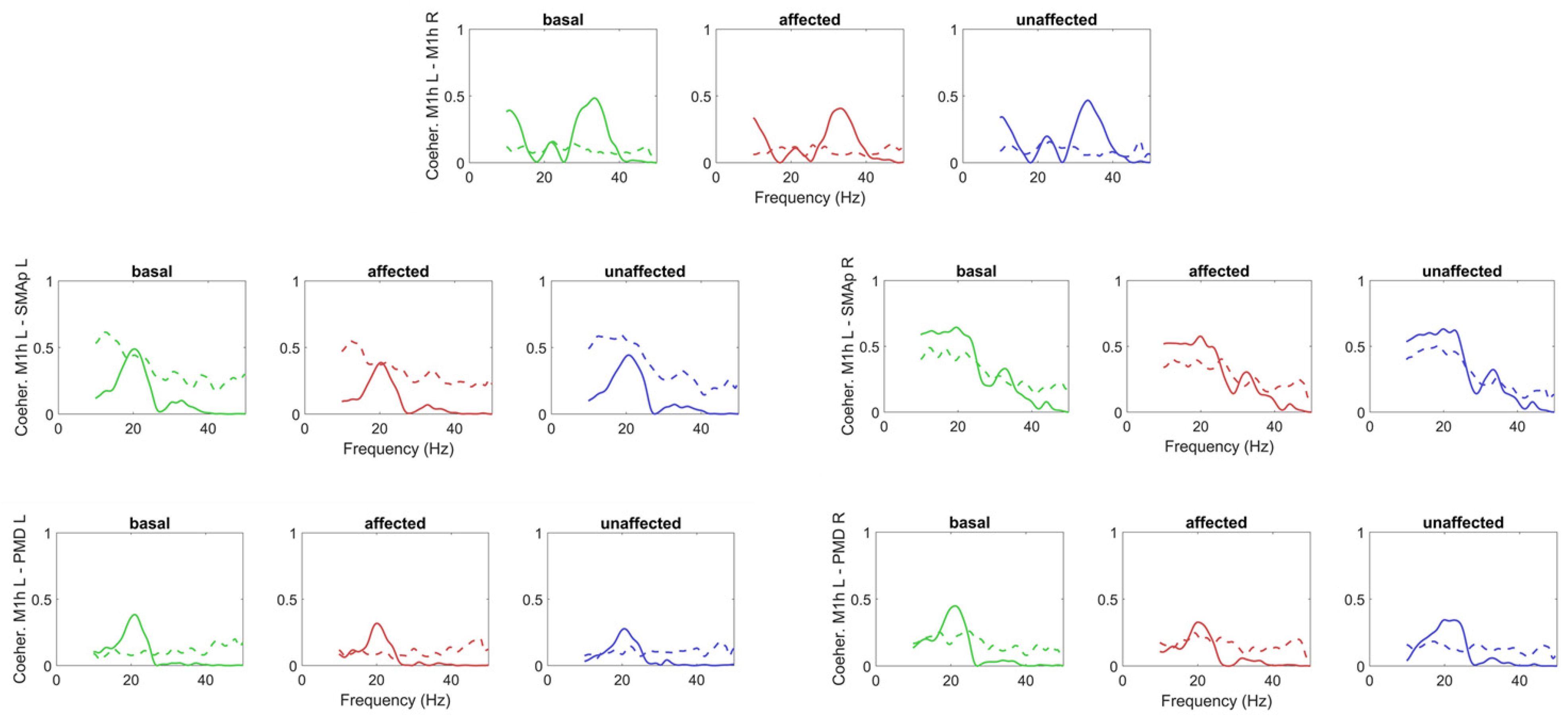
3.1. Model-Free Connectivity Estimation
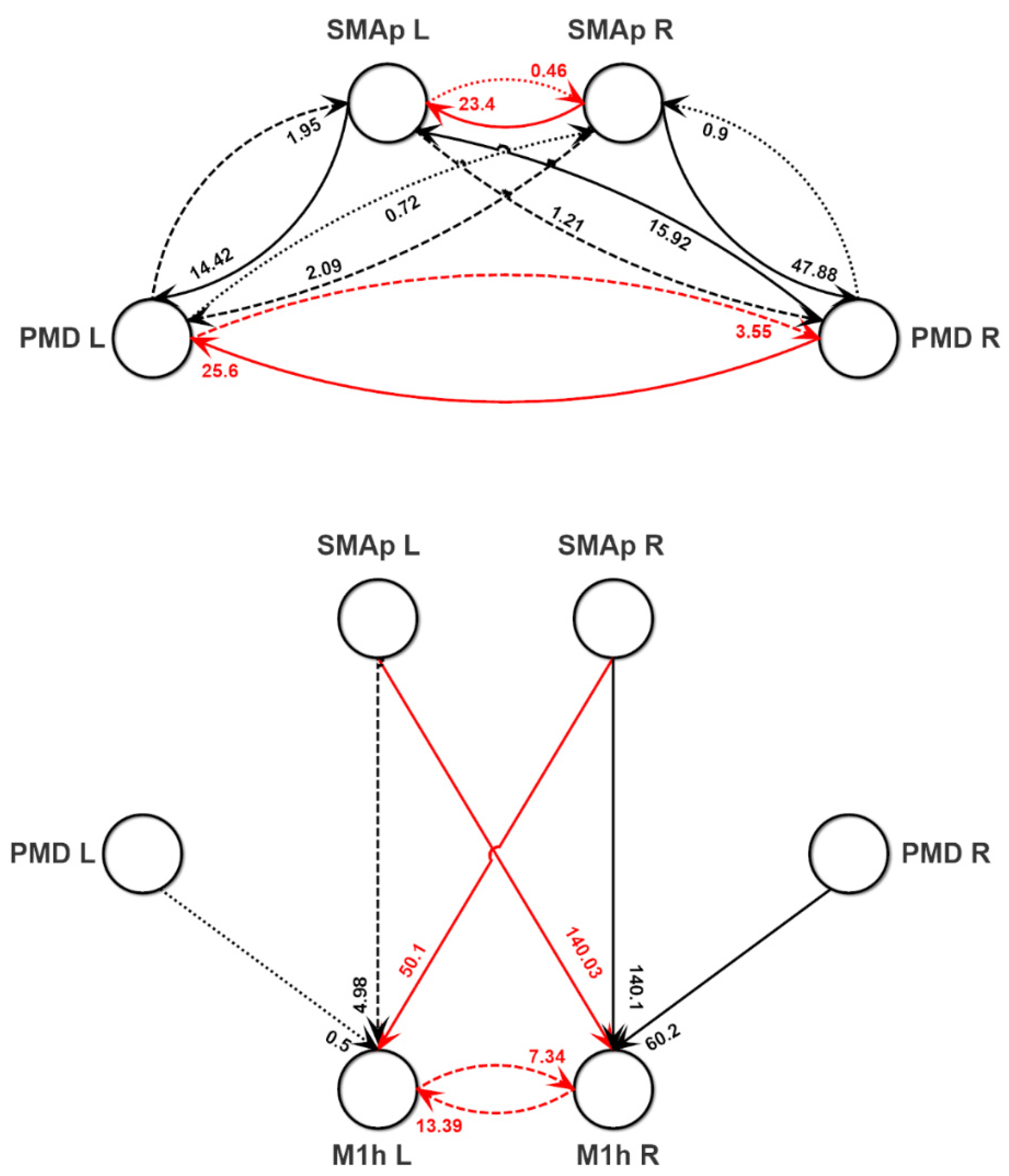
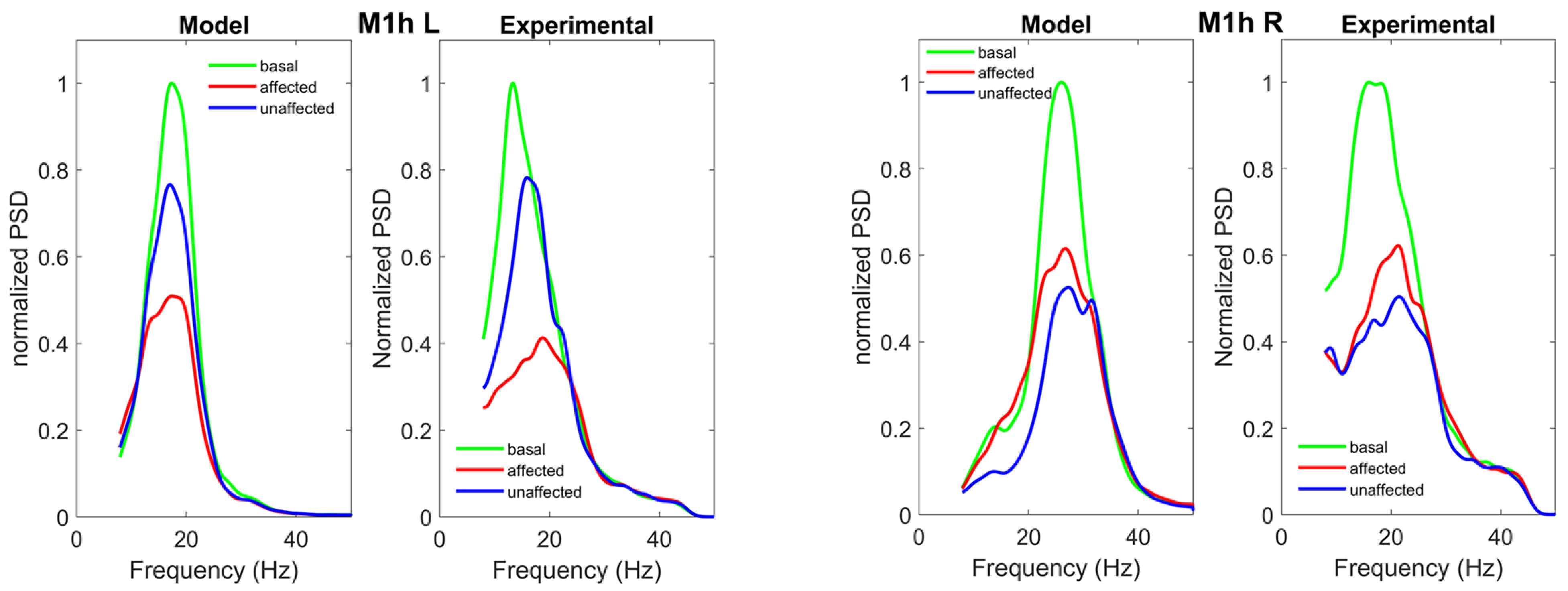
3.2. Parameter Estimation with the NMM Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desowska, A.; Turner, D.L. Dynamics of brain connectivity after stroke. Rev. Neurosci. 2019, 30, 605–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pool, E.-M.; Leimbach, M.; Binder, E.; Nettekoven, C.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Network dynamics engaged in the modulation of motor behavior in stroke patients. Hum. Brain Mapp. 2018, 39, 1078–1092. [Google Scholar] [CrossRef] [Green Version]
- Volz, L.J.; Sarfeld, A.-S.; Diekhoff, S.; Rehme, A.K.; Pool, E.-M.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Motor cortex excitability and connectivity in chronic stroke: A multimodal model of functional reorganization. Brain Struct. Funct. 2015, 220, 1093–1107. [Google Scholar] [CrossRef]
- Bajaj, S.; Butler, A.J.; Drake, D.; Dhamala, M. Brain effective connectivity during motor-imagery and execution following stroke and rehabilitation. NeuroImage Clin. 2015, 8, 572–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grefkes, C.; Eickhoff, S.B.; Nowak, D.A.; Dafotakis, M.; Fink, G.R. Dynamic intra- and Interhemispheric interactions during unilateral and bilateral hand movements assessed with FMRI and DCM. NeuroImage 2008, 41, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Grefkes, C.; Fink, G.R. Reorganization of cerebral networks after stroke: New insights from neuroimaging with connectivity approaches. Brain J. Neurol. 2011, 134, 1264–1276. [Google Scholar] [CrossRef] [Green Version]
- Pool, E.-M.; Rehme, A.K.; Fink, G.R.; Eickhoff, S.B.; Grefkes, C. Network dynamics engaged in the modulation of motor behavior in healthy subjects. NeuroImage 2013, 82, 68–76. [Google Scholar] [CrossRef]
- Rehme, A.K.; Fink, G.R.; von Cramon, D.Y.; Grefkes, C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb. Cortex 1991 2011, 21, 756–768. [Google Scholar] [CrossRef] [Green Version]
- de Vico Fallani, F.; Astolfi, L.; Cincotti, F.; Mattia, D.; la Rocca, D.; Maksuti, E.; Salinari, S.; Babiloni, F.; Vegso, B.; Kozmann, G.; et al. Evaluation of the brain network organization from EEG signals: A preliminary evidence in stroke patient. Anat. Rec. 2007 2009, 292, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Gerloff, C.; Bushara, K.; Sailer, A.; Wassermann, E.M.; Chen, R.; Matsuoka, T.; Waldvogel, D.; Wittenberg, G.F.; Ishii, K.; Cohen, L.G.; et al. Multimodal imaging of brain reorganization in motor areas of the contralesional hemisphere of well recovered patients after capsular stroke. Brain J. Neurol. 