Prospective Evaluation of Low-Dose External Beam Radiotherapy (LD-EBRT) for Painful Trapeziometacarpal Osteoarthritis (Rhizarthrosis) on Pain, Function, and Quality of Life to Calculate the Required Number of Patients for a Prospective Randomized Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endpoints
2.2. LD-EBRT
2.3. Statistical Methods
3. Results
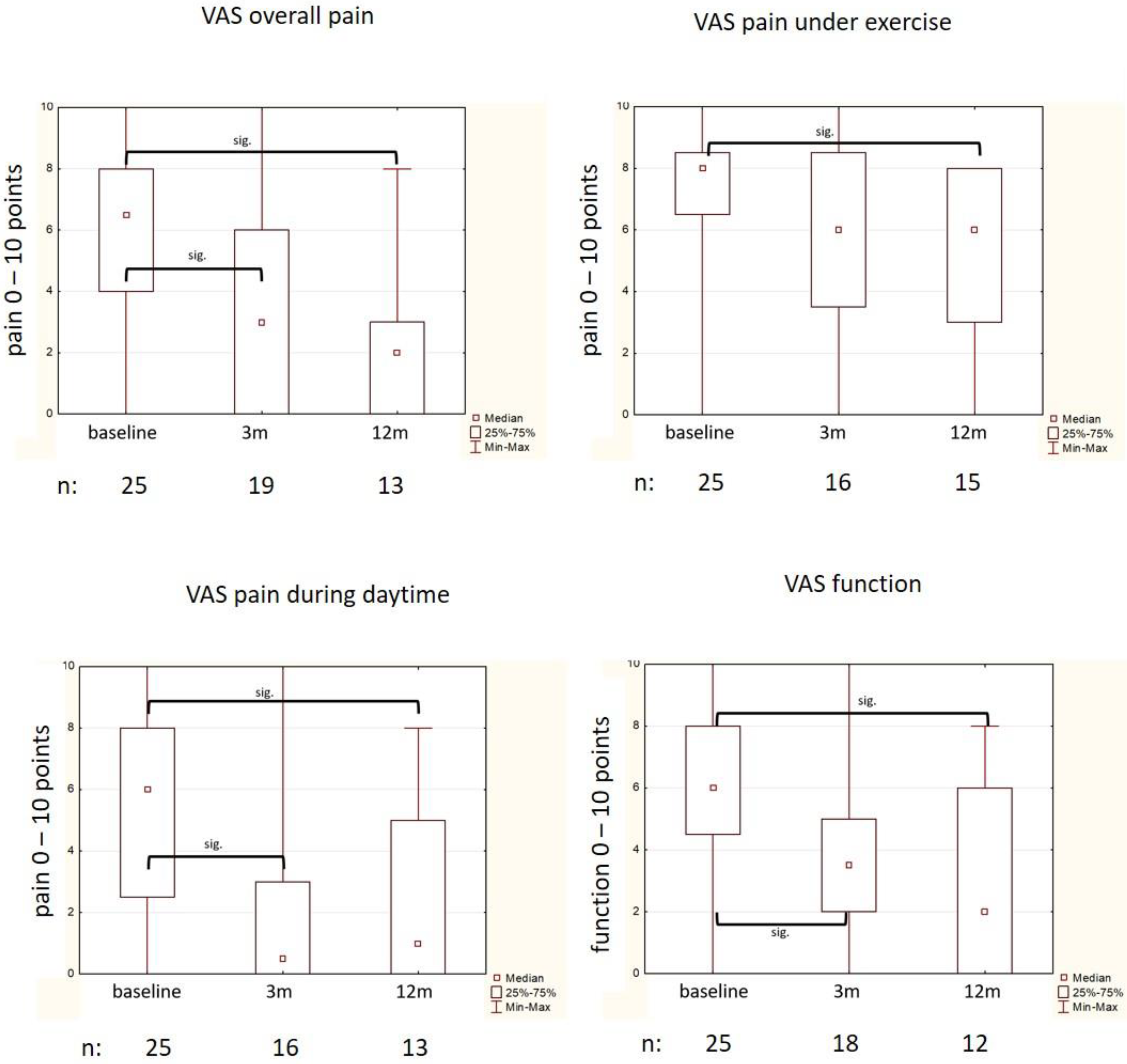
