Polyamines: Functions, Metabolism, and Role in Human Disease Management
Abstract
1. Introduction
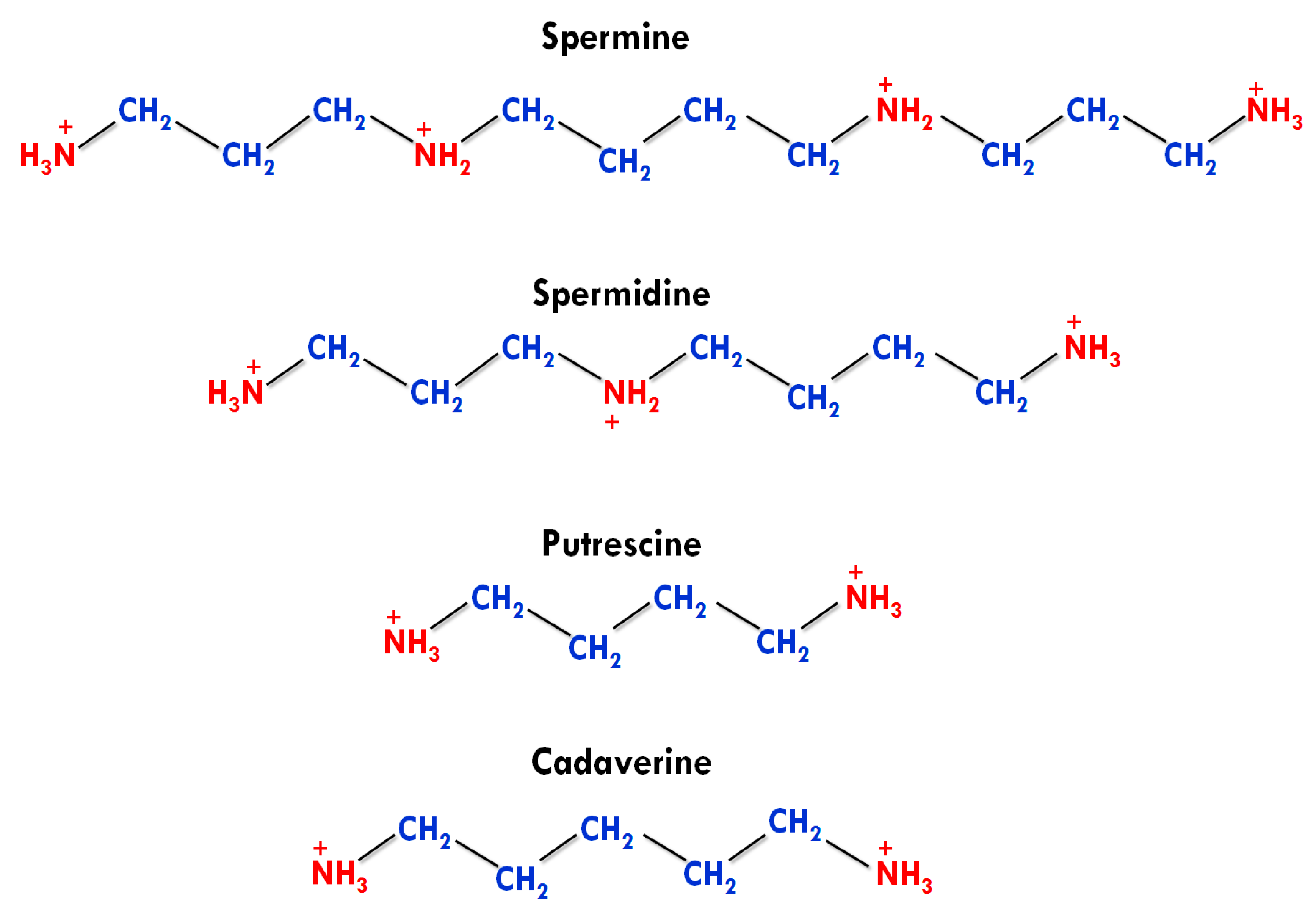
2. Types, Structures, and Functions of PAs
2.1. Types and Structures
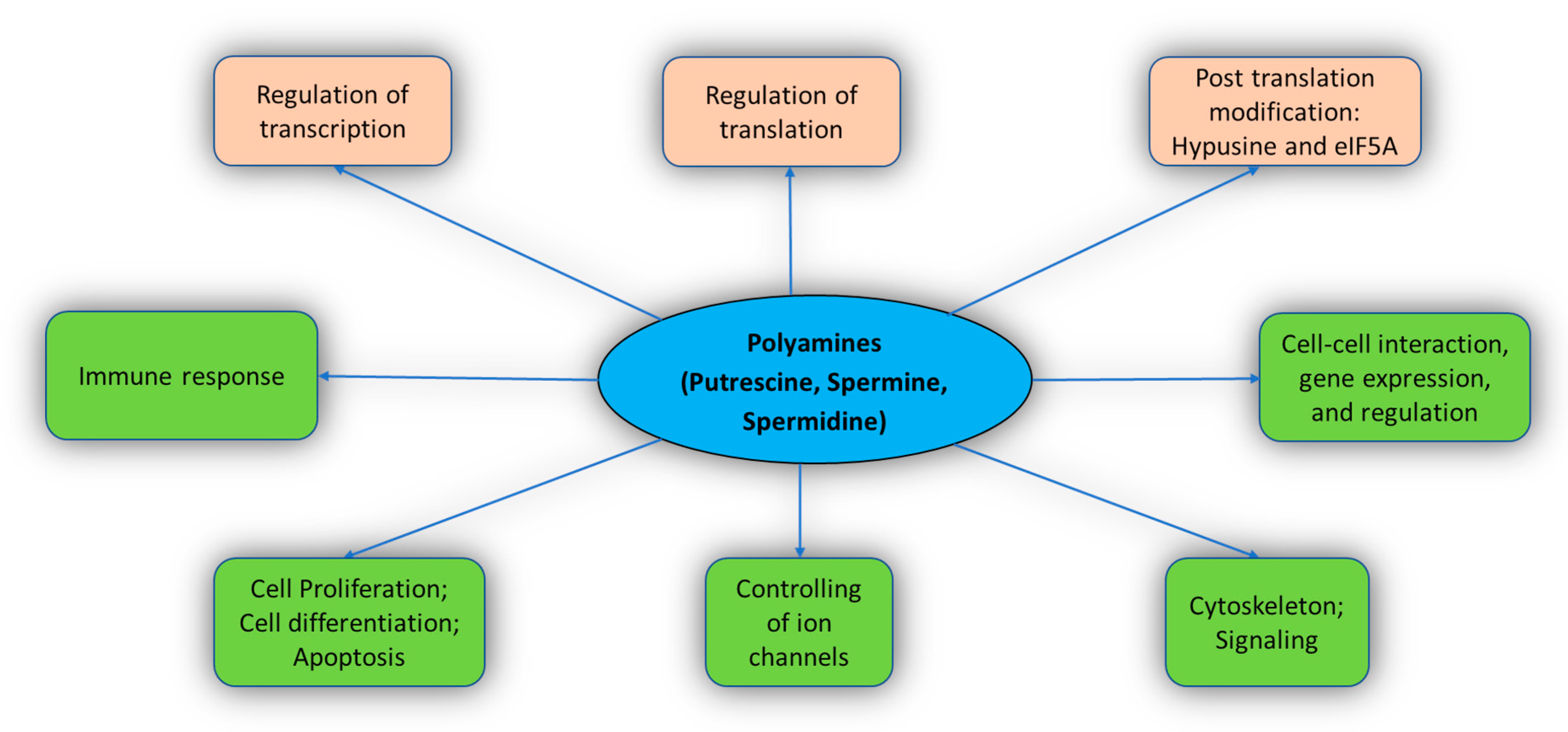
2.2. Functions
2.2.1. Cell Proliferation and Differentiation
2.2.2. Gene Expression and Regulation
2.2.3. Transcription, Translation, and Post-Translation (Hypusine and eIF5A)
2.2.4. Regulating the Function of Ion Channels
Inward Rectifier Potassium (Kir) Channels
Transient Receptor Potential Canonical (TRPC) Channels and Connexins
Ligand-Gated Ion Channels
2.2.5. Immune Response
2.2.6. Regulation of Transglutaminase
3. Metabolic and Transport Pathway of Polyamines in Humans
4. Nutritional Roles of Polyamines in Health Maintenance and Disease Prevention
4.1. Aging and Longevity
4.2. Stress
4.3. Memory
4.4. Cardioprotective Role
4.5. Cancer Prevention
4.6. Huntington’s Disease (HD)
4.7. Alzheimer’s Disease and Parkinson’s Disease
5. Conclusions, Current Problems, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory effects of exogenously applied polyamines on postharvest physiology, antioxidant system and shelf life of fruits: A review. Int. J. Mol. Sci. 2017, 18, 1789. [Google Scholar] [CrossRef]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 1–18. [Google Scholar] [CrossRef]
- Firpo, M.R.; Mounce, B.C. Diverse functions of polyamines in virus infection. Biomolecules 2020, 10, 628. [Google Scholar] [CrossRef]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef]
- Vauquelin, L.N. Experiences sur le sperme humain. Ann. Chim. 1791, 9, 64–80. [Google Scholar]
- Schreiner, P. Ueber eine neue organische Basis in thierischen Organismen. Justus Lieb. Annal. Chem. 1878, 194, 68–84. [Google Scholar] [CrossRef]
- Ladenburg, A.; Abel, J. Ueber das aethylenimin (Spermin?). Berichte Deutschen Chemischen Gesellschaft 1888, 21, 758–766. [Google Scholar] [CrossRef]
- Poehl, A.V.E. Die Physiologisch-Chemischen Grundlagen der Spermintheorie Nebst Klinischem Material zur Therapeutischen Verwendung des Sperminum-Poehl; Wienecke: Sain Petersburg, Russia, 1898. [Google Scholar]
- Rosenheim, O. The isolation of spermine phosphate from semen and testis. Biochem. J. 1924, 18, 1253. [Google Scholar] [CrossRef]
- Gerner, E.W.; Meyskens, F.L. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. 2006, 139, 161–169. [Google Scholar] [CrossRef]
- Hesterberg, R.S.; Cleveland, J.L.; Epling-Burnette, P.K. Role of polyamines in immune cell functions. Med. Sci. 2018, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.T.; Hogarty, M.D. Myc, oncogenic protein translation, and the role of polyamines. Med. Sci. 2018, 6, 41. [Google Scholar] [CrossRef]
- Mathews, M.B.; Hershey, J.W. The translation factor eIF5A and human cancer. Biochimica Biophysica Acta (BBA)-Gene Regul. Mech. 2015, 1849, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.J.; Thomas, T. Cellular and animal model studies on the growth inhibitory effects of polyamine analogues on breast cancer. Med. Sci. 2018, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Pällmann, N.; Braig, M.; Sievert, H.; Preukschas, M.; Hermans-Borgmeyer, I.; Schweizer, M.; Balabanov, S. Biological relevance and therapeutic potential of the hypusine modification system. J. Biol. Chem. 2015, 290, 18343–18360. [Google Scholar] [CrossRef]
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Lenis, Y.Y.; Elmetwally, M.A.; Maldonado-Estrada, J.G.; Bazer, F.W. Physiological importance of polyamines. Zygote 2017, 25, 244. [Google Scholar] [CrossRef]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.A.N.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Baronas, V.A.; Kurata, H.T. Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 2014, 5, 325. [Google Scholar] [CrossRef]
- Pfeffer, L.M.; Yang, C.H.; Murti, A.; McCormack, S.A.; Viar, M.J.; Ray, R.M.; Johnson, L.R. Polyamine depletion induces rapid NF-κB activation in IEC-6 cells. J. Biol. Chem. 2001, 276, 45909–45913. [Google Scholar] [CrossRef]
- Casero, R.A.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Massaro, C.; Thomas, J.; Phanstiel, O. Investigation of polyamine metabolism and homeostasis in pancreatic cancers. Medical Sci. 2017, 5, 1–14. [Google Scholar]
- Tomitori, H.; Usui, T.; Saeki, N.; Ueda, S.; Kase, H.; Nishimura, K.; Igarashi, K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke 2005, 36, 2609–2613. [Google Scholar] [CrossRef] [PubMed]
- Bacchi, C.J.; Yarlett, N. Polyamine metabolism as chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2002, 2, 553–563. [Google Scholar] [CrossRef]
- Pignatti, C.; Tantini, B.; Stefanelli, C.; Flamigni, F. Signal transduction pathways linking polyamines to apoptosis. Amino Acids 2004, 27, 359–365. [Google Scholar] [CrossRef]