2006, 129, 791–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larivière, S.; Ward, N.S.; Boudrias, M.-H. Disrupted functional network integrity and flexibility after stroke: Relation to motor impairments. NeuroImage Clin. 2018, 19, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Pool, E.-M.; Rehme, A.K.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Functional resting-state connectivity of the human motor network: Differences between right- and left-handers. NeuroImage 2015, 109, 298–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pool, E.-M.; Rehme, A.K.; Fink, G.R.; Eickhoff, S.B.; Grefkes, C. Handedness and effective connectivity of the motor system. NeuroImage 2014, 99, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Park, E.; Lee, A.; Im, C.-H.; Kim, Y.-H. Changes in network connectivity during motor imagery and execution. PLoS ONE 2018, 13, e0190715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ursino, M.; Ricci, G.; Magosso, E. Transfer entropy as a measure of brain connectivity: A critical analysis with the help of neural mass models. Front. Comput. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, A.; Klados, M.A.; Styliadis, C.; Foroglou, N.; Polyzoidis, K.; Bamidis, P.D. Investigating the role of alpha and beta rhythms in functional motor networks. Neuroscience 2018, 378, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Carmena, J.M. Neural oscillations: Beta band activity across motor networks. Curr. Opin. Neurobiol. 2015, 32, 60–67. [Google Scholar] [CrossRef]
- Neuper, C.; Wörtz, M.; Pfurtscheller, G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Neuper, C.; Pfurtscheller, G. Event-related dynamics of cortical rhythms: Frequency-specific features and functional correlates. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2001, 43, 41–58. [Google Scholar] [CrossRef]
- Heinrichs-Graham, E.; Wilson, T.W. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. NeuroImage 2016, 134, 514–521. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, V.; Daly, I.; Pichiorri, F.; Mattia, D.; Müller-Putz, G.R.; Neuper, C. Relationship between electrical brain responses to motor imagery and motor impairment in stroke. Stroke 2012, 43, 2735–2740. [Google Scholar] [CrossRef] [Green Version]
- Ursino, M.; Cona, F.; Zavaglia, M. The generation of rhythms within a cortical region: Analysis of a neural mass model. NeuroImage 2010, 52, 1080–1094. [Google Scholar] [CrossRef]