3.1. Subjective Amelioration of Symptoms (Von-Pannewitz Score, VAS)
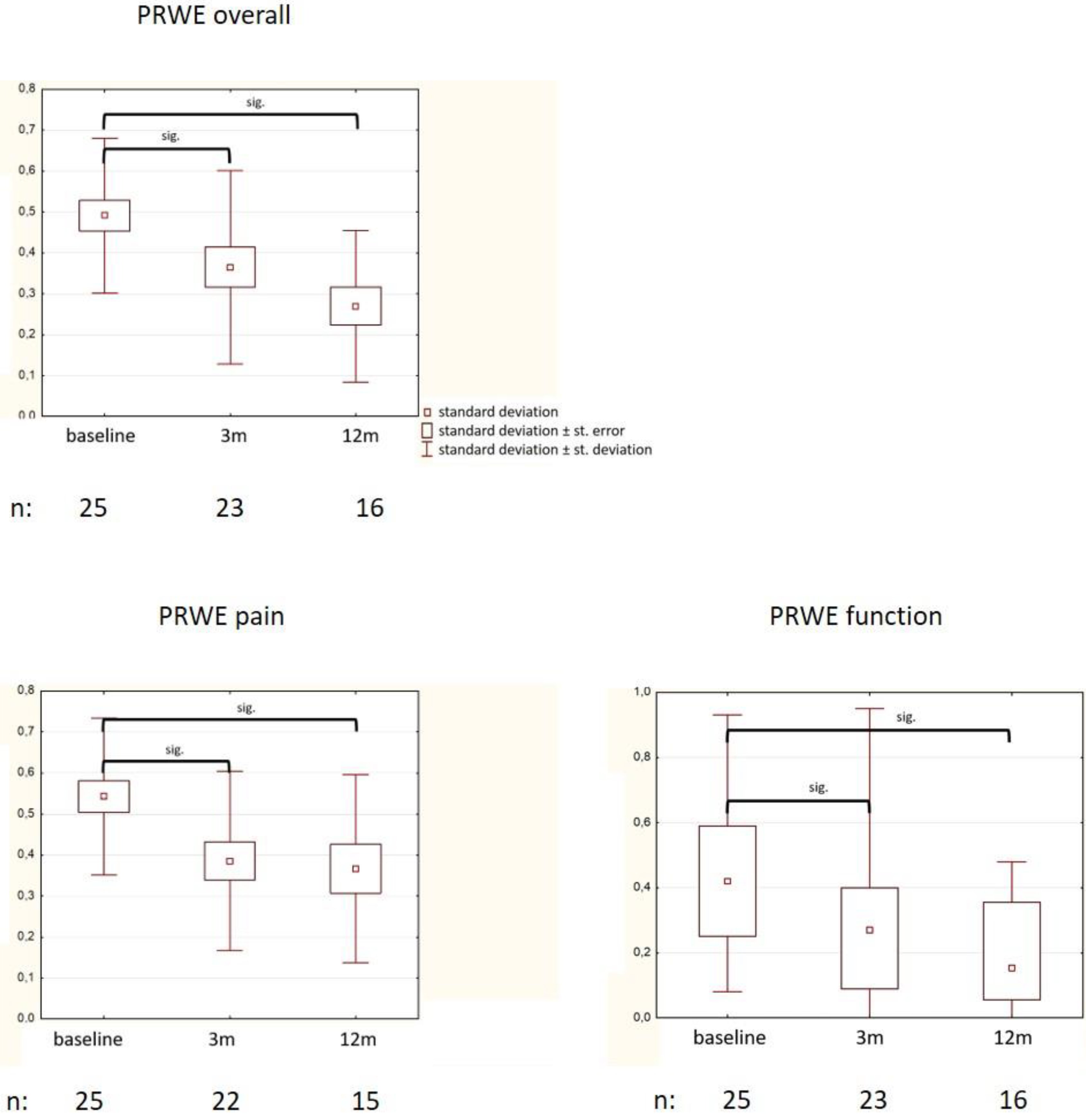
3.2. Changes in QoL (PRWE)
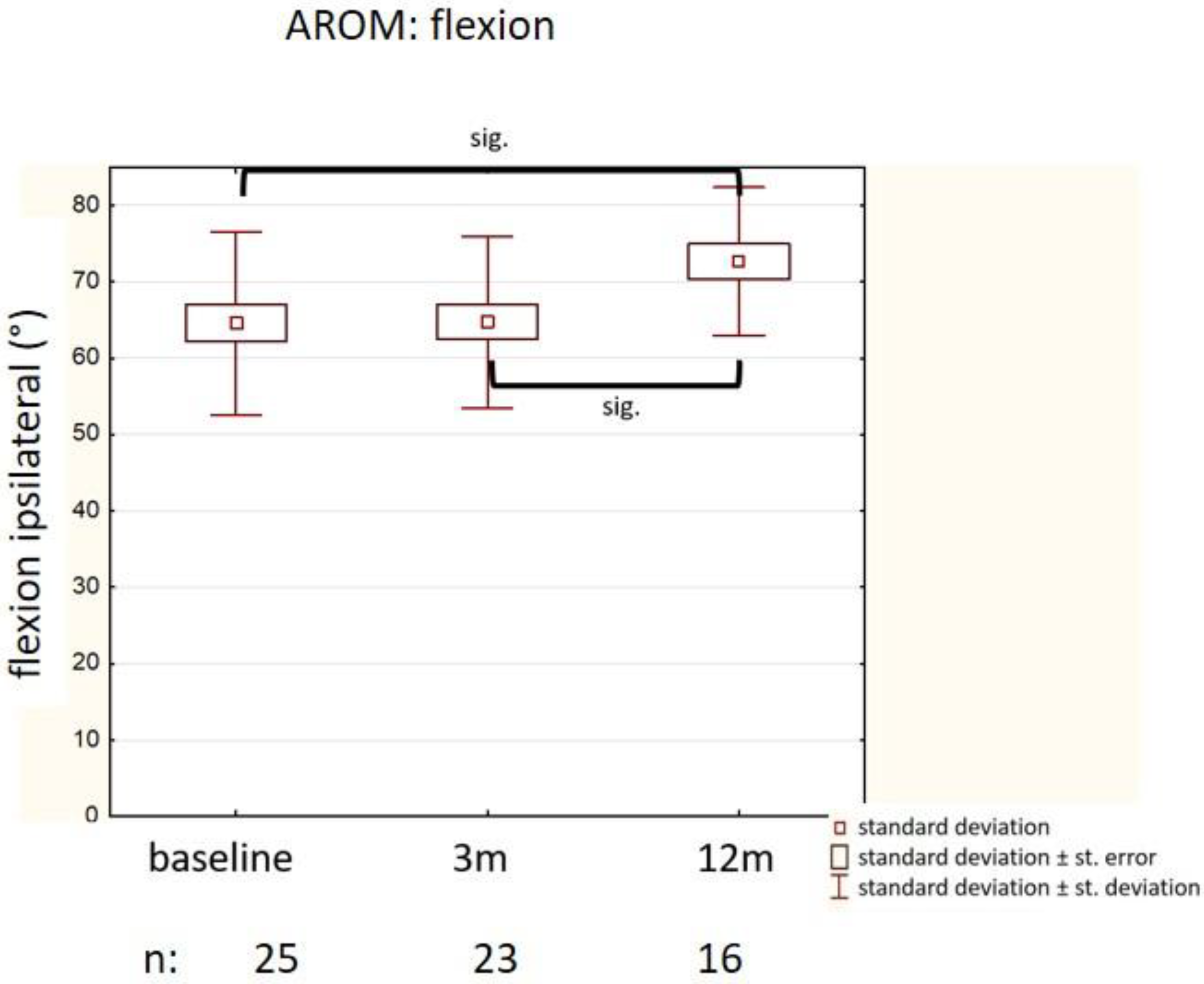
3.3. Improvement of Motion (AROM, Kapandji)
3.4. Changes in Grip Strength
4. Discussion
- Adequate patient selection seems to be crucial. Inclusion of patients with a limited number of affected joints, optimally solitary symptomatic osteoarthritis of the TMC (or another single small finger joint), and select a collective with another prognosis rather than including patients with multiple affected small finger joints. Furthermore, only patients with symptomatic osteoarthritis that has not yet become chronic should be recruited, i.e., with a maximum medical history of 2 years.