- Cai, G.; Sobieszczuk-Nowicka, E.; Aloisi, I.; Fattorini, L.; Serafini-Fracassini, D.; Del Duca, S. Polyamines are common players in different facets of plant programmed cell death. Amino Acids 2015, 47, 27–44. [Google Scholar] [CrossRef]
- Jell, J.; Merali, S.; Hensen, M.L.; Mazurchuk, R.; Spernyak, J.A.; Diegelman, P.; Kisiel, N.D.; Barrero, C.; Deeb, K.K.; Alhonen, A.; et al. Genetically altered expression of spermidine/spermine N1-acetyltransferase affects fat metabolism in mice via acetyl-CoA. J. Biol. Chem. 2007, 282, 8404–8413. [Google Scholar] [CrossRef]
- Jain, V. Role of polyamines in asthma pathophysiology. Med. Sci. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, S.; Mancinelli, R.; Miglietta, S.; Cona, A.; Angelini, R.; Canettieri, G.; Agostinelli, E. Maize polyamine oxidase in the presence of spermine/spermidine induces the apoptosis of LoVo human colon adenocarcinoma cells. Int. J. Oncol. 2019, 54, 2080–2094. [Google Scholar] [CrossRef]
- Agostinelli, E. Biochemical and pathophysiological properties of polyamines. Amino Acids 2020, 52, 111–117. [Google Scholar] [CrossRef]
- Agostinelli, E.; Condello, M.; Tempera, G.; Macone, A.; Bozzuto, G.; Ohkubo, S.; Molinari, A. The combined treatment with chloroquine and the enzymatic oxidation products of spermine overcomes multidrug resistance of melanoma M14 ADR2 cells: A new therapeutic approach. Int. J. Oncol. 2014, 45, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A.; Chérif, A.; Agostinelli, E.; Tanel, A.; Fortier, G. Anti-tumoral effect of native and immobilized bovine serum amine oxidase in a mouse melanoma model. Biochem. Pharm. 2005, 69, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Büyükuslu, N. Dietary polyamines and diseases: Reducing polyamine intake can be beneficial in cancer treatment. J. Nutr. 2015, 2, 27–38. [Google Scholar] [CrossRef][Green Version]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2007, 100, 491–497. [Google Scholar] [CrossRef]
- Atiya Ali, M.; Poortvliet, E.; Strömberg, R.; Yngve, A. Polyamines in foods: Development of a food database. Food Nutr. Res. 2011, 55, 5572. [Google Scholar] [CrossRef]
- Buyukuslu, N.; Hizli, H.; Esin, K.; Garipagaoglu, M. A cross-sectional study: Nutritional polyamines in frequently consumed foods of the Turkish population. Foods 2014, 3, 541–557. [Google Scholar] [CrossRef]
- Kabir, A.; Kumar, G.S. Binding of the biogenic polyamines to deoxyribonucleic acids of varying base composition: Base specificity and the associated energetics of the interaction. PLoS ONE 2013, 8, e70510. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef]
- Uemura, T.; Akasaka, Y.; Ikegaya, H. Correlation of polyamines, acrolein-conjugated lysine and polyamine metabolic enzyme levels with age in human liver. Heliyon 2020, 6, e05031. [Google Scholar] [CrossRef]
- Dever, T.E.; Ivanov, I.P. Roles of polyamines in translation. J. Biol. Chem. 2018, 293, 18719–18729. [Google Scholar] [CrossRef]
- Li, L.; Rao, J.N.; Guo, X.; Liu, L.; Santora, R.; Bass, B.L. Polyamine depletion stabilizes p53 resulting in inhibition of normal intestinal epithelial cell proliferation. Am. J. Physiol. Cell Physiol. 2001, 281, C941–C953. [Google Scholar] [CrossRef]
- Liang, M.; Ekblad, E.; Hellstrand, P.; Nilsson, B.O. Polyamine synthesis inhibition attenuates vascular smooth muscle cell migration. J. Vasc. Res. 2004, 41, 141–147. [Google Scholar] [CrossRef]
- He, Y.; Shimogori, T.; Kashiwagi, K.; Shirahata, A.; Igarashi, K. Inhibition of cell growth by combination of α-difluoromethylornithine and an inhibitor of spermine synthase. J. Biochem. 1995, 117, 824–829. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, A.; Park, M.H. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/ spermine N1-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem. J. 2015, 468, 435–447. [Google Scholar] [CrossRef]
- Nilsson, J.; Gritli-Linde, A.; Heby, O. Skin fibroblasts from spermine synthase-deficient hemizygous gyro male (Gy/Y) mice overproduce spermidine and exhibit increased resistance to oxidative stress but decreased resistance to UV irradiation. Biochem. J. 2000, 352, 381–387. [Google Scholar] [CrossRef]
- Pegg, A.E. Spermidine/spermine-N1-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef]
- Coffino, P. Polyamines in spermiogenesis: Not now, darling. Proc. Natl. Acad. Sci. USA. 2000, 97, 4421–4423. [Google Scholar] [CrossRef]
- Yoshida, M.; Kashiwagi, K.; Kawai, G.; Ishihama, A.; Igarashi, K. Polyamine enhancement of the synthesis of adenylate cyclase at the translational level and the consequential stimulation of the synthesis of the RNA polymerase sigma 28 subunit. J. Biol. Chem. 2001, 276, 16289–16295. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, B.; Swiatlo, E. The expansive effects of polyamines on the metabolism and virulence of Streptococcus pneumoniae. Pneumonia 2021, 13, 1–11. [Google Scholar] [CrossRef]
