LINCS Dataset-Based Repositioning of Dutasteride as an Anti-Neuroinflammation Agent
Abstract
:1. Introduction
2. Materials and Methods
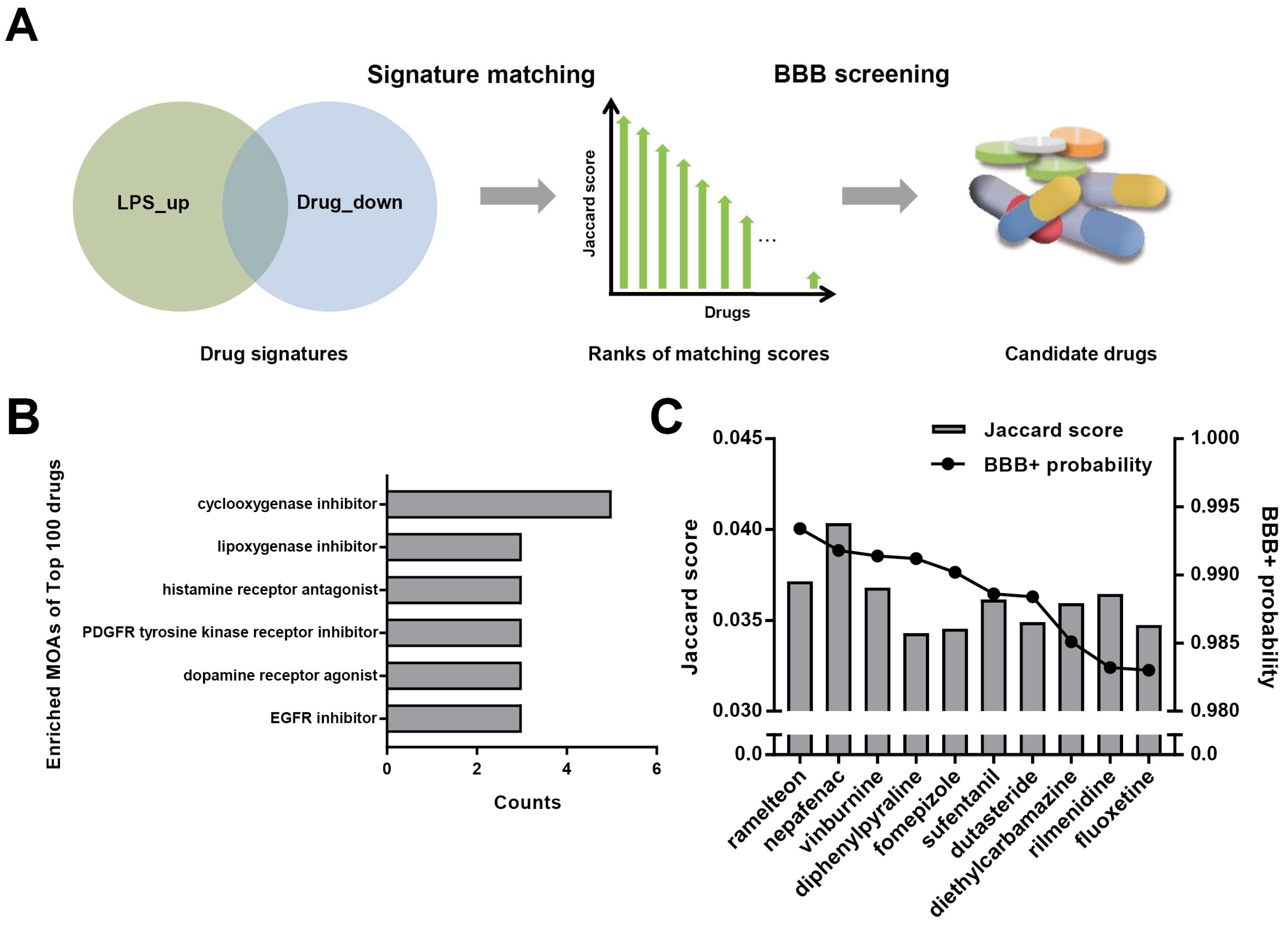
2.1. Signature Matching and BBB Penetration Prediction
2.2. Cell Culture and Drug Administration
2.3. Cell Viability
2.4. ELISA Measurement of TNF-α and IL-6
2.5. Animals and Drug Administration
2.6. Tissue and Blood Samples Collection
2.7. MWM Test
2.8. Immunofluorescence
2.9. Luminex Assay Detection of Multiple Cytokines
2.10. Statistical Analysis
3. Results
3.1. Repurposing of Dutasteride to Treat Neuroinflammation by Signature Matching and BBB Penetration Screening
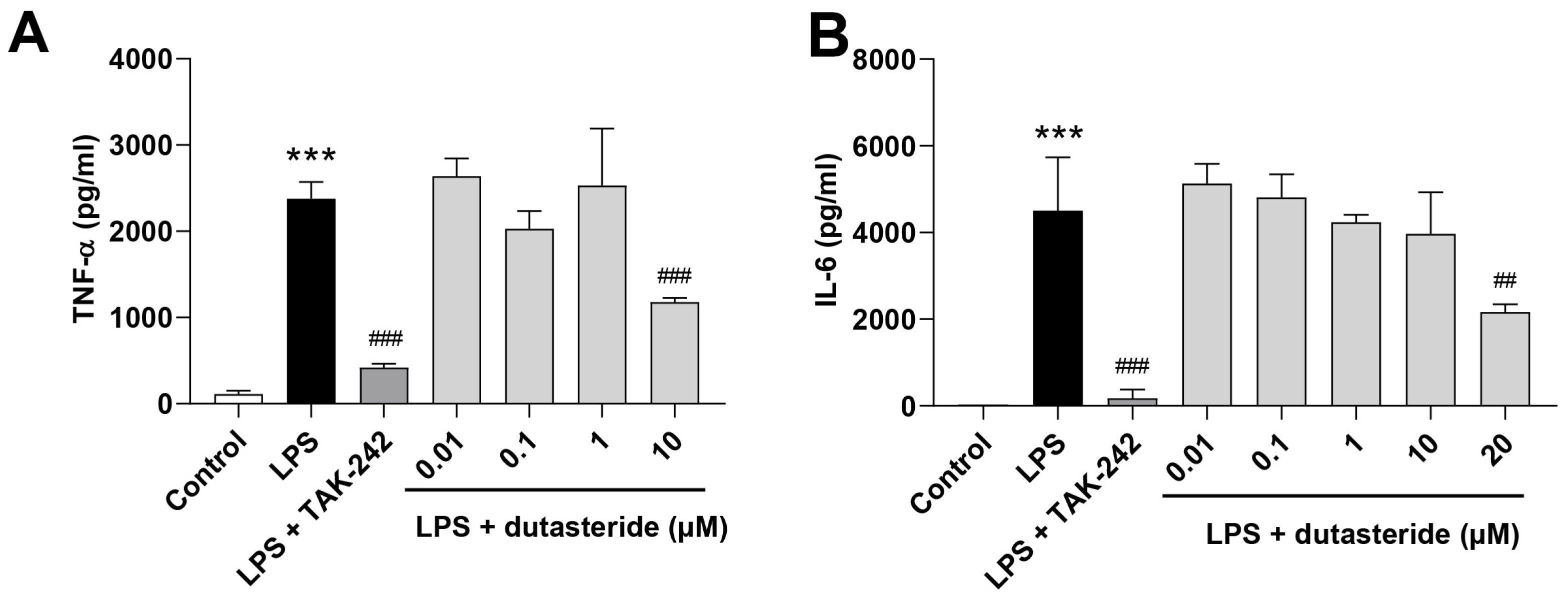
3.2. Dutasteride Reduced Cytokine Secretions in LPS-Activated BV2 Microglial Cells
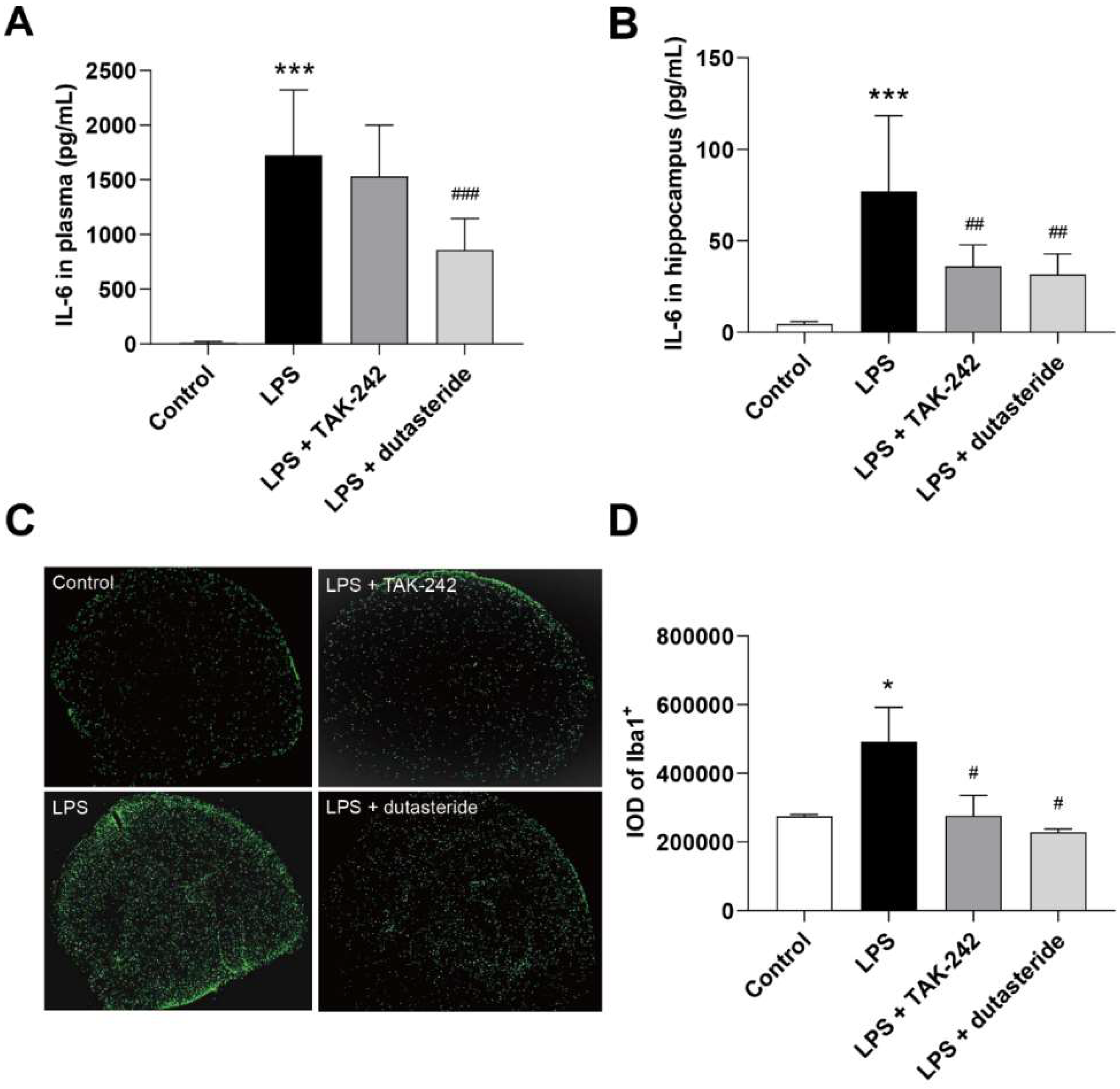
3.3. Dutasteride Decreased Level of IL-6 in Plasma and Hippocampus following LPS Administration
3.4. Dutasteride Decreased Microglia Activation in the Brain of LPS Stimulated Mice
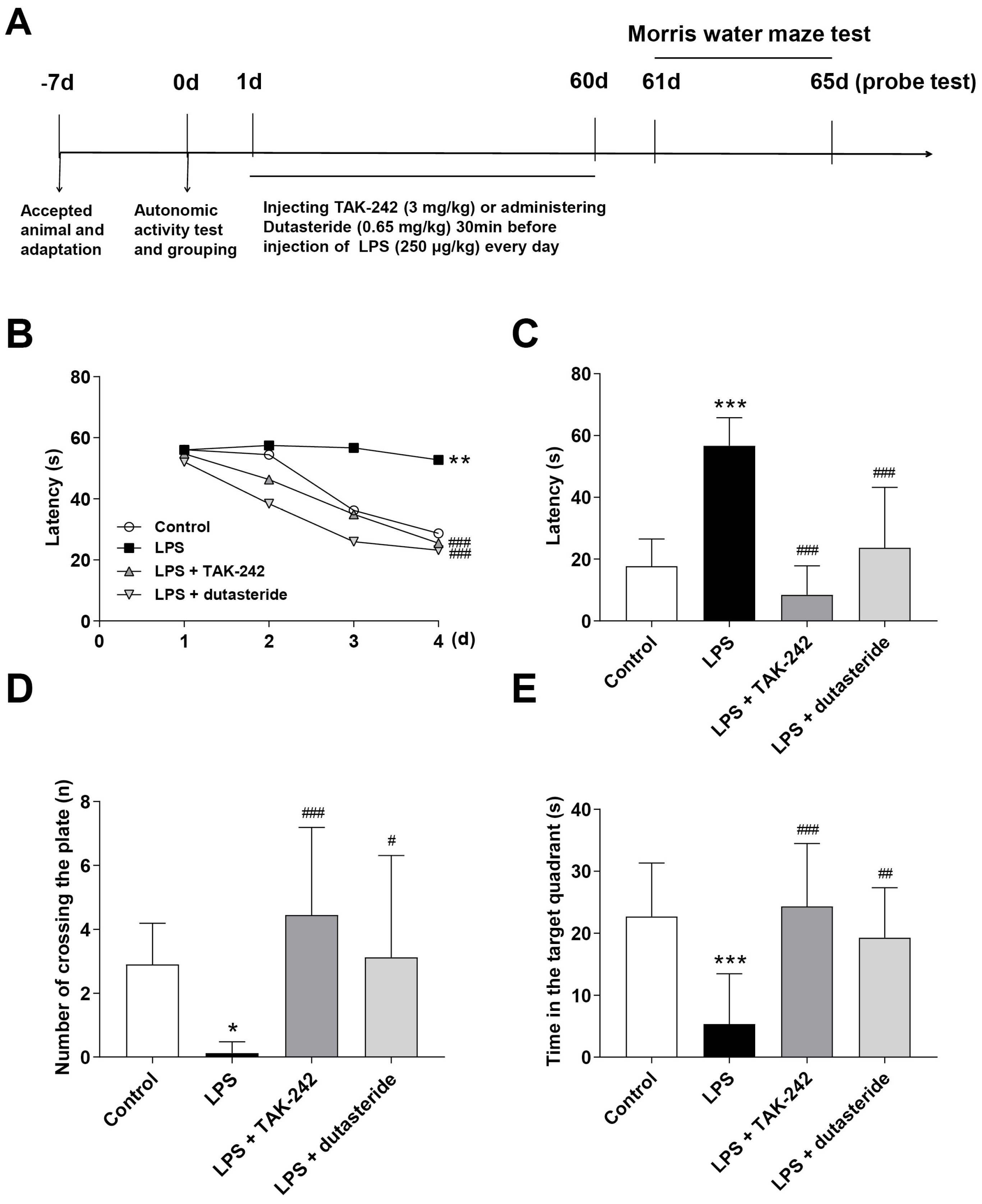
3.5. Effects of Dutasteride on Spatial Learning and Memory in LPS-induced Neuroinflammatory Mouse Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as Regulators of Neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Lehnardt, S. Innate Immunity and Neuroinflammation in the CNS: The Role of Microglia in Toll-like Receptor-Mediated Neuronal Injury. Glia 2009, 58, 253–263. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, J. Neuroinflammation in the Central Nervous System: Symphony of Glial Cells. Glia 2019, 67, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation Induced by Lipopolysaccharide Causes Cognitive Impairment in Mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. IJMS 2019, 20, 2293. [Google Scholar] [CrossRef] [Green Version]
- Valero, J.; Mastrella, G.; Neiva, I.; Sánchez, S.; Malva, J.O. Long-Term Effects of an Acute and Systemic Administration of LPS on Adult Neurogenesis and Spatial Memory. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Badshah, H.; Ali, T.; Kim, M.O. Osmotin Attenuates LPS-Induced Neuroinflammation and Memory Impairments via the TLR4/NFκB Signaling Pathway. Sci. Rep. 2016, 6, 24493. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Vicente-Rodríguez, M.; Gramage, E.; Pita, J.; Pérez-García, C.; Ferrer-Alcón, M.; Uribarri, M.; Ramos, M.P.; Herradón, G. Pleiotrophin Regulates Microglia-Mediated Neuroinflammation. J. Neuroinflamm. 2017, 14, 46. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qian, Y.; Fang, Q.; Zhong, P.; Li, W.; Wang, L.; Fu, W.; Zhang, Y.; Xu, Z.; Li, X.; et al. Saturated Palmitic Acid Induces Myocardial Inflammatory Injuries through Direct Binding to TLR4 Accessory Protein MD2. Nat. Commun. 2017, 8, 13997. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Maqbool, F.; Moeini-Nodeh, S.; Niaz, K.; Abdollahi, M.; Braidy, N.; Nabavi, S.M.; Nabavi, S.F. Targeting the TLR4 Signaling Pathway by Polyphenols: A Novel Therapeutic Strategy for Neuroinflammation. Ageing Res. Rev. 2017, 36, 11–19. [Google Scholar] [CrossRef]
- Hou, Y.; Xie, G.; Liu, X.; Li, G.; Jia, C.; Xu, J.; Wang, B. Minocycline Protects against Lipopolysaccharide-Induced Cognitive Impairment in Mice. Psychopharmacology 2016, 233, 905–916. [Google Scholar] [CrossRef]
- Abareshi, A.; Hosseini, M.; Beheshti, F.; Norouzi, F.; Khazaei, M.; Sadeghnia, H.R.; Boskabady, M.H.; Shafei, M.N.; Anaeigoudari, A. The Effects of Captopril on Lipopolysaccharide Induced Learning and Memory Impairments and the Brain Cytokine Levels and Oxidative Damage in Rats. Life Sci. 2016, 167, 46–56. [Google Scholar] [CrossRef]
- Ajmone-Cat, M.A.; Bernardo, A.; Greco, A.; Minghetti, L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals 2010, 3, 1949–1965. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The Role and Consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Ashburn, T.T.; Thor, K.B. Drug Repositioning: Identifying and Developing New Uses for Existing Drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Keenan, A.B.; Jenkins, S.L.; Jagodnik, K.M.; Koplev, S.; He, E.; Torre, D.; Wang, Z.; Dohlman, A.B.; Silverstein, M.C.; Lachmann, A.; et al. The Library of Integrated Network-Based Cellular Signatures NIH Program: System-Level Cataloging of Human Cells Response to Perturbations. Cell Syst. 2018, 6, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A Next Generation Connectivity Map: L1000 Platform and the First 1,000,000 Profiles. Cell 2017, 171, 1437–1452.e17. [Google Scholar] [CrossRef]