- Pichiorri, F.; Morone, G.; Petti, M.; Toppi, J.; Pisotta, I.; Molinari, M.; Paolucci, S.; Inghilleri, M.; Astolfi, L.; Cincotti, F.; et al. Brain-computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 2015, 77, 851–865. [Google Scholar] [CrossRef]
- Hantson, L.; De Weerdt, W.; De Keyser, J.; Diener, H.C.; Franke, C.; Palm, R.; Van Orshoven, M.; Schoonderwalt, H.; De Klippel, N.; Herroelen, L. The european stroke scale. Stroke 1994, 25, 2215–2219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Toppi, J.; Risetti, M.; Quitadamo, L.R.; Petti, M.; Bianchi, L.; Salinari, S.; Babiloni, F.; Cincotti, F.; Mattia, D.; Astolfi, L. Investigating the effects of a sensorimotor rhythm-based BCI training on the cortical activity elicited by mental imagery. J. Neural Eng. 2014, 11, 035010. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Cincotti, F.; Mattia, D.; Marciani, M.G.; Baccala, L.A.; de Vico Fallani, F.; Salinari, S.; Ursino, M.; Zavaglia, M.; Ding, L.; et al. Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum. Brain Mapp. 2007, 28, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babiloni, F.; Cincotti, F.; Babiloni, C.; Carducci, F.; Mattia, D.; Astolfi, L.; Basilisco, A.; Rossini, P.M.; Ding, L.; Ni, Y.; et al. Estimation of the cortical functional connectivity with the multimodal integration of high-resolution EEG and FMRI data by directed transfer function. NeuroImage 2005, 24, 118–131. [Google Scholar] [CrossRef]
- Baccalá, L.A.; Sameshima, K. Partial directed coherence: A new concept in neural structure determination. Biol. Cybern. 2001, 84, 463–474. [Google Scholar] [CrossRef]
- Baccala, L.A.; Sameshima, K.; Takahashi, D.Y. Generalized Partial Directed Coherence; IEEE: Piscataway, NJ, USA, 2007; pp. 163–166. [Google Scholar]
- Takahashi, D.Y.; Baccalà, L.A.; Sameshima, K. Connectivity inference between neural structures via partial directed coherence. J. Appl. Stat. 2007, 34, 1259–1273. [Google Scholar] [CrossRef]
- Schreiber, T. Measuring information transfer. Phys. Rev. Lett. 2000, 85, 461–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindner, M.; Vicente, R.; Priesemann, V.; Wibral, M. TRENTOOL: A Matlab open source toolbox to analyse information flow in time series data with transfer entropy. BMC Neurosci. 