- Nilsson, J.A.; Keller, U.B.; Baudino, T.A.; Yang, C.; Norton, S.; Old, J.A.; Nilsson, L.M.; Neale, G.; Kramer, D.L.; Porter, C.W.; et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell 2005, 7, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Rao, J.N.; Zou, T.; Liu, L.; Marasa, B.S.; Chen, J.; Turner, D.J.; Passaniti, A.; Wang, J.Y. Induced JunD in intestinal epithelial cells represses CDK4 transcription through its proximal promoter region following polyamine depletion. Biochem. J. 2007, 403, 573–581. [Google Scholar] [CrossRef]
- Vaidya, R.J.; Ray, R.M.; Johnson, L.R. Akt-mediated GSK-3β inhibition prevents migration of polyamine-depleted intestinal epithelial cells via Rac1. Cell Mol. Life Sci. 2006, 63, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liu, L.; Rao, J.N.; Marasa, B.S.; Chen, J.; Xiao, L.; Zhou, H.; Gorospe, M.; Wang, J.Y. Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1. Biochem. J. 2008, 409, 389–398. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ray, R.M.; Johnson, L.R. Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell Signal. 2009, 21, 509–522. [Google Scholar] [CrossRef]
- Kucharzewska, P.; Welch, J.E.; Svensson, K.J.; Belting, M. The polyamines regulate endothelial cell survival during hypoxic stress through PI3K/AKT and MCL-1. Biochem. Biophys. Res. Commun. 2009, 380, 413–418. [Google Scholar] [CrossRef]
- Ramos-Molina, B.; Lambertos, A.; Peñafiel, R. Antizyme inhibitors in polyamine metabolism and beyond: Physiopathological implications. Med. Sci. 2018, 6, 89. [Google Scholar] [CrossRef]
- Sakamoto, A.; Terui, Y.; Yoshida, T.; Yamamoto, T.; Suzuki, H.; Yamamoto, K.; Suzuji, H.; Yamamoto, K.; Ishihama, A.; Igarashi, K.; et al. Three members of polyamine modulon under oxidative stress conditions: Two transcription factors (SoxR and EmrR) and a glutathione synthetic enzyme (GshA). PLoS ONE 2015, 10, e0124883. [Google Scholar] [CrossRef]
- Yamashita, T.; Nishimura, K.; Saiki, R.; Okudaira, H.; Tome, M.; Higashi, K.; Nakamura, M.; Terui, Y.; Fuziwara, K.; Kashiwagi, K.; et al. Role of polyamines at the G1/S boundary and G2/M phase of the cell cycle. Int. J. Biochem. Cell Boil. 2013, 45, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, W.D.; Zhang, Y.; Cottet, S.E.; Bennett, E.M.; Ekstrom, J.L.; Pegg, A.E.; Ealick, S.E. Mechanism of human S-adenosylmethionine decarboxylase proenzyme processing as revealed by the structure of the S68A mutant. Biochemistry 2003, 42, 2386–2395. [Google Scholar] [CrossRef]
- Pegg, A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef]
- Caraglia, M.; Park, M.H.; Wolff, E.C.; Marra, M.; Abbruzzese, A. eIF5A isoforms and cancer: Two brothers for two functions? Amino Acids 2013, 44, 103–109. [Google Scholar] [CrossRef]
- Nishimura, K.; Lee, S.B.; Park, J.H.; Park, M.H. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids 2012, 42, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sievert, H.; Pällmann, N.; Miller, K.K.; Hermans-Borgmeyer, I.; Venz, S.; Sendoel, A.; Balabanov, S. A novel mouse model for inhibition of DOHH-mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis. Models Mech. 2014, 7, 963–976. [Google Scholar] [CrossRef]
- Gutierrez, E.; Shin, B.S.; Woolstenhulme, C.J.; Kim, J.R.; Saini, P.; Buskirk, A.R.; Dever, T.E. eIF5A promotes translation of polyproline motifs. Mol. Cell 2013, 51, 35–45. [Google Scholar] [CrossRef]
- Dever, T.E.; Gutierrez, E.; Shin, B.S. The hypusine-containing translation factor eIF5A. Cri. Rev. Biochem. Mol Biol. 2014, 49, 413–425. [Google Scholar] [CrossRef]
- Schmidt, C.; Becker, T.; Heuer, A.; Braunger, K.; Shanmuganathan, V.; Pech, M.; Berninghausen, O.; Wilson, D.N.; Beckmann, R. Structure of the hypusinylated eukaryotic translation factor eIF-5A bound to the ribosome. Nucleic Acids Res. 2016, 44, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Mandal, S.; Park, M.H. Genome-wide analyses and functional classification of proline repeat-rich proteins: Potential role of eIF5A in eukaryotic evolution. PLoS ONE 2014, 9, e111800. [Google Scholar] [CrossRef]
- Fujimura, K.; Wright, T.; Strnadel, J.; Kaushal, S.; Metildi, C.; Lowy, A.M.; Bouvet, M.; Kelber, J.A.; Klemke, R.L. A hypusine–eIF5A–PEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 2014, 74, 6671–6681. [Google Scholar] [CrossRef]
- Landau, G.; Ran, A.; Bercovich, Z.; Feldmesser, E.; Horn-Saban, S.; Korkotian, E.; Kahana, C. Expression profiling and biochemical analysis suggest stress response as a potential mechanism inhibiting proliferation of polyamine-depleted cells. J. Biol. Chem. 2012, 287, 35825–35837. [Google Scholar] [CrossRef] [PubMed]