- The control group should receive sham irradiation as performed in the Dutch study (“acoustic” irradiation) [17]. Since biological effects cannot be ruled out even with extremely low radiation doses, an effect of LD-EBRT cannot otherwise be disproved in case of comparable efficacy between the two study arms.
- Since the effect of LD-EBRT is not limited to the reduction in pain but also improves the subjective functionality of the hand, we propose the PRWE score as the primary endpoint, which captures both aspects well. We assume a 15% higher efficacy after LD-EBRT than sham irradiation, taking into account the undulating clinical course of symptoms in activated arthrosis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar] [PubMed]
- Van Saase, J.L.; van Romunde, L.K.; Cats, A.; Vandenbroucke, J.P.; Valkenburg, H.A. Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann. Rheum. Dis. 1989, 48, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Niu, J.; Kelly-Hayes, M.; Chaisson, C.E.; Aliabadi, P.; Felson, D.T. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am. J. Epidemiol. 2002, 156, 1021–1027. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Doherty, M.; Leeb, B.F.; Alekseeva, L.; Arden, N.K.; Bijlsma, J.W.; Dinçer, F.; Dziedzic, K.; Häuselmann, H.J.; Herrero-Beaumont, G.; et al. EULAR evidence based recommendations for the management of hand osteoarthritis: Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 2007, 66, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Grifka, J.K.; Zacher, J.; Brown, J.P.; Seriolo, B.; Lee, A.; Moore, A.; Gimona, A. Efficacy and tolerability of lumiracoxib versus placebo in patients with osteoarthritis of the hand. Clin. Exp. Rheumatol. 2004, 22, 589–596. [Google Scholar] [PubMed]
- Østerås, N.; Kjeken, I.; Smedslund, G.; Moe, R.H.; Slatkowsky-Christensen, B.; Uhlig, T.; Hagen, K.B. Exercise for hand osteoarthritis. Cochrane Database Syst. Rev. 2017, CD010388. [Google Scholar] [CrossRef]
- Kjeken, I.; Smedslund, G.; Moe, R.H.; Slatkowsky-Christensen, B.; Uhlig, T.; Hagen, K.B. Systematic review of design and effects of splints and exercise programs in hand osteoarthritis. Arthritis Care Res. (Hoboken) 2011, 63, 834–848. [Google Scholar] [CrossRef]
- Spies, C.K.; Langer, M.; Hahn, P.; Müller, L.P.; Unglaub, F. The Treatment of Primary Arthritis of the Finger and Thumb Joint. Dtsch. Arztebl. Int. 2018, 115, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wajon, A.; Vinycomb, T.; Carr, E.; Edmunds, I.; Ada, L. Surgery for thumb (trapeziometacarpal joint) osteoarthritis. Cochrane Database Syst. Rev. 2015, CD004631. [Google Scholar] [CrossRef]
- Cook, G.S.; Lalonde, D.H. MOC-PSSM CME article: Management of thumb carpometacarpal joint arthritis. Plast. Reconstr. Surg. 2008, 121, S1–S9. [Google Scholar] [CrossRef]
- Hermann, R.M.; Bulling, E.; Kaltenborn, A.; Carl, U.M.; Nitsche, M. Efficacy of percutaneous high-voltage radiotherapy in painful degenerative diseases of the skeleton and tendons: Clinical experiences in a general patient population. Nuklearmediziner 2016, 39, 57–63. [Google Scholar]