- Keenan, A.B.; Wojciechowicz, M.L.; Wang, Z.; Jagodnik, K.M.; Jenkins, S.L.; Lachmann, A.; Ma’ayan, A. Connectivity Mapping: Methods and Applications. Annu. Rev. Biomed. Data Sci. 2019, 2, 69–92. [Google Scholar] [CrossRef]
- Han, H.-W.; Hahn, S.; Jeong, H.Y.; Jee, J.-H.; Nam, M.-O.; Kim, H.K.; Lee, D.H.; Lee, S.-Y.; Choi, D.K.; Yu, J.H.; et al. LINCS L1000 Dataset-Based Repositioning of CGP-60474 as a Highly Potent Anti-Endotoxemic Agent. Sci. Rep. 2018, 8, 14969. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Monteiro, C.D.; Jagodnik, K.M.; Fernandez, N.F.; Gundersen, G.W.; Rouillard, A.D.; Jenkins, S.L.; Feldmann, A.S.; Hu, K.S.; McDermott, M.G.; et al. Extraction and Analysis of Signatures from the Gene Expression Omnibus by the Crowd. Nat. Commun. 2016, 7, 12846. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.; Lash, A. 6. The Gene Expression Omnibus (GEO): A Gene Expression and Hybridization Repository. In The NCBI Handbook; National Center for Biotechnology Information: Bethesda, MD, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21093/ (accessed on 14 September 2021).
- Clark, N.R.; Hu, K.S.; Feldmann, A.S.; Kou, Y.; Chen, E.Y.; Duan, Q.; Ma’ayan, A. The Characteristic Direction: A Geometrical Approach to Identify Differentially Expressed Genes. BMC Bioinform. 2014, 15, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Q.; Reid, S.P.; Clark, N.R.; Wang, Z.; Fernandez, N.F.; Rouillard, A.D.; Readhead, B.; Tritsch, S.R.; Hodos, R.; Hafner, M.; et al. L1000CDS2: LINCS L1000 Characteristic Direction Signatures Search Engine. NPJ Syst. Biol. Appl. 2016, 2. [Google Scholar] [CrossRef]
- Wang, Z.; Lachmann, A.; Keenan, A.B.; Ma’ayan, A. L1000FWD: Fireworks Visualization of Drug-Induced Transcriptomic Signatures. Bioinformatics 2018, 34, 2150–2152. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, F.; Xu, Y.; Li, W.; Tang, Y. Estimation of ADME Properties with Substructure Pattern Recognition. J. Chem. Inf. Model. 2010, 50, 1034–1041. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.; Wu, Z.; Wang, T.; Li, W.; Tang, Y.; Liu, G. In Silico Prediction of Blood-Brain Barrier Permeability of Compounds by Machine Learning and Resampling Methods. ChemMedChem 2018, 13, 2189–2201. [Google Scholar] [CrossRef]