- Lopatin, A.N.; Makhina, E.N.; Nichols, C.G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 1994, 372, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, P.R.; Michael, J.S. Spermine is fit to block inward rectifier (Kir) channels. J. Gen. Physiol. 2003, 122, 481–484. [Google Scholar] [CrossRef]
- Kurata, H.T.; Zhu, E.A.; Nichols, C.G. Locale and chemistry of spermine binding in the archetypal inward rectifier Kir2. 1. J. Gen. Physiol. 2010, 135, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Kurata, H.T.; Akrouh, A.; Li, J.W.; Marton, L.J.; Nichols, C.G. Scanning the topography of polyamine blocker binding in an inwardly rectifying potassium channel. J. Biol. Chem. 2013, 288, 6591–6601. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, X.; Tian, J.; Xiao, Y.; Tian, T.; Xu, F.; Zhu, M.X. TRPC channels: Structure, function, regulation and recent advances in small molecular probes. Pharmacol. Therapeutics 2020, 209, 107497. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moon, S.H.; Shin, Y.C.; Jeon, J.H.; Park, K.J.; Lee, K.P.; So, I. Intracellular spermine blocks TRPC4 channel via electrostatic interaction with C-terminal negative amino acids. Pflügers Archiv-Eur. J. Physiol. 2016, 468, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Benedikt, J.; Inyushin, M.; Kucheryavykh, Y.V.; Rivera, Y.; Kucheryavykh, L.Y.; Nichols, C.G.; Skatchkov, S.N. Intracellular polyamines enhance astrocytic coupling. Neuroreport 2012, 23, 1021. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Bukauskas, F.F.; Benedikt, J.; Inyushin, M.; Kucheryavykh, Y.V. Intracellular spermine prevents acid-induced uncoupling of Cx43 gap junction channels. Neuroreport 2015, 26, 528. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Boil. 2010, 42, 39–51. [Google Scholar] [CrossRef]
- Bowie, D.; Mayer, M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 1995, 15, 453–462. [Google Scholar] [CrossRef]
- Williams, K. Modulation and block of ion channels: A new biology of polyamines. Cell. Signal. 1997, 9, 1–13. [Google Scholar] [CrossRef]
- Han, X.; Tomitori, H.; Mizuno, S.; Higashi, K.; Füll, C.; Fukiwake, T.; Terui, Y.; Leewanich, P.; Nishimura, K.; Toida, T.; et al. Binding of spermine and ifenprodil to a purified, soluble regulatory domain of the N-methyl-D-aspartate receptor. J. Neurochem. 2008, 107, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Miyazaki, M.; Mizuno, S.; Takigawa, M.; Hirose, T.; Nishimura, K.; Toida, T.; Williams, K.; Kashiwagi, K.; Igarashi, K. The pore region of N-methyl-D-aspartate receptors differentially influences stimulation and block by spermine. J. Pharmacol. Exp. Ther. 2008, 327, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Bowie, D. Redefining the classification of AMPA-selective ionotropic glutamate receptors. J. Physiol. 2012, 590, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Shen, F.; Huguenard, J. PKC and polyamine modulation of GluR2-deficient AMPA receptors in immature neocortical pyramidal neurons of the rat. J. Physiol. 2007, 581, 679–691. [Google Scholar] [CrossRef]
- Riboldi, P.; Gerosa, M.; Moroni, G.; Radice, A.; Allegri, F.; Sinico, A.; Meroni, P.L. Anti-DNA antibodies: A diagnostic and prognostic tool for systemic lupus erythematosus? Autoimmunity 2005, 38, 39–45. [Google Scholar] [CrossRef]
- Fineschi, S.; Borghi, M.O.; Riboldi, P.; Gariglio, M.; Buzio, C.; Landolfo, S.; Meroni, P.L. Prevalence of autoantibodies against structure specific recognition protein 1 in systemic lupus erythematosus. Lupus 2004, 13, 463–468. [Google Scholar] [CrossRef]
- Agostinelli, E. Polyamines and transglutaminases: Biological, clinical, and biotechnological perspectives. Amino Acids 2014, 46, 475–485. [Google Scholar] [CrossRef]
- Folk, J.E.; Cole, P.W. Mechanism of action of guinea pig liver transglutaminase: I. Purification and properties of the enzyme: Identification of a functional cysteine essential for activity. J. Biol. Chem. 1966, 241, 5518–5525. [Google Scholar] [CrossRef]
- Fesus, L.; Piacentini, M. Transglutaminase 2: An enigmatic enzyme with diverse functions. Trends Biochem. Sci. 2002, 27, 534–539. [Google Scholar] [CrossRef]
- Telci, D.; Griffin, M. Tissue transglutaminase (TG2)-a wound response enzyme. Front. Biosci. 2006, 11, 867–882. [Google Scholar] [CrossRef]
- Folk, J.E.; Park, M.H.; Chung, S.I.; Schrode, J.; Lester, E.P.; Cooper, H.L. Polyamines as physiological substrates for transglutaminases. J. Biol. Chem. 1980, 255, 3695–3700. [Google Scholar] [CrossRef]