- Javadinia, S.A.; Nazeminezhad, N.; Ghahramani-Asl, R.; Soroosh, D.; Fazilat-Panah, D.; PeyroShabany, B.; Saberhosseini, S.N.; Mehrabian, A.; Taghizadeh-Hesary, F.; Nematshahi, M.; et al. Low-dose radiation therapy for osteoarthritis and enthesopathies: A review of current data. Int. J. Radiat. Biol. 2021, 97, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Kaltenborn, A.; Bulling, E.; Nitsche, M.; Carl, U.M.; Hermann, R.M. The field size matters: Low dose external beam radiotherapy for thumb carpometacarpal osteoarthritis. Strahlenther. Onkol. 2016, 192, 582–588. [Google Scholar] [CrossRef]
- Donaubauer, A.J.; Zhou, J.G.; Ott, O.J.; Putz, F.; Fietkau, R.; Keilholz, L.; Gaipl, U.S.; Frey, B.; Weissmann, T. Low Dose Radiation Therapy, Particularly with 0.5 Gy, Improves Pain in Degenerative Joint Disease of the Fingers: Results of a Retrospective Analysis. Int J. Mol. Sci. 2020, 21, 5854. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.T.; Broerse, J.J.; Zoetelief, J.; Klein, C.; Seegenschmiedt, H.M. Estimation of the carcinogenic risk of radiotherapy of benign diseases from shoulder to heel. Radiother. Oncol. 2005, 76, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Minten, M.J.M.; Leseman-Hoogenboom, M.M.; Kloppenburg, M.; Kortekaas, M.C.; Leer, J.W.; Poortmans, P.M.P.; van den Hoogen, F.H.J.; den Broeder, A.A.; van den Ende, C.H.M. Lack of beneficial effects of low-dose radiation therapy on hand osteoarthritis symptoms and inflammation: A randomised, blinded, sham-controlled trial. Osteoarthr. Cartil. 2018, 26, 1283–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, O.J.; Micke, O.; Mücke, R.; Niewald, M.; Rödel, F.; Schäfer, U.; Seegenschmiedt, M.H.; Arenas, M.; Frey, B.; Gaipl, U.S. German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). Low-dose radiotherapy: Mayday, mayday. We’ve been hit! Strahlenther. Onkol. 2019, 195, 285–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ARTHROAD-trial: Multicentric prospective and randomized single-blinded trial on the effect of low-dose radiotherapy for painful osteoarthritis depending on the dose: Results after three months’ follow-up. Strahlenther. Onkol. 2021, accepted.
- Pannewitz, G. Die Röntgentherapie der Arthritis deformans. In Ergebnisse der Medizinischen Strahlenforschung; Holfeder, H., Holthausen, H., Jüngling, O., Martius, H., Schinz, H.R., Eds.; Thieme: Leipzig, Germany, 1933; pp. 61–126. [Google Scholar]
- John, M.; Angst, F.; Awiszus, F.; Pap, G.; Macdermid, J.C.; Simmen, B.R. The patient-rated wrist evaluation (PRWE): Cross-cultural adaptation into German and evaluation of its psychometric properties. Clin. Exp. Rheumatol. 2008, 26, 1047–1058. [Google Scholar]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C. Development of an upper extremity outcome measure: The DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am. J. Ind. Med. 1996, 30, 372. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Hautmann, M.G.; Rechner, P.; Neumaier, U.; Süß, C.; Dietl, B.; Putz, F.J.; Behr, M.; Kölbl, O.; Steger, F. Radiotherapy for osteoarthritis-an analysis of 295 joints treated with a linear accelerator. Strahlenther. Onkol. 2020, 196, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Keilholz, L.; Seegenschmiedt, M.H.; Sauer, R. Radiotherapy for painful degenerative joint disorders. Indications, technique and clinical results. Strahlenther. Onkol. 