Depression, Anxiety, and Social Environmental Adversity as Potential Modulators of the Immune Tumor Microenvironment in Breast Cancer Patients
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Goding Sauer, A.; Ortiz, A.P.; Fedewa, S.A.; Pinheiro, P.S.; Tortolero-Luna, G.; Martinez-Tyson, D.; Jemal, A.; Siegel, R.L. Cancer statistics for hispanics/latinos, 2018. CA Cancer J. Clin. 2018, 68, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Irwin, M.R. From Stress to Inflammation and Major Depressive Disorder A Social Signal Transduction Theory of Depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Antonova, L.; Aronson, K.; Mueller, C.R. Stress and breast cancer: From epidemiology to molecular biology. Breast Cancer Res. 2011, 13, 208. [Google Scholar] [CrossRef]
- Obeid, E.I.; Conzen, S.D. The role of adrenergic signaling in breast cancer biology. Cancer Biomark. 2013, 13, 161–169. [Google Scholar] [CrossRef]
- Armaiz-Pena, G.N.; Lutgendorf, S.K.; Cole, S.W.; Sood, A.K. Neuroendocrine modulation of cancer progression. Brain Behav. Immun. 2009, 23, 10–15. [Google Scholar] [CrossRef]
- Antoni, M.H.; Lutgendorf, S.K.; Cole, S.W.; Dhabhar, F.S.; Sephton, S.E.; McDonald, P.G.; Sood, A.K.; Stefanek, M. The influence of bio-behavioural factors on tumour biology: Pathways and mechanisms. Nat. Rev. Cancer 2006, 6, 240–248. [Google Scholar] [CrossRef]
- Powell, N.D.; Tarr, A.J.; Sheridan, J.F. Psychosocial stress and inflammation in cancer. Brain Behav. Immun. 2013, 30, S41–S47. [Google Scholar] [CrossRef]
- Pilevarzadeh, M.; Amirshahi, M.; Afsargharehbagh, R.; Rafiemanesh, H.; Hashemi, S.M.; Balouchi, A. Global prevalence of depression among breast cancer patients: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 176, 519–533. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular Pathways: Beta-Adrenergic Signaling in Cancer. Clin. Cancer Res. 2012, 18, 1201–1206. Available online: http://clincancerres.aacrjournals.org/content/18/5/1201.abstract (accessed on 1 October 2020). [CrossRef] [PubMed]
- Bouchard, L.C.; Antoni, M.H.; Blomberg, B.B.; Stagl, J.M.; Gudenkauf, L.M.; Jutagir, D.R.; Carver, C.S.; Derhagopian, R.P.; Glück, S.; Lechner, S.; et al. Postsurgical Depressive Symptoms and Proinflammatory Cytokine Elevations in Women Undergoing Primary Treatment for Breast Cancer. Psychosom. Med. 2016, 78, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Pace, T.W.; Liu, T.; Felger, J.C.; Mister, D.; Doho, G.H.; Kohn, J.N.; Barsevick, A.M.; Long, Q.; Miller, A.H. Predictors of depression in breast cancer patients treated with radiation: Role of prior chemotherapy and nuclear factor kappa B. Cancer 2013, 119, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, C.; LeRoy, A.; Karuga, M.; Armer, J.M. Behavioral Symptoms after Breast Cancer Treatment: A Biobehavioral Approach. J. Pers. Med. 2015, 5, 280–295. [Google Scholar] [CrossRef]
- Qiao, Y.; He, H.; Jonsson, P.; Sinha, I.; Zhao, C.; Dahlman-Wright, K. AP-1 is a key regulator of proinflammatory cytokine TNFα-mediated triple-negative breast cancer progression. J. Biol. Chem. 2016, 291, 5068–5079. [Google Scholar] [CrossRef]
- Hsieh, N.-T.; Huang, C.-Y.; Li, C.-I.; Weng, Y.-T.; Lee, M.-F. MED28 Regulates Proinflammatory Cytokine-induced Epithelial–Mesenchymal Transition and Invasion in Human Breast Cancer Cells. FASEB J. 2017, 31, 809.16. [Google Scholar] [CrossRef]
- Roberts, A.L.; Gilman, S.E.; Breslau, J.; Breslau, N.; Koenen, K.C. Race/ethnic differences in exposure to traumatic events, development of post-traumatic stress disorder, and treatment-seeking for post-traumatic stress disorder in the United States. Psychol. Med. 2011, 41, 71–83. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Bortolato, B.; Hyphantis, T.; Valpoine, S.; Caravalho, A. Depression in cancer: The many biobehavioral pathways driving tumor progression. Cancer Treat. Rev. 2017, 52, 58–70. [Google Scholar] [CrossRef]
- Sotelo, J.L.; Musselman, D.; Nemeroff, C. The biology of depression in cancer and the relationship between depression and cancer progression. Int. Rev. Psychiatry 2014, 26, 16–30. [Google Scholar] [CrossRef]
- Aldea, M.; Craciun, L.; Tomuleasa, C.; Crivii, C. The role of depression and neuroimmune axis in the prognosis of cancer patients. J BUON 2014, 19, 5–14. [Google Scholar] [PubMed]
- Rua Rodrigues, A.; Cristina Trufelli, D.; Fonseca, F.; de Carvalho, L.; Giglio, A. Fatigue in Patients With Advanced Terminal Cancer Correlates with Inflammation, Poor Quality of Life and Sleep, and Anxiety/Depression. Am. J. Hosp. Palliat. Med. 2015, 33, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Armer, J.S.; Schrepf, A.; Cuneo, M.G.; Christensen, D.; Thaker, P.H.; Slavich, G.M.; Lutgendorf, S.K. Anxiety levels parallel changes in inflammation over time in ovarian cancer patients. Brain Behav. Immun. 2017, 66, e27–e28. [Google Scholar] [CrossRef]