- Ruan, Q.; Johnson, G.V. Transglutaminase 2 in neurodegenerative disorders. Front. Biosci. 2007, 12, 891–904. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caccamo, D.; Currò, M.; Ferlazzo, N.; Condello, S.; Ientile, R. Monitoring of transglutaminase2 under different oxidative stress conditions. Amino Acids 2012, 42, 1037–1043. [Google Scholar] [CrossRef]
- Fujita, K.; Shibayama, K.; Yamauchi, M.; Kato, T.; Ando, M.; Takahashi, H.; Nagata, Y. Alteration of enzymatic activities implicating neuronal degeneration in the spinal cord of the motor neuron degeneration mouse during postnatal development. Neurochem. Res. 1998, 23, 557–562. [Google Scholar] [CrossRef]
- Campisi, A.; Caccamo, D.; Volti, G.L.; Curro, M.; Parisi, G.; Avola, R.; Ientile, R. Glutamate-evoked redox state alterations are involved in tissue transglutaminase upregulation in primary astrocyte cultures. FEBS Letters 2004, 578, 80–84. [Google Scholar] [CrossRef]
- Takano, K.; Shiraiwa, K.; Moriyama, M.; Nakamura, Y. Transglutaminase 2 expression induced by lipopolysaccharide stimulation together with NO synthase induction in cultured astrocytes. Neurochem. Int. 2010, 57, 812–818. [Google Scholar] [CrossRef]
- Gamble, L.D.; Hogarty, M.D.; Liu, X.; Ziegler, D.S.; Marshall, G.M.; Norris, M.D.; Haber, M. Polyamine pathway inhibition as a novel therapeutic approach to treating neuroblastoma. Front. Oncol. 2012, 2, 162. [Google Scholar] [CrossRef]
- Liu, Y.C.; Liu, Y.L.; Su, J.Y.; Liu, G.Y.; Hung, H.C. Critical factors governing the difference in antizyme-binding affinities between human ornithine decarboxylase and antizyme inhibitor. PLoS ONE 2011, 6, e19253. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Liu, J.; Xing, F. Antizyme inhibitor 1: A potential carcinogenic molecule. Cancer Sci. 2017, 108, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Albeck, S.; Dym, O.; Unger, T.; Snapir, Z.; Bercovich, Z.; Kahana, C. Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. Protein Sci. 2008, 17, 793–802. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Iyo, A.; Piletz, J.E.; Regunathan, S. Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochimica Biophysica Acta (BBA) Gen. Sub. 2004, 1670, 156–164. [Google Scholar] [CrossRef]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine contents in current foods: A basis for polyamine reduced diet and a study of its long term observance and tolerance in prostate carcinoma patients. Amino Acids 2007, 33, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, C.; Gómez-Tamayo, J.C.; Nebel, J.C.; Pardo, L.; Gonzalez, A. Identifying human diamine sensors for death related putrescine and cadaverine molecules. PLoS Comp. Biol. 2018, 14, e1005945. [Google Scholar] [CrossRef]
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, N.N.; Van den Haute, C.; Vanhoutte, R.; Sannerud, R.; Azfar, M.; Mayer, R.; Calabuig, Á.C.; Swinnen, J.V.; Agostinis, P.; Baekelandt, V.; et al. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J. Biol. Chem. 2021, 296, 100182. [Google Scholar] [CrossRef]
- Moriyama, Y.; Hatano, R.; Moriyama, S.; Uehara, S. Vesicular polyamine transporter as a novel player in amine-mediated chemical transmission. Biochimica Biophysica Acta (BBA) Biomem. 2020, 1862, 183208. [Google Scholar] [CrossRef]
- Palmer, A.J.; Wallace, H.M. The polyamine transport system as a target for anticancer drug development. Amino Acids 2010, 38, 415–422. [Google Scholar] [CrossRef]
- Kibe, R.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef]
- Muth, A.; Kamel, J.; Kaur, N.; Shicora, A.C.; Ayene, I.S.; Gilmour, S.K.; Phanstiel IV, O. Development of polyamine transport ligands with improved metabolic stability and selectivity against specific human cancers. J. Med. Chem. 2013, 56, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Corral, M.; Wallace, H.M. Upregulation of polyamine transport in human colorectal cancer cells. Biomolecules 2020, 10, 499. [Google Scholar] [CrossRef]
- Sala-Rabanal, M.; Li, D.C.; Dake, G.R.; Kurata, H.T.; Inyushin, M.; Skatchkov, S.N.; Nichols, C.G. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol. Pharm. 2013, 10, 1450–1458. [Google Scholar] [CrossRef]
- Viña, J.; Borrás, C.; Miquel, J. Theories of ageing. IUBMB Life 2007, 59, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonaci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Boil. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z. Polyamines control eIF5A hypusination, TFEB translation, and autophagy to reverse B cell senescence. Mol. Cell 2019, 76, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Puleston, D.J.; Buck, M.D.; Geltink, R.I.K.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Pearce, E.L. Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab. 2019, 30, 352–363. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1, 2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef]