- Gárate, I.; García-Bueno, B.; Madrigal, J.L.M.; Caso, J.R.; Alou, L.; Gómez-Lus, M.L.; Leza, J.C. Toll-like 4 Receptor Inhibitor TAK-242 Decreases Neuroinflammation in Rat Brain Frontal Cortex after Stress. J. Neuroinflamm. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Cai, J.; Wang, C.; Ruan, W.; Guan, G.; Pan, H.; Li, J.; Qian, C.; Chen, J.; Wang, L.; et al. Fluoxetine Attenuates Neuroinflammation in Early Brain Injury after Subarachnoid Hemorrhage: A Possible Role for the Regulation of TLR4/MyD88/NF-ΚB Signaling Pathway. J. Neuroinflamm. 2018, 15, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, X.; Zeng, J.; Yuan, J.; Wang, Z.; Zhou, W.; Zhang, Y. LW-AFC Effects on N-Glycan Profile in Senescence-Accelerated Mouse Prone 8 Strain, a Mouse Model of Alzheimer’s Disease. Aging Dis. 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, A.C.; Rocha, N.P.; Nicoli, J.R.; Vieira, L.Q.; Teixeira, M.M.; Teixeira, A.L. Absence of Gut Microbiota Influences Lipopolysaccharide-Induced Behavioral Changes in Mice. Behav. Brain Res. 2016, 312, 186–194. [Google Scholar] [CrossRef]
- Lei, B.; Mace, B.; Dawson, H.N.; Warner, D.S.; Laskowitz, D.T.; James, M.L. Anti-Inflammatory Effects of Progesterone in Lipopolysaccharide-Stimulated BV-2 Microglia. PLoS ONE 2014, 9, e103969. [Google Scholar] [CrossRef] [Green Version]
- Franco, R.; Fernández-Suárez, D. Alternatively Activated Microglia and Macrophages in the Central Nervous System. Progress Neurobiol. 2015, 131, 65–86. [Google Scholar] [CrossRef]
- Henn, A. The Suitability of BV2 Cells as Alternative Model System for Primary Microglia Cultures or for Animal Experiments Examining Brain Inflammation. ALTEX 2009, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Cui, M.; Zhong, S.; Feng, C.; Nwobodo, A.K.; Chen, B.; Song, Y.; Wang, Y. Dihydroartemisinin Ameliorates LPS-Induced Neuroinflammation by Inhibiting the PI3K/AKT Pathway. Metab. Brain Dis. 2020, 35, 661–672. [Google Scholar] [CrossRef]
- Lopes, P.C. LPS and Neuroinflammation: A Matter of Timing. Inflammopharmacology 2016, 24, 291–293. [Google Scholar] [CrossRef]