- Lamkin, D.M.; Sung, H.Y.; Yang, G.S.; David, J.M.; Ma, J.C.; Cole, S.W.; Sloan, E.K. α2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology 2017, 51, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.D.; Koopman, C.; Classen, C.; Spiegel, D. Traumatic stress, life events, and emotional support in women with metastatic breast cancer: Cancer-related traumatic stress symptoms associated with past and current stressors. Heal. Psychol. 1999, 18, 555–560. [Google Scholar] [CrossRef]
- Han, T.J.; Felger, J.C.; Lee, A.; Mister, D.; Miller, A.H.; Torres, M.A. Association of childhood trauma with fatigue, depression, stress, and inflammation in breast cancer patients undergoing radiotherapy. Psychooncology 2015, 25, 187–193. [Google Scholar] [CrossRef]
- Mundy-Bosse, B.L.; Thornton, L.M.; Yang, H.-C.; Andersen, B.L.; Carson, W.E. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011, 270, 80–87. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Hughes, S.; Jaremka, L.M.; Alfano, C.M.; Glaser, R.; Povoski, S.P.; Lipari, A.M.; Agnese, D.M.; Farrar, W.B.; Yee, L.D.; Kiecolt-Glaser, J.K. Social support predicts inflammation, pain, and depressive symptoms: Longitudinal relationships among breast cancer survivors. Psychoneuroendocrinology 2014, 42, 38–44. [Google Scholar] [CrossRef]
- Renzi, C.; Vadilonga, V.; Gandini, S.; Perinel, G.; Rotmensz, N.; Didier, F.; Rescigno, M.; Pravettoni, G. Stress exposure in significant relationships is associated with lymph node status in breast cancer. PLoS ONE 2016, 11, e0149443. [Google Scholar] [CrossRef][Green Version]
- Valente, V.B.; Verza, F.A.; Lopes, F.Y.; Ferreira, J.Z.; Dos Santos, P.S.; Sundefeld, M.L.; Biasoli, É.R.; Miyahara, G.I.; Soubhia, A.M.; de Andrade, M.; et al. Stress hormones concentrations in the normal microenvironment predict risk for chemically induced cancer in rats. Psychoneuroendocrinology 2018, 1, 229–238. [Google Scholar] [CrossRef] [PubMed]
- McDonald, P.G.; O’Connell, M.; Lutgendorf, S.K. Psychoneuroimmunology and cancer: A decade of discovery, paradigm shifts, and methodological innovations. Brain Behav. Immun. 2013, 30, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.K.; Srivastava, S.K.; Poosarla, T.; Dyess, D.L.; Holliday, N.P.; Singh, A.P.; Singh, S. Inflammation, immunosuppressive microenvironment and breast cancer: Opportunities for cancer prevention and therapy. Ann. Transl. Med. 2019, 7, 593. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): A meta-analysis. Can. Med. Assoc. J. 2011, 184, E191–E196. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.D.; Fox, R.S.; Malcarne, V.L.; Roesch, S.; Champagne, B.R.; Sadler, G.R. The Psychometric Properties of the Generalized Anxiety Disorder-7 scale in Hispanic Americans with English or Spanish Language Preference. Cult. Divers. Ethn. Minor. Psychol. 2014, 20, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.M.; Stockton, P.; Krupnick, J.L.; Green, B.L. Development, use, and psychometric properties of the Trauma History Questionnaire. J. Loss Trauma 2011, 16, 258–283. [Google Scholar] [CrossRef]
- Roy, C.A.; Perry, J.C. Instruments for the assessment of childhood trauma in adults. J. Nerv. Ment. Dis. 2004, 192, 343–351. [Google Scholar] [CrossRef]
- Our World 2021: Emerging Hope: National & Regional Demographics. La Salle Academy. 2021. Available online: https://lasalle-academy.libguides.com/ourworldpoverty/demographics (accessed on 25 May 2021).
- Lutgendorf, S.K.; Andersen, B.L. Biobehavioral approaches to cancer progression and survival: Mechanisms and interventions. Am. Psychol. 2015, 70, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Maass, S.W.M.C.; Roorda, C.; Berendsen, A.J.; Verhaak, P.F.M.; De Bock, G.H. The prevalence of long-term symptoms of depression and anxiety after breast cancer treatment: A systematic review. Maturitas 2015, 82, 100–108. [Google Scholar] [CrossRef]
- Mersky, J.; Janczewski, C. Racial and ethnic differences in the prevalence of adverse childhood experiences: Findings from a low-income sample of U.S. women. Child Abuse Negl. 2017, 76, 480–487. [Google Scholar] [CrossRef]
- Llabre, M.M.; Schneiderman, N.; Gallo, L.C.; Arguelles, W.; Daviglus, M.L.; Gonzalez, F. Childhood Trauma and Adult Risk Factors and Disease in Hispanics/Latinos in the US: Results From the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Sociocultural Ancillary Study. Psychosom. Med. 2017, 79, 172–180. [Google Scholar] [CrossRef]
- Jiao-Mei, X.; Wen, G.; Feng-Lin, C. Quality of life among breast cancer survivors 2 years after diagnosis: The relationship with adverse childhood events and posttraumatic growth. Cancer Nurs. 2016, 39, E32–E39. [Google Scholar] [CrossRef]