- Hibbs, M.L.; Xu, H.; Stacker, S.A.; Springer, T.A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science 1991, 251, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Whitcup, S.M.; Chan, C.C.; Kozhich, A.T.; Magone, M.T. Blocking ICAM-1 (CD54) and LFA-1 (CD11a) inhibits experimental allergic conjunctivitis. Clin. Immunol. 1999, 93, 107–113. [Google Scholar] [CrossRef]
- Kano, Y.; Soda, K.; Konishi, F. Suppression of LFA-1 expression by spermine is associated with enhanced methylation of ITGAL, the LFA-1 promoter area. PLoS ONE 2013, 8, e56056. [Google Scholar] [CrossRef]
- Goll, M.G.; Bestor, T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Uehara, Y.; Kurishita, A.; Tawa, R.; Sakurai, H. Biological significance of DNA methylation in the ageing process. Age Ageing 1993, 22, S34–S43. [Google Scholar] [CrossRef]
- Fabrizio, P.; Pozza, F.; Pletcher, S.D.; Gendron, C.M.; Longo, V.D. Regulation of longevity and stress resistance by Sch9 in yeast. Science 2001, 292, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Minois, N.; Carmona-Gutierrez, D.; Bauer, M.A.; Rockenfeller, P.; Eisenberg, T.; Brandhorst, S.; Sigrist, S.J.; Kroemer, G.; Madeo, F. Spermidine promotes stress resistance in Drosophila melanogaster through autophagy-dependent and-independent pathways. Cell Death Dis. 2012, 3, e401. [Google Scholar] [CrossRef] [PubMed]
- Bagatini, P.B.; Saur, L.; Rodrigues, M.F.; Bernardino, G.C.; Paim, M.F.; Coelho, G.P.; Vieira da Silva, D.; Mattos de Oliveira, R.; Schirmer, H.; Souto, A.A.; et al. The role of calcium channel blockers and resveratrol in the prevention of paraquat-induced parkinsonism in Drosophila melanogaster: A locomotor analysis. Invert. Neurosci. 2011, 11, 43–51. [Google Scholar] [CrossRef]
- Cerrada-Gimenez, M.; Pietilä, M.; Loimas, S.; Pirinen, E.; Hyvönen, M.T.; Keinänen, T.A.; Alhonen, L. Continuous oxidative stress due to activation of polyamine catabolism accelerates aging and protects against hepatotoxic insults. Transgenic Res. 2011, 20, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Malaterre, J.; Strambi, C.; Aouane, A.; Strambi, A.; Rougon, G.; Cayre, M. A novel role for polyamines in adult neurogenesis in rodent brain. Eur. J. Neurosci. 2004, 20, 317–330. [Google Scholar] [CrossRef]
- Skatchkov, S.N.; Woodbury-Fariña, M.A.; Eaton, M. The role of glia in stress: Polyamines and brain disorders. Psychiatr. Clin. 2014, 37, 653–678. [Google Scholar]
- Schreiber, R.C.; Boeshore, K.L.; Laube, G.; Veh, R.W.; Zigmond, R.E. Polyamines increase in sympathetic neurons and non-neuronal cells after axotomy and enhance neurite outgrowth in nerve growth factor-primed PC12 cells. Neuroscience 2004, 128, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Scheunemann, L.; Eisenberg, T.; Mertel, S.; Bhukel, A.; Koemans, T.S.; Kramer, J.M.; Liu, K.S.U.; Schroeder, S.; Stunnenberg, H.G.; et al. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat. Neurosci. 2013, 16, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Fabbrin, S.B.; Girardi, B.A.; de Lorena Wendel, A.; Valin, C.C.I.; Pillat, M.M.; Viero, F.T.; Rubin, M.A. Spermidine-induced improvement of memory consolidation involves PI3K/Akt signaling pathway. Brain Res. Bull. 2020, 164, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Signor, C.; Girardi, B.A.; Wendel, A.L.; Frühauf, P.K.S.; Pillat, M.M.; Ulrich, H.; Rubin, M.A. Spermidine improves the persistence of reconsolidated fear memory and neural differentiation in vitro: Involvement of BDNF. Neurobiol. Learn. Mem. 2017, 140, 82–91. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Mello, C.F.; Signor, C.; Rubin, M.A. Polyaminergic agents modulate the reconsolidation of conditioned fear. Neurobiol. Learn. Mem. 2013, 104, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Girardi, B.A.; Ribeiro, D.A.; Signor, C.; Muller, M.; Gais, M.A.; Mello, C.F.; Rubin, M.A. Spermidine-induced improvement of reconsolidation of memory involves calcium-dependent protein kinase in rats. Learn. Mem. 2016, 23, 21–28. [Google Scholar] [CrossRef]
- Camera, K.; Mello, C.F.; Ceretta, A.P.C.; Rubin, M.A. Systemic administration of polyaminergic agents modulate fear conditioning in rats. Psychopharmacology 2007, 192, 457–464. [Google Scholar] [CrossRef]
- Berlese, D.B.; Sauzem, P.D.; Carati, M.C.; Guerra, G.P.; Stiegemeier, J.A.; Mello, C.F.; Rubin, M.A. Time-dependent modulation of inhibitory avoidance memory by spermidine in rats. Neurobiol. Learn. Mem. 2005, 83, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and memory processing. Neuropharmacology 2014, 76, 677–683. [Google Scholar] [CrossRef]