- Dudley, J.T.; Sirota, M.; Shenoy, M.; Pai, R.K.; Roedder, S.; Chiang, A.P.; Morgan, A.A.; Sarwal, M.M.; Pasricha, P.J.; Butte, A.J. Computational Repositioning of the Anticonvulsant Topiramate for Inflammatory Bowel Disease. Sci. Transl. Med. 2011, 3, 96ra76. [Google Scholar] [CrossRef] [Green Version]
- Vanderstocken, G.; Dvorkin-Gheva, A.; Shen, P.; Brandsma, C.-A.; Obeidat, M.; Bossé, Y.; Hassell, J.A.; Stampfli, M.R. Identification of Drug Candidates to Suppress Cigarette Smoke-Induced Inflammation via Connectivity Map Analyses. Am. J. Respir. Cell Mol. Biol. 2018, 58, 727–735. [Google Scholar] [CrossRef]
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent Advancements in Liposomes Targeting Strategies to Cross Blood-Brain Barrier (BBB) for the Treatment of Alzheimer’s Disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-Based Blood-Brain-Barrier (BBB) Crossing Strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, D.C.; Brichacek, A.L.; Mohammad, A.S.; Griffith, J.; Lucke-Wold, B.P.; Benkovic, S.A.; Geldenhuys, W.J.; Lockman, P.R.; Brown, C.M. Targeting the Blood-Brain Barrier to Prevent Sepsis-Associated Cognitive Impairment. J. Cent. Nerv Syst. Dis. 2019, 11, 117957351984065. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.K.; Jermakowicz, A.; Mookhtiar, A.K.; Nemeroff, C.B.; Schürer, S.C.; Ayad, N.G. Drug Repositioning in Glioblastoma: A Pathway Perspective. Front. Pharmacol. 2018, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Wang, J.; Wang, D.; Guo, L.; Yu, D. Melatonin Receptor Agonist Ramelteon Suppresses LPS-Induced Neuroinflammation in Astrocytes. ACS Chem. Neurosci. 2021, 12, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wang, F.; Zhang, Y.; Wei, S.; Liu, J.; Sun, H.; Wang, G.; Liu, C. Microglial MT1 Activation Inhibits LPS-induced Neuroinflammation via Regulation of Metabolic Reprogramming. Aging Cell 2021, 20. [Google Scholar] [CrossRef]
- Corti, O.; Blomgren, K.; Poletti, A.; Beart, P.M. Autophagy in Neurodegeneration: New Insights Underpinning Therapy for Neurological Diseases. J. Neurochem. 2020, 154, 354–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vas, Á.; Gulyás, B. Eburnamine Derivatives and the Brain. Med. Res. Rev. 2005, 25, 737–757. [Google Scholar] [CrossRef]