- Fagundes, C.; Lindgren, M.; Shapiro, C.; Kiecolt-Glaser, J. Child Maltreatment and Breast Cancer Survivors: Social Support Makes a Difference for Quality of Life, Fatigue, and Cancer Stress. Eur. J. Cancer 2011, 48, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Breiding, M.J.; Smith, S.G.; Basile, K.C.; Walters, M.L.; Chen, J.; Merrick, M.T. Prevalence and characteristics of sexual violence, stalking, and intimate partner violence victimization—National Intimate Partner and Sexual Violence Survey, United States, 2011. Am. J. Public Health 2015, 105, e11–e12. [Google Scholar]
- Cuevas, C.A.; Sabina, C.; Milloshi, R. Interpersonal victimization among a national sample of Latino women. Violence Against Women 2012, 18, 377–403. [Google Scholar] [CrossRef]
- Dey, A.; Hankey Giblin, P.A. Insights into Macrophage Heterogeneity and Cytokine-Induced Neuroinflammation in Major Depressive Disorder. Pharmaceuticals 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.G.; Stojadinovic, A.; Mason, J.; Avital, I.; Bilchik, A.; Bruecher, B.; Jewett, A.; Protic, M.; Nissan, A.; Izadjoo, M.; et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: Existing theories. J. Cancer 2013, 4, 84–95. [Google Scholar] [CrossRef]
- Dushyanthen, S.; Beavis, P.A.; Savas, P.; Teo, Z.L.; Zhou, C.; Mansour, M.; Loi, S.; Darcy, P.K. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015, 13, 202. [Google Scholar] [CrossRef]
- Ali, H.R.; Chlon, L.; Pharoah, P.D.P.; Markowetz, F.; Caldas, C. Patterns of Immune Infiltration in Breast Cancer and Their Clinical Implications: A Gene-Expression-Based Retrospective Study. PLoS Med. 2016, 13, e1002194. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Fu, L.; Zhai, L.; Zhu, J.; Han, Y.; Cao, X.; Zhang, Y.; Zhang, P.; Jiang, Z.; et al. Tumor-educated B cells selectively promote breast cancer lymph node metastasis by HSPA4-targeting IgG. Nat. Med. 2019, 25, 312–322. [Google Scholar] [CrossRef]
- Ishigami, E.; Sakakibara, M.; Sakakibara, J.; Masuda, T.; Fujimoto, H.; Hayama, S.; Otsuka, M. Coexistence of regulatory B cells and regulatory T cells in tumor-infiltrating lymphocyte aggregates is a prognostic factor in patients with breast cancer. Breast Cancer 2019, 26, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Baldwin, J. Hidden Wounds? Inflammatory Links Between Childhood Trauma and Psychopathology. Annu. Rev. Psychol. 2017, 68, 517–544. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Pariante, C.M.; Caspi, A.; Taylor, A.; Poulton, R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA 2015, 104, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.N.; Bauer, M.R.; Wiley, J.F.; Hammen, C.; Krull, J.L.; Crespi, C.M.; Stanton, A.L. Chronic and episodic stress predict physical symptom bother following breast cancer diagnosis. J. Behav. Med. 2017, 40, 875–885. [Google Scholar] [CrossRef] [PubMed]
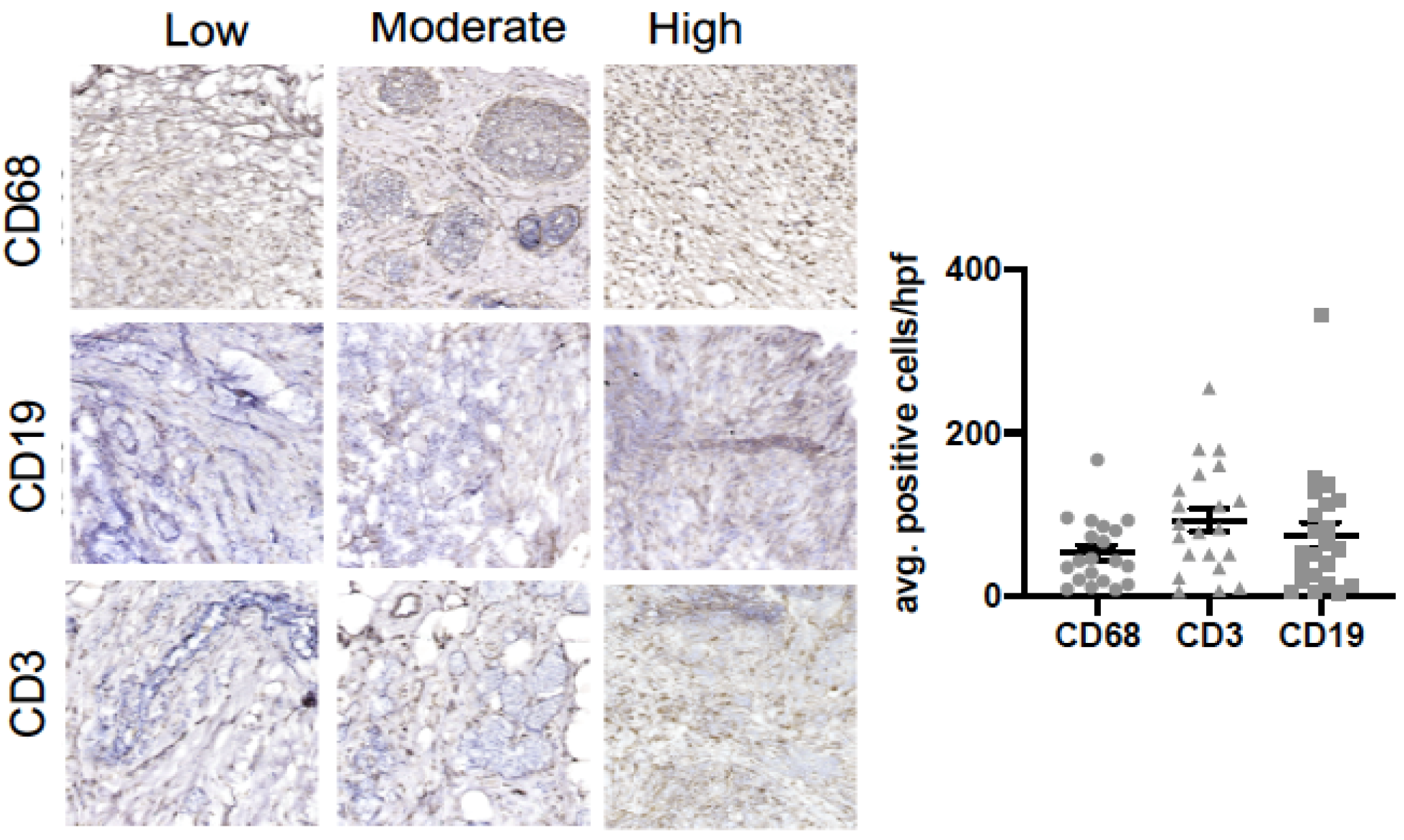
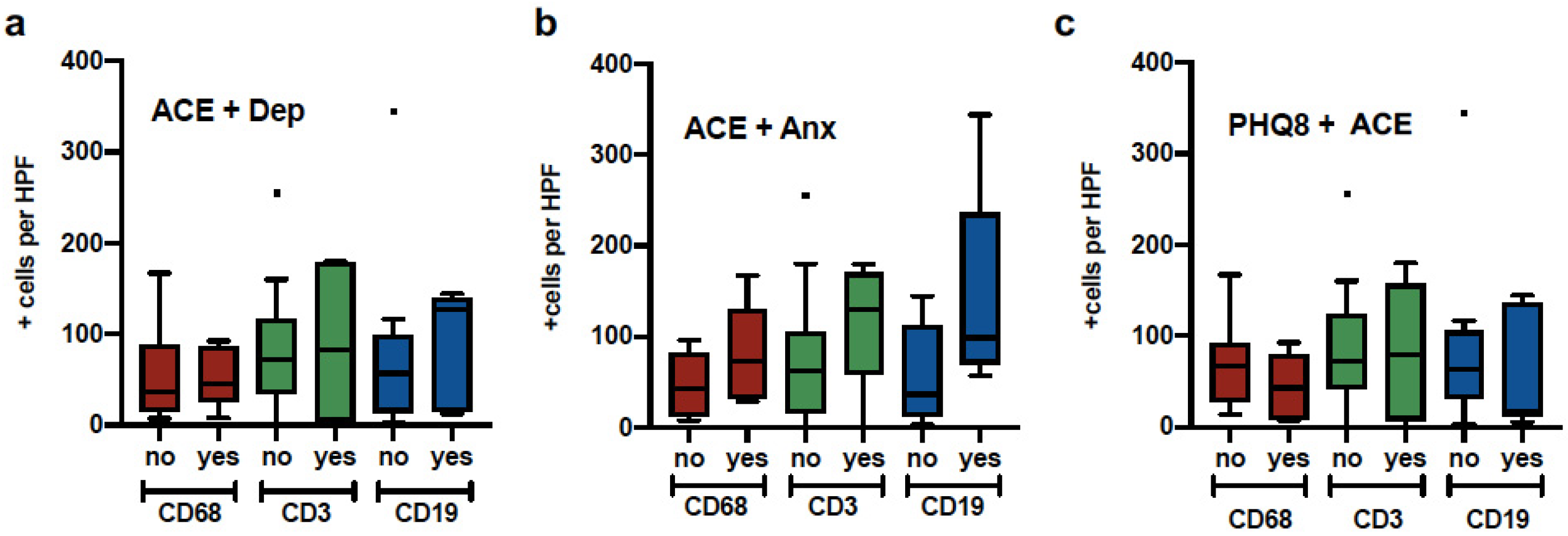
| Variables | Mean/SD, N/% | PHQ-8 (N = 33) | GAD-7 (N = 33) | HX. Depression (N = 33) | ACE (N = 33) | THQ (N = 33) | ACE + Depression (N = 33) | THQ + Depression (N = 33) |
|---|---|---|---|---|---|---|---|---|
| Socio-Demographic Characteristics | ||||||||
| Age | 62.8 (SD = 10.58) | −0.223 | −0.194 | 0.073 | 0.099 | |||
| Civil Status: Married | 18 (57.6%) | 1.312 | 0.788 | 0.001 | 0.418 | 0.034 | 0.137 | |
| With Employment | 8 (24.2%) | 0.792 | 1.676 | 0.090 | 1.161 | 1.676 | 0.015 | |
| Income (≤19,000) | 14 (42.4%) | 0.248 | 0.698 | 1.146 | 2.068 | 4.467 * | 1.146 | |
| Income Enough (Yes) | 14 (42.4%) | 0.104 | 0.001 | 0.001 | 0.020 | 0.034 | 0.137 | |
| Clinical Characteristics | ||||||||
| Tumor Size (cm) | 3.19 (SD = 2.8) | −0.242 | −0.350 | −0.096 | −0.231 | |||
| Lymph Nodes | 7 (21.2%) | 0.046 | 0.377 | 0.326 | 0.326 | 0.029 | 0.326 | |
| Chemo-Therapy | 9 (27.3%) | 0.280 | 0.009 | 1.296 | 3.764* | 0.836 | 0.836 | |
| Radiotherapy | 3 (9.1%) | 0.172549 | 0.014 | 0.647 | 0.416 | 0.027 | 0.416 | |
| Cancer Hormonal Therapy | 6 (18.2%) | 2.363 | 0.141 | 0.933 | 0.117 | 0.117 | 0.933 | |
| Menopause Status (Yes) | 21(63.6%) | 2.444 | 0.932 | 5.917 * | 2.437 | 6.559 * | 5.917 * | |
| BMI | 30.59 (SD = 4.8) | 0.128 | 0.105 | −0.102 | 0.055 | |||
| Mental Health Diagnosis (Yes) | 9 (27.3%) | 0.027 | 1.088 | 7.637 ** | 4.641 * | 13.206 ** | 7.637 ** | |
| Current Mental Health Services | 5 (15.2%) | 1.886 | 1.245 | 0.001 | 0.157 | 0.262 | 0.001 | |
| Mental Health Services (Past) | 13 (39.4%) | 0.016 | 1.172 | 4.406 * | 1.528 | 5.629 ** | 4.406 * | |
| Current Self-Reported Medication Use | ||||||||
| Ssri’s | 7 (21.2%) | 0.090 | 0.287 | 0.287 | 0.007 | 0.012 | 0.044 | |
| Aspirin | 11 (33.3%) | 1.320 | 1.450 | 0.090 | 0.687 | 0.071 | 0.253 | |
| Statins | 9 (27.3%) | 0.557 | 0.556 | 0.007 | 0.229 | 0.053 | 0.132 | |
| High Blood Pressure Medication | 16 (48.5%) | 0.831 | 0.267 | 1.410 | 1.637 | 1.962 | 0.046 | |