- Frühauf-Perez, P.K.; Temp, F.R.; Pillat, M.M.; Signor, C.; Wendel, A.L.; Ulrich, H.; Rubin, M.A. Spermine protects from LPS-induced memory deficit via BDNF and TrkB activation. Neurobiol. Learn. Mem. 2018, 149, 135–143. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014; pp. 223–250. [Google Scholar]
- Barros, D.M.; e Souza, T.M.; De Souza, M.M.; Choi, H.; e Silva, T.D.; Lenz, G.; Izquierdo, I. LY294002, an inhibitor of phosphoinositide 3-kinase given into rat hippocampus impairs acquisition, consolidation and retrieval of memory for one-trial step-down inhibitory avoidance. Behaviour. Pharmacol. 2001, 12, 629–634. [Google Scholar] [CrossRef]
- Lin, C.H.; Yeh, S.H.; Lin, C.H.; Lu, K.T.; Leu, T.H.; Chang, W.C.; Gean, P.W. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron 2001, 31, 841–851. [Google Scholar] [CrossRef]
- Nakai, T.; Nagai, T.; Tanaka, M.; Itoh, N.; Asai, N.; Enomoto, A.; Asai, M.; Yamada, S.; Saifullah, A.B.; Sokabe, M.; et al. Girdin phosphorylation is crucial for synaptic plasticity and memory: A potential role in the interaction of BDNF/TrkB/Akt signaling with NMDA receptor. J. Neurosci. 2014, 34, 14995–15008. [Google Scholar] [CrossRef]
- Alberini, C.M.; Kandel, E.R. The regulation of transcription in memory consolidation. CSH Perspect. Biol. 2015, 7, a021741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Won, J.; Karlsson, M.G.; Zhou, M.; Rogerson, T.; Balaji, J.; Silva, A.J. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 2009, 12, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Fukushima, H.; Mukawa, T.; Toyoda, H.; Wu, L.J.; Zhao, M.G.; Kida, S. Upregulation of CREB-mediated transcription enhances both short-and long-term memory. J. Neurosci. 2011, 31, 8786–8802. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martínez, S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front. Mol. Neurosci. 2015, 8, 46. [Google Scholar] [CrossRef]
- Guerra, G.P.; Mello, C.F.; Bochi, G.V.; Pazini, A.M.; Fachinetto, R.; Dutra, R.C.; Rubin, M.A. Hippocampal PKA/CREB pathway is involved in the improvement of memory induced by spermidine in rats. Neurobiol. Learn. Mem. 2011, 96, 324–332. [Google Scholar] [CrossRef]
- Guerra, G.P.; Mello, C.F.; Bochi, G.V.; Pazini, A.M.; Rosa, M.M.; Ferreira, J.; Rubin, M.A. Spermidine-induced improvement of memory involves a cross-talk between protein kinases C and A. J. Neurochem. 2012, 122, 363–373. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Koga, H.; Kaushik, S.; Cuervo, A.M. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 2011, 10, 205–215. [Google Scholar] [CrossRef]
- Lakatta, E.G. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 2003, 107, 346–354. [Google Scholar] [CrossRef]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Gioscia-Ryan, R.A.; Hearon, C.M., Jr.; Seals, D.R. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Develop. 2013, 134, 314–320. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Madeo, F. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part III: Cellular and molecular clues to heart and arterial aging. Circulation 2003, 107, 490–497. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. College Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Linke, W.A.; Hamdani, N. Gigantic business: Titin properties and function through thick and thin. Circ. Res 2014, 114, 1052–1068. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, B.; Bansard, J.Y.; Ecalard, J.P.; Moulinoux, J.P. Treating metastatic castration-resistant prostate cancer with novel polyamine-free oral nutritional supplementation: Phase I study. BioMedicine 2013, 3, 114–119. [Google Scholar] [CrossRef]
- Bell, R.F.; Borzan, J.; Kalso, E.; Simonnet, G. Food, pain, and drugs: Does it matter what pain patients eat? Pain 2012, 153, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Rivat, C.; Richebé, P.; Laboureyras, E.; Laulin, J.P.; Havouis, R.; Noble, F.; Simonnet, G. Polyamine deficient diet to relieve pain hypersensitivity. Pain 2008, 137, 125–137. [Google Scholar] [CrossRef]
- Ferrier, J.; Bayet-Robert, M.; Pereira, B.; Daulhac, L.; Eschalier, A.; Pezet, D.; Balayssac, D. A polyamine-deficient diet prevents oxaliplatin-induced acute cold and mechanical hypersensitivity in rats. PLoS ONE 2013, 8, e77828. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Yanase, T.; Nakagawa, H.; Matsuo, S.; Ohnishi, Y.; Yamasaki, S. Effect of polyamine-deficient chow on Trypanosoma brucei brucei infection in rats. J. Parasitol. 2009, 95, 781–786. [Google Scholar] [CrossRef]
- Gerner, E.W. Impact of dietary amino acids and polyamines on intestinal carcinogenesis and chemoprevention in mouse models. Biochem. Soc. Transacc. 2007, 35, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, B.G.; Havouis, R.; Moulinoux, J.P. Polyamine reduced diet (PRD) nutrition therapy in hormone refractory prostate cancer patients. Biomed. Pharmacother. 2010, 64, 363–368. [Google Scholar] [CrossRef]
