The Route of Motor Recovery in Stroke Patients Driven by Exoskeleton-Robot-Assisted Therapy: A Path-Analysis
Abstract
:1. Introduction
2. Materials and Methods
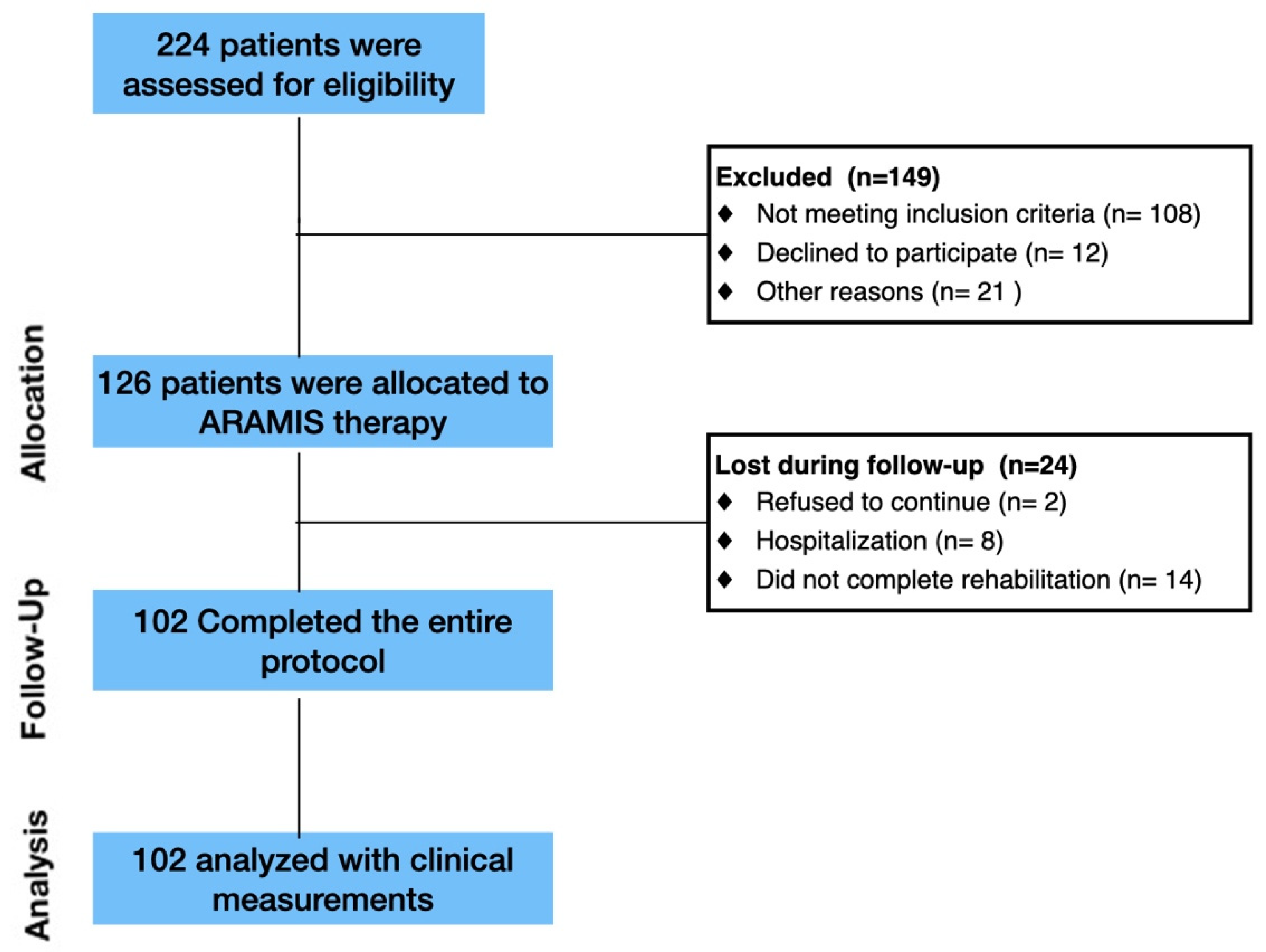
2.1. Enrollment
2.2. ARAMIS Hard/Software Structure
2.3. Design and Procedure
2.4. Data Analysis
3. Results
3.1. Clinical Data
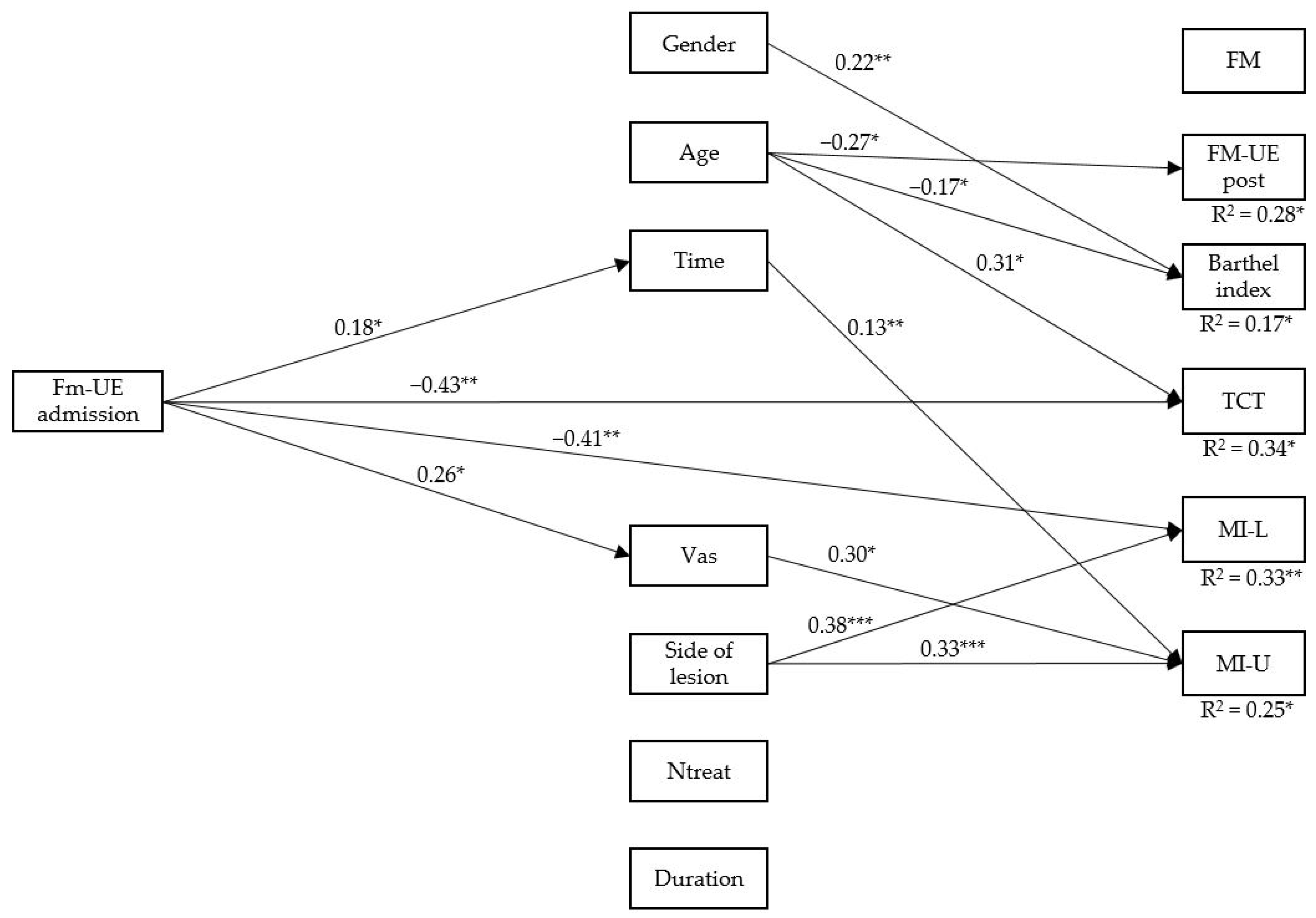
3.2. Path Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertani, R.; Melegari, C.; De Cola, M.C.; Bramanti, A.; Bramanti, P.; Calabrò, R.S. Effects of robot-assisted upper limb rehabilitation in stroke patients: A systematic review with meta-analysis. Neurol. Sci. 2017, 38, 1561–1569. [Google Scholar] [CrossRef]
- Masiero, S.; Celia, A.; Rosati, G.; Armani, M. Robotic-Assisted Rehabilitation of the Upper Limb after Acute Stroke. Arch. Phys. Med. Rehabil. 2007, 88, 142–149. [Google Scholar] [CrossRef]
- Lo, H.S.; Xie, S.Q. Exoskeleton robots for upper-limb rehabilitation: State of the art and future prospects. Med. Eng. Phys. 2012, 34, 261–268. [Google Scholar] [CrossRef]
- Pignolo, L. Robotics in neuro-rehabilitation. J. Rehabil. Med. 2009, 41, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, V.S.; Krakauer, J.W. Robotic neurorehabilitation: A computational motor learning perspective. J. Neuroeng. Rehabil. 2009, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Colizzi, L.; Lidonnici, A.; Pignolo, L. The ARAMIS project: A concept robot and technical design. J. Rehabil. Med. 2009, 41, 1011–1015. [Google Scholar] [CrossRef] [Green Version]
- Dolce, G.; Lucca, L.F.; Pignolo, L. Robot-assisted rehabilitation of the paretic upper limb: Rationale of the ARAMIS project. J. Rehabil. Med. 2009, 41, 1007–1010. [Google Scholar] [CrossRef] [Green Version]
- Stinear, C.; Barber, P.A.; Coxon, J.; Fleming, M.; Byblow, W.D. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain 2008, 131, 1381–1390. [Google Scholar] [CrossRef] [Green Version]
- Saleh, S.; Fluet, G.; Qiu, Q.; Merians, A.; Adamovich, S.V.; Tunik, E. Neural Patterns of Reorganization after Intensive Robot-Assisted Virtual Reality Therapy and Repetitive Task Practice in Patients with Chronic Stroke. Front. Neurol. 2017, 8, 452. [Google Scholar] [CrossRef] [Green Version]