- Karabiyik, C.; Frake, R.A.; Park, S.J.; Pavel, M.; Rubinsztein, D.C. Autophagy in Ageing and Ageing-Related Neurodegenerative Diseases. Ageing Neurodegener. Dis. 2021. [Google Scholar] [CrossRef]
- Lapa, G.B.; Mathews, T.A.; Harp, J.; Budygin, E.A.; Jones, S.R. Diphenylpyraline, a Histamine H1 Receptor Antagonist, Has Psychostimulant Properties. Eur. J. Pharmacol. 2005, 506, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K. H1-Antihistamines and Inflammation: H1-Antihistamines and Inflammation. Clin. Exp. Allergy. 2001, 31, 1341–1343. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Q.; Han, C.; Yang, Y. Sufentanil Attenuates Inflammation and Oxidative Stress in Sepsis-Induced Acute Lung Injury by Downregulating KNG1 Expression. Mol. Med. Rep. 2020. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, C.-Y.; Lin, H.-Q.; Hu, J.-S.; Ip, T.-M.; Chi-Cheong Wan, D. Development of an Enzyme-Linked Immunosorbent Assay for Keap1-Nrf2 Interaction Inhibitors Identification. Redox Biol. 2020, 34, 101573. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.W.S.; de França, M.E.R.; Rodrigues, G.B.; Barbosa, K.P.S.; Nunes, A.K.S.; Pastor, A.F.; Oliveira, A.G.V.; Oliveira, W.H.; Luna, R.L.A.; Peixoto, C.A. Diethylcarbamazine Reduces Chronic Inflammation and Fibrosis in Carbon Tetrachloride- (CCl4-) Induced Liver Injury in Mice. Mediat. Inflamm. 2014, 696383. [Google Scholar] [CrossRef]
- Rodrigues, G.B.; Oliveira, E.E.; Junior, F.J.B.M.; dos Santos, L.A.M.; de Oliveira, W.H.; de França, M.E.R.; Lós, D.B.; Gabínio, B.M.; de Lira, F.C.M.L.; Peixoto, C.A. Characterization and Evaluation of Nanoencapsulated Diethylcarbamazine in Model of Acute Hepatic Inflammation. Int. Immunopharmacol. 2017, 50, 330–337. [Google Scholar] [CrossRef]
- Singh, A.; Chokriwal, A.; Sharma, M.M.; Jain, D.; Saxena, J.; Stephen, B.J. Therapeutic Role and Drug Delivery Potential of Neuroinflammation as a Target in Neurodegenerative Disorders. ACS Chem. Neurosci. 2017, 8, 1645–1655. [Google Scholar] [CrossRef]