2011, 12, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehme, A.K.; Eickhoff, S.B.; Wang, L.E.; Fink, G.R.; Grefkes, C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage 2011, 55, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Hellige, J.B.; Taylor, A.K.; Eng, T.L. Interhemispheric interaction when both hemispheres have access to the same stimulus information. J. Exp. Psychol. Hum. Percept. Perform. 1989, 15, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Güntürkün, O. When one hemisphere takes control: Metacontrol in pigeons (Columba Livia). PLoS ONE 2009, 4, e5307. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.S.; Hynd, G.W. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychol. Rev. 2005, 15, 59–71. [Google Scholar] [CrossRef]
- Kinsbourne, M. Hemispheric specialization and the growth of human understanding. Am. Psychol. 1982, 37, 411–420. [Google Scholar] [CrossRef]
- Welcome, S.E.; Chiarello, C. How dynamic is interhemispheric interaction? Effects of task switching on the across-hemisphere advantage. Brain Cogn. 2008, 67, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Cona, F.; Zavaglia, M.; Massimini, M.; Rosanova, M.; Ursino, M. A neural mass model of interconnected regions simulates rhythm propagation observed via TMS-EEG. NeuroImage 2011, 57, 1045–1058. [Google Scholar] [CrossRef]
- Siebner, H.R.; Peller, M.; Lee, L. Applications of combined TMS-PET studies in clinical and basic research. Suppl. Clin. Neurophysiol. 2003, 56, 63–72. [Google Scholar] [CrossRef]
- Ferbert, A.; Priori, A.; Rothwell, J.C.; Day, B.L.; Colebatch, J.G.; Marsden, C.D. Interhemispheric inhibition of the human motor cortex. J. Physiol. 1992, 453, 525–546. [Google Scholar] [CrossRef]
- Wassermann, E.M.; Fuhr, P.; Cohen, L.G.; Hallett, M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology 1991, 41, 1795–1799. [Google Scholar] [CrossRef]
- Kinsbourne, M. The cerebral basis of lateral asymmetries in attention. Acta Psychol. 1970, 33, 193–201. [Google Scholar] [CrossRef]
- Sack, A.T.; Camprodon, J.A.; Pascual-Leone, A.; Goebel, R. The dynamics of interhemispheric compensatory processes in mental imagery. Science 2005, 308, 702–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grefkes, C.; Nowak, D.A.; Eickhoff, S.B.; Dafotakis, M.; Küst, J.; Karbe, H.; Fink, G.R. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 2008, 63, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Baron, J.-C.; Rowe, J.B. Motor imagery after stroke: Relating outcome to motor network connectivity. Ann. Neurol. 2009, 66, 604–616. [Google Scholar] [CrossRef]
- Pichiorri, F.; Petti, M.; Caschera, S.; Astolfi, L.; Cincotti, F.; Mattia, D. An EEG index of sensorimotor interhemispheric coupling after unilateral stroke: Clinical and neurophysiological study. Eur. J. Neurosci. 2018, 47, 158–163. [Google Scholar] [CrossRef]
