Thalamus Atrophy in the Peri-Pregnancy Period in Clinically Stable Multiple Sclerosis Patients: Preliminary Results
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. MRI Acquisition and Processing
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paavilainen, T.; Kurki, T.; Parkkola, R.; Färkkilä, M.; Salonen, O.; Dastidar, P.; Elovaara, I.; Airas, L. Magnetic resonance imaging of the brain used to detect early post-partum activation of multiple sclerosis. Eur. J. Neurol. 2007, 14, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Paavilainen, T.; Kurki, T.; Färkkilä, M.; Salonen, O.; Parkkola, R.; Airas, L. Lower brain diffusivity in postpartum period compared to late pregnancy: Results from a prospective imaging study of multiple sclerosis patients. Neuroradiology 2012, 54, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Houtchens, M.; Bove, R.; Healy, B.; Houtchens, S.; Kaplan, T.B.; Mahlanza, T.; Chitnis, T.; Bakshi, R. MRI activity in MS and completed pregnancy. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e890. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.; Healy, B.C.; Dupuy, S.L.; Chu, R.; Chitnis, T.; Bakshi, R.; Houtchens, M. Quantitative MRI analysis of cerebral lesions and atrophy in post-partum patients with multiple sclerosis. J. Neurol. Sci. 2018, 392, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Bontempi, D.; Benini, S.; Signoroni, A.; Svanera, M.; Muckli, L. Cerebrum: A fast and fully-volumetric Convolutional Encoder-decodeR for weakly-supervised sEgmentation of BRain strUctures from out-of-the-scanner MRI. Med. Image Anal. 2020, 62, 101688. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.S.; Fotenos, A.F.; Csernansky, J.G.; Morris, J.C.; Buckner, R.L. Open Access Series of Imaging Studies: Longitudinal MRI Data in Nondemented and Demented Older Adults. J. Cogn. Neurosci. 2010, 22, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar] [CrossRef]
- Roy, A.G.; Conjeti, S.; Navab, N.; Wachinger, C. QuickNAT: A fully convolutional network for quick and accurate segmentation of neuroanatomy. NeuroImage 2019, 186, 713–727. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Burggraaff, J.; Liu, Y.; Prieto, J.C.; Simoes, J.; de Sitter, A.; Ruggieri, S.; Brouwer, I.; Lissenberg-Witte, B.I.; Rocca, M.A.; Valsasina, P.; et al. Manual and automated tissue segmentation confirm the impact of thalamus atrophy on cognition in multiple sclerosis: A multicenter study. NeuroImage Clin. 2021, 29, 102549. [Google Scholar] [CrossRef] [PubMed]