- Wu, C.; Kapoor, A. Dutasteride for the Treatment of Benign Prostatic Hyperplasia. Expert Opin. Pharmacother. 2013, 14, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Hordinsky, M.; Whiting, D.; Stough, D.; Hobbs, S.; Ellis, M.L.; Wilson, T.; Rittmaster, R.S. The Importance of Dual 5α-Reductase Inhibition in the Treatment of Male Pattern Hair Loss: Results of a Randomized Placebo-Controlled Study of Dutasteride versus Finasteride. J. Am. Acad. Dermatol. 2006, 55, 1014–1023. [Google Scholar] [CrossRef]
- Litim, N.; Bourque, M.; Al Sweidi, S.; Morissette, M.; Di Paolo, T. The 5α-Reductase Inhibitor Dutasteride but Not Finasteride Protects Dopamine Neurons in the MPTP Mouse Model of Parkinson’s Disease. Neuropharmacology 2015, 97, 86–94. [Google Scholar] [CrossRef]
- Mhaouty-Kodja, S. Role of the Androgen Receptor in the Central Nervous System. Mol. Cell Endocrinol. 2018, 465, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-Mediated Neuroinflammation in Neurodegenerative Diseases. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef]
- Tanaka, M.; Ogaeri, T.; Samsonov, M.; Sokabe, M. The 5α-Reductase Inhibitor Finasteride Exerts Neuroprotection Against Ischemic Brain Injury in Aged Male Rats. Transl. Stroke Res. 2019, 10, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Litim, N. Effect of the 5α-Reductase Enzyme Inhibitor Dutasteride in the Brain of Intact and Parkinsonian Mice. Neuropharmacology 2015, 97, 86–94. [Google Scholar] [CrossRef]
- Traish, A.M. Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It’s Time to Sound the Alarm. World J. Mens. Health 2020, 38, 323. [Google Scholar] [CrossRef] [Green Version]
- Fertig, R.; Shapiro, J.; Bergfeld, W.; Tosti, A. Investigation of the Plausibility of 5-Alpha-Reductase Inhibitor Syndrome. Skin Appendage Disord. 2016, 2, 120–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasibhushana, R.B.; Shankaranarayana Rao, B.S.; Srikumar, B.N. Repeated Finasteride Administration Induces Depression-like Behavior in Adult Male Rats. Behav. Brain Res. 2019, 365, 185–189. [Google Scholar] [CrossRef]