- Chollet, F.; DiPiero, V.; Wise, R.J.; Brooks, D.J.; Dolan, R.J.; Frackowiak, R.S. The functional anatomy of motor recovery after stroke in humans: A study with positron emission tomography. Ann. Neurol. 1991, 29, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Diekhoff-Krebs, S.; Pool, E.-M.; Sarfeld, A.-S.; Rehme, A.K.; Eickhoff, S.B.; Fink, G.R.; Grefkes, C. Interindividual differences in motor network connectivity and behavioral response to ITBS in stroke patients. NeuroImage Clin. 2017, 15, 559–571. [Google Scholar] [CrossRef]
- Ward, N.S.; Brown, M.M.; Thompson, A.J.; Frackowiak, R.S.J. Neural correlates of motor recovery after stroke: A longitudinal FMRI study. Brain J. Neurol. 2003, 126, 2476–2496. [Google Scholar] [CrossRef]
- Weiller, C.; Chollet, F.; Friston, K.J.; Wise, R.J.; Frackowiak, R.S. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann. Neurol. 1992, 31, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Fridman, E.A.; Hanakawa, T.; Chung, M.; Hummel, F.; Leiguarda, R.C.; Cohen, L.G. Reorganization of the human ipsilesional premotor cortex after stroke. Brain J. Neurol. 2004, 127, 747–758. [Google Scholar] [CrossRef]
- Johansen-Berg, H.; Rushworth, M.F.S.; Bogdanovic, M.D.; Kischka, U.; Wimalaratna, S.; Matthews, P.M. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. USA 2002, 99, 14518–14523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.C.; Kiebel, S.J.; Friston, K.J. Dynamic causal modelling of induced responses. NeuroImage 2008, 41, 1293–1312. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Kiebel, S.J.; Kilner, J.M.; Ward, N.S.; Stephan, K.E.; Wang, W.-J.; Friston, K.J. A dynamic causal model for evoked and induced responses. NeuroImage 2012, 59, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-C.; Kilner, J.M.; Friston, K.J.; Kiebel, S.J.; Jolly, R.K.; Ward, N.S. Nonlinear coupling in the human motor system. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 8393–8399. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Preller, K.H.; Mathys, C.; Cagnan, H.; Heinzle, J.; Razi, A.; Zeidman, P. Dynamic causal modelling revisited. NeuroImage 2019, 199, 730–744. [Google Scholar] [CrossRef]
- Kiebel, S.J.; Garrido, M.I.; Friston, K.J. Dynamic causal modelling of evoked responses: The role of intrinsic connections. NeuroImage 2007, 36, 332–345. [Google Scholar] [CrossRef]