| Contraceptives | 1 (3%) | 3.223 | 0.277 | 0.277 | 2.750 | 0.448 | 1.586 | |
| Hormone Replacement | 5 (15.2%) | 0.797 | 1.244 | 5.305 * | 0.157 | 2.460 | 1.244 | |
| Sleep Hours Per Day (≥8 or <6) | 7 (21.2%)26 (78.8%) | 1.676 | 2.392 | 1.172 | 0.755 | 3.030 | 1.172 | |
| Smoking Behavior in the Past 6 Months (Yes) | 4 (12.1%) | 0.001 | 7.880 ** | 2.25 | 1.70 | 0.836 | 0.214 | |
| Alcohol Consumption in the Past 6 Months (Yes) | 10 (30.3%) | 5.18 * | 0.663 | 3.03 | 0.054 | 2.63 | 5.62 * |
| Variables | CD68 Macrophages | CD3 T-Cells | CD19 B-Cells |
|---|---|---|---|
| Known biological mediators/moderators | |||
| Age | 0.090 | 0.528 ** | −0.460 * |
| BMI | 0.184 | 0.2816 | −0.012 |
| Menopause status | 9.000 * | 21.500 | 21.000 |
| Tumor size | 0.511 | −0.118 | 0.011 |
| Cancer treatment: Chemotherapy | 0.000 ** | 9.000 | 4.000 |
| Cancer treatment: Radiation Therapy | 13.000 | 12.000 | 12.000 |
| Cancer treatment: Hormonal Therapy | 10.000 | 10.000 | 9.000 |
| Lymph node status | 3.000 * | 19.000 | 7.000 |
| SSRI’s | 41.000 | 167.000 | 40.000 |
| Aspirin | 15.000 | 51.5000 | 41.000 |
| Statins | 40.000 | 64.000 | 45.000 |
| High blood pressure medication | 33.000 | 44.000 | 39.000 |
| Contraceptives | 2.000 | 4.000 | 2.000 |
| Hormone replacement | 26.000 | 23.000 | 24.000 |
| Sleep hours per day | 33.000 | 28.500 | 35.000 |
| Known behavioral mediators/moderators | |||
| Current use of stress management skills | 37.000 | 42.5000 | 39.000 |
| Mental health services (present) | 16.000 | 9.500 | 1.000 * |
| Mental health services (past) | 15.000 * | 35.500 | 39.000 |
| Smoking behavior | 22.000 | 21.000 | 25.000 |
| Alcohol consumption | 29.000 | 39.000 | 37.000 |
| Variables | CD68 Macrophages | CD3 T-Cells | CD19 B-Cells |
|---|---|---|---|
| PHQ-8 total score (depression symptoms) | 0.224 | 0.466 * | 0.581 |
| Depression symptoms (current) | 20.000 | 33.500 | 33.000 |
| Hx. of Depression | 32.000 | 49.000 | 44.000 |
| GAD-7 total score (anxiety symptoms) | 0.098 | 0.013 | 0.189 |
| Adverse Childhood Events (ACE, total score) | 0.805 | 0.886 | 0.457 |
| Trauma History Questionnaire (THQ, total score) | −0.054 | 0.015 | 0.173 |
| THQ/Crime-related events (total score) | 0.163 | 0.067 | 0.216 |
| THQ/Crime-related events (average age) | |||
| THQ/General disasters and trauma (total score) | - | 0.166 | 0.109 |
| THQ/General disasters and trauma (average age) | |||