- Diviccaro, S.; Giatti, S.; Borgo, F.; Barcella, M.; Borghi, E.; Trejo, J.L.; Garcia-Segura, L.M.; Melcangi, R.C. Treatment of Male Rats with Finasteride, an Inhibitor of 5alpha-Reductase Enzyme, Induces Long-Lasting Effects on Depressive-like Behavior, Hippocampal Neurogenesis, Neuroinflammation and Gut Microbiota Composition. Psychoneuroendocrinology 2019, 99, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Keleku-Lukwete, N.; Suzuki, M.; Yamamoto, M. An Overview of the Advantages of KEAP1-NRF2 System Activation During Inflammatory Disease Treatment. Antioxid. Redox Signal. 2018, 29, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 Suppresses Macrophage Inflammatory Response by Blocking Proinflammatory Cytokine Transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Scollick, C.; Traore, K.; Yates, M.; Trush, M.A.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2-Dependent Protection from LPS Induced Inflammatory Response and Mortality by CDDO-Imidazolide. Biochem. Biophys. Res. Commun. 2006, 351, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.-C.; Zhao, J.; Liu, Y.-T.; Liu, T.; Tao, M.-M.; You, Q.-D.; Jiang, Z.-Y. CPUY192018, a Potent Inhibitor of the Keap1-Nrf2 Protein-Protein Interaction, Alleviates Renal Inflammation in Mice by Restricting Oxidative Stress and NF-ΚB Activation. Redox Biol. 2019, 26, 101266. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Han, L.; Gao, S.; Xiao, Z.; Zhou, Q.; Cheng, X.; Zhang, Y.; Zhou, W. LINCS Dataset-Based Repositioning of Dutasteride as an Anti-Neuroinflammation Agent. Brain Sci. 2021, 11, 1411. https://doi.org/10.3390/brainsci11111411
Luo D, Han L, Gao S, Xiao Z, Zhou Q, Cheng X, Zhang Y, Zhou W. LINCS Dataset-Based Repositioning of Dutasteride as an Anti-Neuroinflammation Agent. Brain Sciences. 2021; 11(11):1411. https://doi.org/10.3390/brainsci11111411
Chicago/Turabian StyleLuo, Dan, Lu Han, Shengqiao Gao, Zhiyong Xiao, Qingru Zhou, Xiaorui Cheng, Yongxiang Zhang, and Wenxia Zhou. 2021. "LINCS Dataset-Based Repositioning of Dutasteride as an Anti-Neuroinflammation Agent" Brain Sciences 11, no. 11: 1411. https://doi.org/10.3390/brainsci11111411
APA StyleLuo, D., Han, L., Gao, S., Xiao, Z., Zhou, Q., Cheng, X., Zhang, Y., & Zhou, W. (2021). LINCS Dataset-Based Repositioning of Dutasteride as an Anti-Neuroinflammation Agent. Brain Sciences, 11(11), 1411. https://doi.org/10.3390/brainsci11111411