- Pinotsis, D.A.; Loonis, R.; Bastos, A.M.; Miller, E.K.; Friston, K.J. Bayesian modelling of induced responses and neuronal rhythms. Brain Topogr. 2019, 32, 569–582. [Google Scholar] [CrossRef] [Green Version]
- van Wijk, B.C.M.; Cagnan, H.; Litvak, V.; Kühn, A.A.; Friston, K.J. Generic dynamic causal modelling: An illustrative application to Parkinson’s disease. NeuroImage 2018, 181, 818–830. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Aranibar, A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr. Clin. Neurophysiol. 1977, 42, 817–826. [Google Scholar] [CrossRef]
- Rau, C.; Plewnia, C.; Hummel, F.; Gerloff, C. Event-related desynchronization and excitability of the ipsilateral motor cortex during simple self-paced finger movements. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2003, 114, 1819–1826. [Google Scholar] [CrossRef]
- Takemi, M.; Masakado, Y.; Liu, M.; Ushiba, J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J. Neurophysiol. 2013, 110, 1158–1166. [Google Scholar] [CrossRef] [Green Version]
- Byrne, Á.; O’Dea, R.D.; Forrester, M.; Ross, J.; Coombes, S. Next-generation neural mass and field modeling. J. Neurophysiol. 2020, 123, 726–742. [Google Scholar] [CrossRef]
- Byrne, Á.; Brookes, M.J.; Coombes, S. A mean field model for movement induced changes in the beta rhythm. J. Comput. Neurosci. 2017, 43, 143–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabska-Barwińska, A.; Zygierewicz, J. A model of event-related EEG synchronization changes in beta and gamma frequency bands. J. Theor. Biol. 2006, 238, 901–913. [Google Scholar] [CrossRef]
- Mangia, A.L.; Ursino, M.; Lannocca, M.; Cappello, A. Transcallosal inhibition during motor imagery: Analysis of a neural mass model. Front. Comput. Neurosci. 2017, 11, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fries, P. Rhythms for cognition: Communication through coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendling, F.; Bartolomei, F.; Bellanger, J.J.; Chauvel, P. Epileptic fast activity can be explained by a model of impaired GABAergic dendritic inhibition. Europ. J. Neurosci. 2002, 15, 1499–1508. [Google Scholar] [CrossRef]
- Moran, R.J.; Stephan, K.E.; Kiebel, S.J.; Rombach, N.; O’Connor, W.T.; Murphy, K.J.; Reilly, R.B.; Friston, K.J. Bayesian estimation of synaptic physiology from the spectral responses of neural masses. NeuroImage 2008, 42, 272–284. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Value | Meaning |
|---|---|---|
| e0 | 2.5 Hz | Saturation of the sigmoid |
| r | 0.56 mV−1 | Parameter related with the central slope of the sigmoid |