- Choo, P.L.; Gallagher, H.L.; Morris, J.; Pomeroy, V.M.; Van Wijck, F. Correlations between arm motor behavior and brain function following bilateral arm training after stroke: A systematic review. Brain Behav. 2015, 5, e00411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandolfi, M.; Formaggio, E.; Geroin, C.; Storti, S.F.; Galazzo, I.B.; Bortolami, M.; Saltuari, L.; Picelli, A.; Waldner, A.; Manganotti, P.; et al. Quantification of Upper Limb Motor Recovery and EEG Power Changes after Robot-Assisted Bilateral Arm Training in Chronic Stroke Patients: A Prospective Pilot Study. Neural Plast. 2018, 2018, 8105480. [Google Scholar] [CrossRef] [Green Version]
- Pignolo, L.; Lucca, L.F.; Basta, G.; Serra, S.; Pugliese, M.E.; Sannita, W.G.; Dolce, G. A new treatment in the rehabilitation of the paretic upper limb after stroke: The ARAMIS prototype and treatment protocol. Ann. Dell’istituto Super. Sanita 2016, 52, 301–308. [Google Scholar] [CrossRef]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Cerasa, A.; Pignolo, L.; Gramigna, V.; Serra, S.; Olivadese, G.; Rocca, F.; Perrotta, P.; Dolce, G.; Quattrone, A.; Tonin, P. Exoskeleton-Robot Assisted Therapy in Stroke Patients: A Lesion Mapping Study. Front. Aging Neurosci. 2018, 12, 44. [Google Scholar] [CrossRef]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherwin, F.S. The functional independence measure: A new tool for rehabilitation. Adv. Clin. Rehabil. 1987, 1, 6–18. [Google Scholar]
- Coupar, F.; Pollock, A.; Rowe, P.; Weir, C.; Langhorne, P. Predictors of upper limb recovery after stroke: A systematic review and meta-analysis. Clin. Rehabil. 2011, 26, 291–313. [Google Scholar] [CrossRef]
- Zeleňák, K.; Krajina, A.; Meyer, L.; Fiehler, J.; ESMINT Artificial Intelligence and Robotics Ad hoc Committee; Behme, D.; Bulja, D.; Caroff, J.; Chotai, A.; Da Ros, V.; et al. How to Improve the Management of Acute Ischemic Stroke by Modern Technologies, Artificial Intelligence, and New Treatment Methods. Life 2021, 11, 488. [Google Scholar] [CrossRef]
- Wan, S.; Goudos, S. Faster R-CNN for multi-class fruit detection using a robotic vision system. Comput. Netw. 2020, 168, 107036. [Google Scholar] [CrossRef]