1998, 174, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, R.; Seegenschmiedt, M.H.; Sauer, R. Radiotherapy of osteoarthritis. Indication, technique and clinical results. Orthopade 2004, 33, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.W.; Na, Y.J.; Lee, Y.J.; An, S.; Lee, J.E.; Jung, M.; Kim, H.; Nam, S.Y.; Kim, C.S.; Yang, K.H.; et al. Comprehensive analysis of time- and dose-dependent patterns of gene expression in a human mesenchymal stem cell line exposed to low-dose ionizing radiation. Oncol. Rep. 2008, 19, 135–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessio, N.; Del Gaudio, S.; Capasso, S.; Di Bernardo, G.; Cappabianca, S.; Cipollaro, M.; Peluso, G.; Galderisi, U. Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget 2015, 6, 8155–8166. [Google Scholar] [CrossRef] [Green Version]
- Miousse, I.R.; Shao, L.; Chang, J.; Feng, W.; Wang, Y.; Allen, A.R.; Turner, J.; Stewart, B.; Raber, J.; Zhou, D.; et al. Exposure to low-dose (56)Fe-ion radiation induces long-term epigenetic alterations in mouse bone marrow hematopoietic progenitor and stem cells. Radiat. Res. 2014, 182, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Mean */Median# (SD */IQR#) | Min/Max | |
|---|---|---|
| Age | # 66 (18) | 47/80 |
| Sex: | ||
| Female | 17 (68%) | |
| Male | 8 (32%) | |
| BMI * | * 27.29 (4.14) | 19/35.5 |
| Previous medication: | ||
| None | 9 | |
| NSAI | 14 | |
| Steroids | 2 | |
| Previous conservative therapies: | ||
| Physiotherapy | 4 | |
| Orthesis/splints | 10 | |
| Injections | 4 | |
| Duration of symptoms before irradiation | # 12 (20) | 1/240 |
| <3 months | 4 | |
| 3–12 months | 11 | |
| >12 months | 10 | |
| VAS pain | ||
| - Overall pain | # 7 (4) | 0/10 |
| - Under exercise | # 8 (1) | 0/10 |
| - At night | # 3 (6) | 0/8 |
| - During day | # 6 (5) | 0/10 |
| - At rest | # 5 (6) | 0/9 |
| - Warm-up pain | # 0 (6) | 0/8 |
| VAS function | * 5.76 (2.66) | 0/10 |
| PRWE pain | * 0.54 (0.19) | 0/0.8 |
| PRWE function | * 0.44 (0.22) | 0.08/0.93 |
| PRWE total | * 0.5 (0.19) | 0.05/0.82 |
| AROM | Mean/Median (SD/IQR) | Min/Max | |
|---|---|---|---|
| Adduction (ipsilateral) (cm) | # 1.5 (0.8) | 0.5/2.5 | # p = 0.62 |
| Adduction (contralateral) (cm) | * 1.16 (0.56) | 0.3/2.5 | |
| Abduction (ipsilateral) (°) | # 65 (20) | 40/85 | * p = 0.01 |
| Abduction (contralateral) (°) | * 76.76 (11.9) | 55/110 | |
| Flexion (ipsilateral) (°) | * 64.6 (11.9) | 40/90 | * p = 0.0003 |
| Flexion (contralateral) (°) | * 77.8 (11.7) | 50/100 | |
| Extension (ipsilateral) (°) | # 8 (5) | 0/20 | # p = 0.003 |
| Extension (contralateral) (°) | # 10 (5) | 5/40 | |
| Kapandji (ipsilateral) | # 7.5 (2.5) | 4/9 | # p = 0.13 |
| Kapandji (contralateral) | # 8.5 (2) | 4/10 | |
| Grip strength (ipsilateral) (kg) | * 23.8 (11.9) | 5/46 | # p = 0.07 |
| Grip strength (contralateral) (kg) | # 25 (18) | 10/52 | |
| Pinch grip (ipsilateral) (kPa) | # 10 (13) | 0/30 | # p = 0.003 |
| Pinch grip (contralateral) (kPa) | # 15 (12) | 2/32 |
| N | Technique | Dose | Results | Commentary | |
|---|---|---|---|---|---|
| randomized studies | |||||
| Minten [17] | 56 | MVX | 6 × 1 Gy 3 × per week 6 × sham | Response at 3 mo: 29% (LD-EBRT) vs. 36% (n.s.) | No TMC arthrosis |
| ARTHRORAD [19] | 158 hands | MVX | 6 × 0.5 Gy 2 × per week 6 × 0.05 Gy | VAS reduction at 3 m: 18.9 points (SD 27.2) vs. 15.8 (SD 25.5) (n.s.) | Small finger joints, no exclusive recruitment of TMC arthrosis |