| T | 16.6 ms | delay |
| Ge | 5.17 mV | Synaptic gain excitatory |
| Gs | 4.45 mV | Synaptic gain inhibitory slow |
| Gf | 57.1 mV | Synaptic gain inhibitory fast |
| Parameter | SMAp L | SMAp R | PMD L | PMD R | Meaning |
|---|---|---|---|---|---|
| ωe | 76.14 s−1 | 76.14 s−1 | 62.97 s−1 | 62.97 s−1 | Reciprocal of a time constant |
| ωs | 33.95 s−1 | 33.95 s−1 | 24.07 s−1 | 24.07 s−1 | |
| ωf | 336.8 s−1 | 336.8 s−1 | 734.9 s−1 | 734.9 s−1 | |
| Cep | 34.90 | 5.55 | 47.41 | 26.26 | Internal connectivity constant |
| Cpe | 12.02 | 5.46 | 29.04 | 50.73 | |
| Csp | 13.94 | 53.58 | 78.70 | 227.61 | |
| Cps | 6.92 | 53.98 | 68.80 | 123.99 | |
| Cfs | 10.38 | 5.25 | 18.52 | 4.62 | |
| Cfp | 45.02 | 40.91 | 80.80 | 55.06 | |
| Cpf | 39.06 | 28.36 | 34.24 | 72.65 | |
| Cff | 22.83 | 5.67 | 5.44 | 4.74 |
| Parameter | Rest | Movement Affected Hand | Movement Unaffected Hand | Meaning |
|---|---|---|---|---|
| 0 | 24.66 | 0 | Input mean value to a ROI | |
| 0 | 190.29 | 111.87 | ||
| 0 | 277.28 | 0 | ||
| 0 | 21.09 | 482.99 |
| Parameter | M1h L | M1h R | Meaning |
|---|---|---|---|
| ωe | 60.78 s−1 | 60.78 s−1 | Reciprocal of a time constant |
| ωs | 68.24 s−1 | 68.24 s−1 | |
| ωf | 689.50 s−1 | 689.50 s−1 | |
| Cep | 176 | 64 | Internal connectivity constant |
| Cpe | 63 | 56 | |
| Csp | 172 | 329 | |
| Cps | 114 | 116 | |
| Cfs | 20 | 20 | |
| Cfp | 44 | 204 | |
| Cpf | 68 | 60 | |
| Cff | 36 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ursino, M.; Ricci, G.; Astolfi, L.; Pichiorri, F.; Petti, M.; Magosso, E. A Novel Method to Assess Motor Cortex Connectivity and Event Related Desynchronization Based on Mass Models. Brain Sci. 2021, 11, 1479. https://doi.org/10.3390/brainsci11111479
Ursino M, Ricci G, Astolfi L, Pichiorri F, Petti M, Magosso E. A Novel Method to Assess Motor Cortex Connectivity and Event Related Desynchronization Based on Mass Models. Brain Sciences. 2021; 11(11):1479. https://doi.org/10.3390/brainsci11111479
Chicago/Turabian StyleUrsino, Mauro, Giulia Ricci, Laura Astolfi, Floriana Pichiorri, Manuela Petti, and Elisa Magosso. 2021. "A Novel Method to Assess Motor Cortex Connectivity and Event Related Desynchronization Based on Mass Models" Brain Sciences 11, no. 11: 1479. https://doi.org/10.3390/brainsci11111479
APA StyleUrsino, M., Ricci, G., Astolfi, L., Pichiorri, F., Petti, M., & Magosso, E. (2021). A Novel Method to Assess Motor Cortex Connectivity and Event Related Desynchronization Based on Mass Models. Brain Sciences, 11(11), 1479. https://doi.org/10.3390/brainsci11111479