- Koskimäki, F.; Bernard, J.; Yong, J.; Arndt, N.; Carroll, T.; Lee, S.-K.; Reder, A.T.; Javed, A. Gray matter atrophy in multiple sclerosis despite clinical and lesion stability during natalizumab treatment. PLoS ONE 2018, 13, e0209326. [Google Scholar] [CrossRef] [PubMed]
- Oatridge, A.; Holdcroft, A.; Saeed, N.; Hajnal, J.V.; Puri, B.K.; Fusi, L.; Bydder, G.M. Change in Brain Size during and after Pregnancy: Study in Healthy Women and Women with Preeclampsia. Am. J. Neuroradiol. 2002, 23, 19–26. [Google Scholar] [PubMed]
- Cifelli, A.; Arridge, M.; Jezzard, P.; Esiri, M.M.; Palace, J.; Matthews, P.M. Thalamic neurodegeneration in multiple sclerosis. Ann. Neurol. 2002, 52, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Hammond, K.E.; Metcalf, M.; Carvajal, L.; Okuda, D.; Srinivasan, R.; Vigneron, D.; Nelson, S.J.; Pelletier, D. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann. Neurol. 2008, 64, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.J.; Cen, S.Y.; Bs, S.K.; Liu, S.; Kornak, J.; Shi, Y.; Zheng, L.; Hauser, S.L.; Pelletier, D. Thalamic atrophy in multiple sclerosis: A magnetic resonance imaging marker of neurodegeneration throughout disease. Ann. Neurol. 2018, 83, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Reddel, S.W.; Miller, D.H.; Chataway, J.; Riminton, S.; Barnett, Y.; Masters, L.; Barnett, M.H.; Hardy, T.A. The corpus callosum in the diagnosis of multiple sclerosis and other CNS demyelinating and inflammatory diseases. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, N.; Matthews, P.M.; Filippi, M.; Agosta, F.; De Luca, M.; Bartolozzi, M.L.; Guidi, L.; Ghezzi, A.; Montanari, E.; Cifelli, A.; et al. Evidence of early cortical atrophy in MS: Relevance to white matter changes and disability. Neurology 2003, 60, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Raz, E.; Cercignani, M.; Sbardella, E.; Totaro, P.; Pozzilli, C.; Bozzali, M.; Pantano, P. Gray- and White-Matter Changes 1 Year after First Clinical Episode of Multiple Sclerosis: MR Imaging. Radiology 2010, 257, 448–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rocca, M.A.; Comi, G.; Filippi, M. The Role of T1-Weighted Derived Measures of Neurodegeneration for Assessing Disability Progression in Multiple Sclerosis. Front. Neurol. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Gonzalez, C.; Healy, B.C.; Glanz, B.I.; Weiner, H.L.; Chitnis, T. Discontinuation of disease-modifying therapy for patients with relapsing-remitting multiple sclerosis: Effect on clinical and MRI outcomes. Mult. Scler. Relat. Disord. 2019, 35, 119–127. [Google Scholar] [CrossRef] [PubMed]
| Pre-Pregnancy | Post-Partum | Test Statistics | p-Value | |