| observational studies | |||||
| Hautmann [24] | 35 (159 finger joints) | MVX | 6 × 1 Gy (6 × 0.5 Gy) 3 × per week | All patients 46% response during LD-EBRT, 65% within 2 years | No subgroup analysis for TMC |
| Donaubauer [15] | 236 + 127 | kV | 6 × 0.5 Gy (6 × 1 Gy) 2 × per week | 70% at least 20% response within 6 months Worse response in case of TMC arthrosis | Response dependent on involvement patterns of small finger joints |
| Keilholz [25,26] | 19 (20) | kV | 12 × 1 Gy split course 3 × per week | 6 weeks after LD-EBRT: 55% response in pain | All TMC arthrosis |
| Kaltenborn [14] | 84 (101) | MVX | 6 × 1 Gy 2 × per week | 70% at least partial response up to 1 year (30% complete response) | All TMC arthrosis |
| Own data | 25 | MVX | 6 × 0.5 Gy 2 × per week | Response at 3 mo: VAS pain sig. reduced from 7 (IQR 4) to 3 (IQR 6) PRWE pain from 0.54 (SD 0.19) to 0.32 (SD 0.22, p = 0.02) PRWE function from 0.44 (SD 0.22) to 0.29 (SD 0.22, p = 0.03) | |
| T Tests | Means: Difference between Two Independent Means (Two Groups) | |
|---|---|---|
| Analysis | A priori: Compute required sample size | |
| Input: | Tails | Two |
| Effect size d | 0.2250000 | |
| α error | 0.05 | |
| Power (1-β err prob) | 0.8 | |
| Allocation ratio N2/N1 | 1 | |
| Output: | Noncentrality parameter δ | 2.8102491 |
| Critical t | 1.9637852 | |
| Df | 622 | |
| Sample size group 1 | 312 | |
| Sample size group 2 | 312 | |
| Total sample size | 624 | |
| Actual power | 0.8012088 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermann, R.M.; Trillmann, A.; Becker, J.-N.; Kaltenborn, A.; Nitsche, M.; Ruettermann, M. Prospective Evaluation of Low-Dose External Beam Radiotherapy (LD-EBRT) for Painful Trapeziometacarpal Osteoarthritis (Rhizarthrosis) on Pain, Function, and Quality of Life to Calculate the Required Number of Patients for a Prospective Randomized Study. Med. Sci. 2021, 9, 66. https://doi.org/10.3390/medsci9040066
Hermann RM, Trillmann A, Becker J-N, Kaltenborn A, Nitsche M, Ruettermann M. Prospective Evaluation of Low-Dose External Beam Radiotherapy (LD-EBRT) for Painful Trapeziometacarpal Osteoarthritis (Rhizarthrosis) on Pain, Function, and Quality of Life to Calculate the Required Number of Patients for a Prospective Randomized Study. Medical Sciences. 2021; 9(4):66. https://doi.org/10.3390/medsci9040066
Chicago/Turabian StyleHermann, Robert Michael, Annika Trillmann, Jan-Niklas Becker, Alexander Kaltenborn, Mirko Nitsche, and Mike Ruettermann. 2021. "Prospective Evaluation of Low-Dose External Beam Radiotherapy (LD-EBRT) for Painful Trapeziometacarpal Osteoarthritis (Rhizarthrosis) on Pain, Function, and Quality of Life to Calculate the Required Number of Patients for a Prospective Randomized Study" Medical Sciences 9, no. 4: 66. https://doi.org/10.3390/medsci9040066
APA StyleHermann, R. M., Trillmann, A., Becker, J.-N., Kaltenborn, A., Nitsche, M., & Ruettermann, M. (2021). Prospective Evaluation of Low-Dose External Beam Radiotherapy (LD-EBRT) for Painful Trapeziometacarpal Osteoarthritis (Rhizarthrosis) on Pain, Function, and Quality of Life to Calculate the Required Number of Patients for a Prospective Randomized Study. Medical Sciences, 9(4), 66. https://doi.org/10.3390/medsci9040066