- Collin, C.; Wade, D.; Davies, S.; Horne, V. The Barthel ADL Index: A reliability study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef]
- Collin, C.; Wade, D. Assessing motor impairment after stroke: A pilot reliability study. J. Neurol. Neurosurg. Psychiatry 1990, 53, 576–579. [Google Scholar] [CrossRef] [Green Version]
- Hamrin, E.; Lindmark, B. Evaluation of Functional Capacity after Stroke as a Basis for Active Intervention. Scand. J. Caring Sci. 1988, 2, 113–122. [Google Scholar] [CrossRef]
- Franchignoni, F.; Tesio, L.; Ricupero, C.; Martino, M.T. Trunk Control Test as an Early Predictor of Stroke Rehabilitation Outcome. Stroke 1997, 28, 1382–1385. [Google Scholar] [CrossRef]
- Aisen, M.L.; Krebs, H.I.; Hogan, N.; McDowell, F.; Volpe, B. The Effect of Robot-Assisted Therapy and Rehabilitative Training on Motor Recovery Following Stroke. Arch. Neurol. 1997, 54, 443–446. [Google Scholar] [CrossRef]
- Volpe, B.; Krebs, H.; Hogan, N.; Edelstein, L.; Diels, C.; Aisen, M. A novel approach to stroke rehabilitation. Neurology 2000, 54, 1938–1944. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus (Version 7.2) [Computer Software]; Muthén & Muthén: Los Angeles, CA, USA, 2014. [Google Scholar]
- Hu, L.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Modeling Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Maydeu-Olivares, A. Maximum Likelihood Estimation of Structural Equation Models for Continuous Data: Standard Errors and Goodness of Fit. Struct. Equ. Modeling Multidiscip. J. 2017, 24, 383–394. [Google Scholar] [CrossRef]
- Saes, M.; Meskers, C.G.M.; Daffertshofer, A.; van Wegen, E.E.H.; Kwakkel, G. 4D-EEG consortium Are early measured resting-state EEG parameters predictive for upper limb motor impairment six months poststroke? Clin. Neurophysiol. 2021, 132, 56–62. [Google Scholar] [CrossRef]
- Pohl, J.; Held, J.P.O.; Verheyden, G.; Alt Murphy, M.; Engelter, S.; Flöel, A.; Keller, T.; Kwakkel, G.; Nef, T.; Ward, N.; et al. Consensus-Based Core Set of Outcome Measures for Clinical Motor Rehabilitation After Stroke-A Delphi Study. Front. Neurol. 2020, 11, 875. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Kwakkel, G.; van Wegen, E.E.; Ket, J.C.; Heymans, M.W. Early prediction of outcome of activities of daily living after stroke: A systematic review. Stroke 2011, 42, 1482–1488. [Google Scholar] [CrossRef] [Green Version]