|---|---|---|---|---|
| Number of patients, n | 12 | 12 | ||
| Age (years), mean ± SD (range) a | 31.4 ± 3.4 | |||
| (26.7 to 38.0) | ||||
| Weight (kilogram), mean ± SD (range) | 63 ± 9.98 | 65 ± 7.8 | 0.938 | |
| (58.8 to 72.5) | (61.4 to 68.2) | |||
| Disease duration (years), mean ± SD (range) b | 5.7 ± 4.9 | |||
| (1.1 to 14.9) | ||||
| Time of MRI (months), mean ± SD (range) c,d | 3.1 ± 2.4 | 3.9 ± 3.5 | ||
| (0.2 to 6.8) c | (0.5 to 12.3) d | |||
| EDSS score, median (IQR) (range) | 1.5 (1.0) | 1.5 (0.5) | Z = 0.89 | 0.371 |
| (1.0 to 4.0) | (1.0 to 5.0) | |||
| T25FW (seconds), mean ± SD (range) | 4.40 ± 0.64 | 4.52 ± 0.88 | Z = 0.08 | >0.999 |
| (3.33 to 5.13) | (3.30 to 5.60) | |||
| MRI activity | ||||
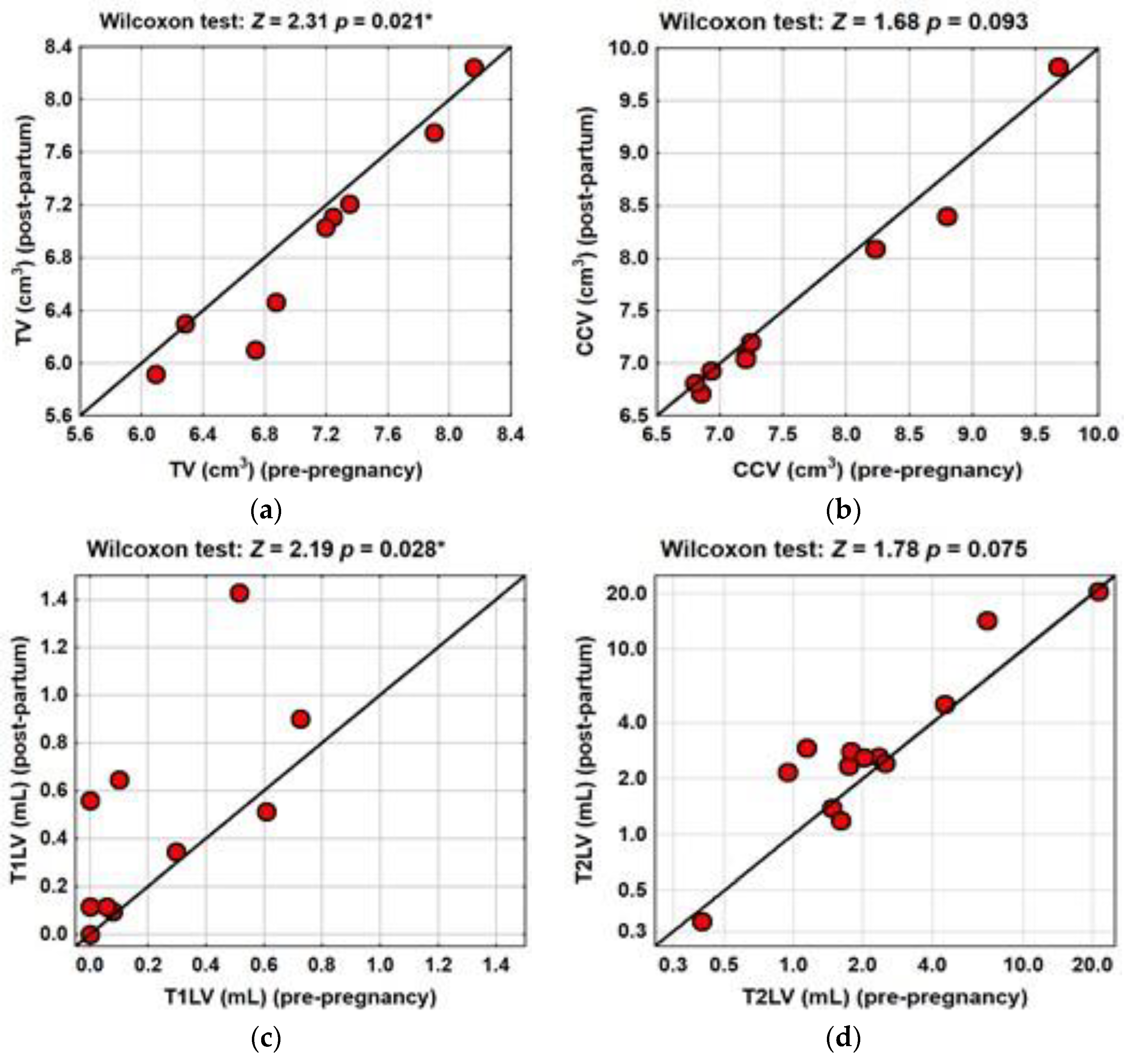
| TV (cm3), median (IQR) (range) | 7.04 (1.25) | 6.74 (1.25) | Z = 2.31 | 0.021 * |
| (5.90 to 8.16) | (5.92 to 8.2) | |||
| CCV (cm3), median (IQR) (range) | 7.20 (1.54) | 7.05 (1.33) | Z = 1.68 | 0.093 |
| (6.15 to 9.68) | (6.71 to 9.82) | |||
| T1LV (mL), median (IQR) (range) | 0.09 (0.46) | 0.34 (0.55) | Z = 2.19 | 0.028 * |
| (0.00 to 0.7) | (0.00 to 1.43) | |||
| T2LV (mL), median (IQR) (range) | 1.78 (2.22) | 2.60 (2.21) | Z = 1.78 | 0.075 |
| (0.40 to 21.22) | (0.34 to 20.47) | |||
| Number of scans with new Gd+ lesions (across all pregnancies), n (%) | 6/13 e (46.2) | |||
| Number of scans with new T2 lesions (across all pregnancies), n (%) | 10/13 e (76.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rościszewska-Żukowska, I.; Podyma, M.; Stasiołek, M.; Siger, M. Thalamus Atrophy in the Peri-Pregnancy Period in Clinically Stable Multiple Sclerosis Patients: Preliminary Results. Brain Sci. 2021, 11, 1270. https://doi.org/10.3390/brainsci11101270
Rościszewska-Żukowska I, Podyma M, Stasiołek M, Siger M. Thalamus Atrophy in the Peri-Pregnancy Period in Clinically Stable Multiple Sclerosis Patients: Preliminary Results. Brain Sciences. 2021; 11(10):1270. https://doi.org/10.3390/brainsci11101270
Chicago/Turabian StyleRościszewska-Żukowska, Iwona, Marek Podyma, Mariusz Stasiołek, and Małgorzata Siger. 2021. "Thalamus Atrophy in the Peri-Pregnancy Period in Clinically Stable Multiple Sclerosis Patients: Preliminary Results" Brain Sciences 11, no. 10: 1270. https://doi.org/10.3390/brainsci11101270
APA StyleRościszewska-Żukowska, I., Podyma, M., Stasiołek, M., & Siger, M. (2021). Thalamus Atrophy in the Peri-Pregnancy Period in Clinically Stable Multiple Sclerosis Patients: Preliminary Results. Brain Sciences, 11(10), 1270. https://doi.org/10.3390/brainsci11101270