| THQ/Physical and Sexual experiences (total score) | 0.067 | 0.288 | −0.034 |
| THQ/Physical and Sexual experiences (average age) | |||
| Comorbid ACE + Hx. of Depression | 30.000 | 32.500 | 26.000 |
| Comorbid ACE + Anxiety | 20.000 | 23.500 | 16.000 |
| Comorbid THQ/Crime + Hx. of Depression | 24.000 | 28.000 | 34.000 |
| Comorbid THQ/Crime + Anxiety | 18.000 | 22.000 | 10.000 * |
| Comorbid THQ/Disaster and trauma + Hx. of Depression | 32.000 | 49.500 | 34.000 |
| Comorbid THQ/Disaster and trauma + Anxiety | 20.000 | 23.500 | 16.000 |
| Comorbid THQ/Physical and sexual abuse + Hx. of Depression | 32.000 | 49.500 | 34.000 |
| Comorbid THQ/Physical and sexual abuse + Anxiety | 12.000 | 8.000 * | 20.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Figueroa, E.M.; Acevedo, K.I.; Peña-Vargas, C.I.; Torres-Blasco, N.; Flores, I.; Colón-Echevarria, C.B.; Maldonado, L.; Rodríguez, Z.; Aquino-Acevedo, A.N.; Jim, H.; et al. Depression, Anxiety, and Social Environmental Adversity as Potential Modulators of the Immune Tumor Microenvironment in Breast Cancer Patients. Med. Sci. 2021, 9, 46. https://doi.org/10.3390/medsci9020046
Castro-Figueroa EM, Acevedo KI, Peña-Vargas CI, Torres-Blasco N, Flores I, Colón-Echevarria CB, Maldonado L, Rodríguez Z, Aquino-Acevedo AN, Jim H, et al. Depression, Anxiety, and Social Environmental Adversity as Potential Modulators of the Immune Tumor Microenvironment in Breast Cancer Patients. Medical Sciences. 2021; 9(2):46. https://doi.org/10.3390/medsci9020046
Chicago/Turabian StyleCastro-Figueroa, Eida M., Karina I. Acevedo, Cristina I. Peña-Vargas, Normarie Torres-Blasco, Idhaliz Flores, Claudia B. Colón-Echevarria, Lizette Maldonado, Zindie Rodríguez, Alexandra N. Aquino-Acevedo, Heather Jim, and et al. 2021. "Depression, Anxiety, and Social Environmental Adversity as Potential Modulators of the Immune Tumor Microenvironment in Breast Cancer Patients" Medical Sciences 9, no. 2: 46. https://doi.org/10.3390/medsci9020046
APA StyleCastro-Figueroa, E. M., Acevedo, K. I., Peña-Vargas, C. I., Torres-Blasco, N., Flores, I., Colón-Echevarria, C. B., Maldonado, L., Rodríguez, Z., Aquino-Acevedo, A. N., Jim, H., Lazaro, M. I., & Armaiz-Peña, G. N. (2021). Depression, Anxiety, and Social Environmental Adversity as Potential Modulators of the Immune Tumor Microenvironment in Breast Cancer Patients. Medical Sciences, 9(2), 46. https://doi.org/10.3390/medsci9020046