- König, I.R.; Ziegler, A.; Bluhmki, E.; Hacke, W.; Bath, P.M.; Sacco, R.L.; Diener, H.C.; Weimar, C.; Virtual International Stroke Trials Archive (VISTA) Investigators. Predicting long-term outcome after acute ischemic stroke: A simple index works in patients from controlled clinical trials. Stroke 2008, 39, 1821–1826. [Google Scholar] [CrossRef]
- Luk, J.K.; Cheung, R.T.; Ho, S.; Li, L. Does Age Predict Outcome in Stroke Rehabilitation? A Study of 878 Chinese Subjects. Cerebrovasc. Dis. 2006, 21, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Poggesi, A.; Insalata, G.; Papi, G.; Rinnoci, V.; Donnini, I.; Martini, M.; Falsini, C.; Hakiki, B.; Romoli, A.; Barbato, C.; et al. Gender differences in post-stroke functional outcome at discharge from an intensive rehabilitation hospital. Eur. J. Neurol. 2021, 28, 1601–1608. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, S.H.; Noh, S.-E.; Bang, H.J.; Lee, K.-M. Robotic-Assisted Shoulder Rehabilitation Therapy Effectively Improved Poststroke Hemiplegic Shoulder Pain: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2019, 100, 1015–1022. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Fink, J.N.; Frampton, C.M.; Lyden, P.; Lees, K.R.; Virtual International Stroke Trials Archive Investigators. Does hemispheric lateralization influence functional and cardiovascular outcomes after stroke? An analysis of placebo-treated patients from prospective acute stroke trials. Stroke 2008, 39, 3335–3340. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Gu, Z.; Ni, Q. Cognitive computing and wireless communications on the edge forhealthcare service robots. Comp. Communic. 2020, 149, 99–106. [Google Scholar] [CrossRef]
- Iannuzzi, D.; Grant, A.; Corriveau, H.; Boissy, P.; Michaud, F. Specification of an integrated information architecture for a mobile teleoperated robot for home telecare. Inform. Heal. Soc. Care 2016, 41, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.-C.; Zheng, Y.; Lu, W.-W.; Zhang, T.-Y.; Wang, D.-F.; Xu, D.-S.; Li, Z.-Y. Prospects for intelligent rehabilitation techniques to treat motor dysfunction. Neural Regen. Res. 2021, 16, 264–269. [Google Scholar] [CrossRef]

| Variables | |||
| Number | 102 | ||
| Gender (% male) | 62% | ||
| Age at injury (years) | 63.6 ± 13 | ||
| Hemispheric lesion (% left) | 68% | ||
| Days from event | 21.9 ± 15.1 | ||
| Length of stay IRU (days) | 44.4 ± 20.8 | ||
| Number of sessions | 22.7 ± 10.6 | ||
| VAS at admission | 49.9 ± 25.1 | ||
| FM-UE at admission | 36.7 ± 20.7 | ||
| Before Rehabilitation | After Rehabilitation | Delta Improvement | |
| FM-UE | 36.7 ± 20.7 | 63.2 ± 25.5 | +26.6 ± 18.7 |
| FIM | 51.9 ± 24.3 | 83.5 ± 22.5 | +31.6 ± 20.2 |
| Barthel-Index | 23.8 ± 15.6 | 67.4 ± 26.2 | +43.6 ± 23.1 |
| TCT | 36.1 ± 20.8 | 80.8 ± 18.7 | +44.1 ± 17.7 |
| MI-C | 49.9 ± 40.4 | 70.5 ± 30.9 | +20.5 ± 19.3 |
| MI-I | 61.74 ± 42.6 | 74.9 ± 33.4 | +13.1 ± 20.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignolo, L.; Servidio, R.; Basta, G.; Carozzo, S.; Tonin, P.; Calabrò, R.S.; Cerasa, A. The Route of Motor Recovery in Stroke Patients Driven by Exoskeleton-Robot-Assisted Therapy: A Path-Analysis. Med. Sci. 2021, 9, 64. https://doi.org/10.3390/medsci9040064
Pignolo L, Servidio R, Basta G, Carozzo S, Tonin P, Calabrò RS, Cerasa A. The Route of Motor Recovery in Stroke Patients Driven by Exoskeleton-Robot-Assisted Therapy: A Path-Analysis. Medical Sciences. 2021; 9(4):64. https://doi.org/10.3390/medsci9040064
Chicago/Turabian StylePignolo, Loris, Rocco Servidio, Giuseppina Basta, Simone Carozzo, Paolo Tonin, Rocco Salvatore Calabrò, and Antonio Cerasa. 2021. "The Route of Motor Recovery in Stroke Patients Driven by Exoskeleton-Robot-Assisted Therapy: A Path-Analysis" Medical Sciences 9, no. 4: 64. https://doi.org/10.3390/medsci9040064
APA StylePignolo, L., Servidio, R., Basta, G., Carozzo, S., Tonin, P., Calabrò, R. S., & Cerasa, A. (2021). The Route of Motor Recovery in Stroke Patients Driven by Exoskeleton-Robot-Assisted Therapy: A Path-Analysis. Medical Sciences, 9(4), 64. https://doi.org/10.3390/medsci9040064