- Linsalata, M.; Russo, F. Nutritional factors and polyamine metabolism in colorectal cancer. Nutrition 2008, 24, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.J.; Wertheim, B.C.; Gerner, E.W.; Thomson, C.A.; Rock, C.L.; Thompson, P.A. Dietary polyamine intake and risk of colorectal adenomatous polyps. Am. J. Clin. Nutr. 2012, 96, 133–141. [Google Scholar] [CrossRef]
- Huang, C.Y.; Fang, Y.J.; Abulimiti, A.; Yang, X.; Li, L.; Liu, K.Y.; Zhang, C.X. Dietary Polyamines Intake and Risk of Colorectal Cancer: A Case-Control Study. Nutrition 2020, 12, 3575. [Google Scholar]
- Dang, C.V.; Reddy, E.P.; Shokat, K.M.; Soucek, L. Drugging the ‘undruggable’ cancer targets. Nat. Rev. Cancer 2017, 17, 502–508. [Google Scholar] [CrossRef]
- LoGiudice, N.; Le, L.; Abuan, I.; Leizorek, Y.; Roberts, S.C. Alpha-difluoromethylornithine, an irreversible inhibitor of polyamine biosynthesis, as a therapeutic strategy against hyperproliferative and infectious diseases. Med. Sci. 2018, 6, 12. [Google Scholar] [CrossRef]
- Lam, S.K.; Yan, S.; Xu, S.; Ho, J.C.M. Targeting polyamine as a novel therapy in xenograft models of malignant pleural mesothelioma. Lung Cancer 2020, 148, 138–148. [Google Scholar] [CrossRef]
- Wallick, C.J.; Gamper, I.; Thorne, M.; Feith, D.J.; Takasaki, K.Y.; Wilson, S.M.; Bachmann, A.S. Key role for p27 Kip1, retinoblastoma protein Rb, and MYCN in polyamine inhibitor-induced G 1 cell cycle arrest in MYCN-amplified human neuroblastoma cells. Oncogene 2005, 24, 5606–5618. [Google Scholar] [CrossRef]
- DiFiglia, M. Excitotoxic injury of the neostriatum: A model for Huntington’s disease. Trends Neurosci. 1990, 13, 286–289. [Google Scholar] [CrossRef]
- Velloso, N.A.; Dalmolin, G.D.; Gomes, G.M.; Rubin, M.A.; Canas, P.M.; Cunha, R.A.; Mello, C.F. Spermine improves recognition memory deficit in a rodent model of Huntington’s disease. Neurobiol. Learn. Mem. 2009, 92, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Rosi, S.; Ferguson, R.; Fishman, K.; Allen, A.; Raber, J.; Fike, J.R. The polyamine inhibitor alpha-difluoromethylornithine modulates hippocampus-dependent function after single and combined injuries. PLoS ONE 2012, 7, e31094. [Google Scholar] [CrossRef]
- Tunali, N.E.; Tüfekçi, M.A. A26 Polyamine metabolism in huntington’s disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, A9. [Google Scholar]
- Shaw, K.N.; Commins, S.; O’Mara, S.M. Lipopolysaccharide causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav. Brain Res. 2001, 124, 47–54. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, D.Y.; Choi, I.S.; Kim, K.H.; Kim, Y.H.; Kim, H.M.; Hong, J.T. Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J. Neuroinflamm. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Frühauf, P.K.S.; Ineu, R.P.; Tomazi, L.; Duarte, T.; Mello, C.F.; Rubin, M.A. Spermine reverses lipopolysaccharide-induced memory deficit in mice. J. Neuroinflamm. 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Roberson, E.D.; Mucke, L. 100 years and counting: Prospects for defeating Alzheimer’s disease. Science 2006, 314, 781–784. [Google Scholar] [CrossRef]
- Inoue, K.; Tsutsui, H.; Akatsu, H.; Hashizume, Y.; Matsukawa, N.; Yamamoto, T.; Toyo’Oka, T. Metabolic profiling of Alzheimer’s disease brains. Sci. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yatin, S.M.; Yatin, M.; Varadarajan, S.; Ain, K.B.; Butterfield, D.A. Role of spermine in amyloid β-peptide-associated free radical-induced neurotoxicity. J. Neurosci. Res. 2001, 63, 395–401. [Google Scholar] [CrossRef]
- Klyubin, I.; Wang, Q.; Reed, M.N.; Irving, E.A.; Upton, N.; Hofmeister, J.; Rowan, M.J. Protection against Aβ-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol. Aging 2011, 32, 614–623. [Google Scholar] [CrossRef]
- Gross, J.A.; Fiori, L.M.; Labonté, B.; Lopez, J.P.; Turecki, G. Effects of promoter methylation on increased expression of polyamine biosynthetic genes in suicide. J. Psychiatr. Res. 2013, 47, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, N.M.; Ju, S.; Verbitsky, M.; Ross, B.; Geddie, M.L.; Rockenstein, E.; Small, S.A. Polyamine pathway contributes to the pathogenesis of Parkinson disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16970–16975. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. https://doi.org/10.3390/medsci9020044
Sagar NA, Tarafdar S, Agarwal S, Tarafdar A, Sharma S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Medical Sciences. 2021; 9(2):44. https://doi.org/10.3390/medsci9020044
Chicago/Turabian StyleSagar, Narashans Alok, Swarnava Tarafdar, Surbhi Agarwal, Ayon Tarafdar, and Sunil Sharma. 2021. "Polyamines: Functions, Metabolism, and Role in Human Disease Management" Medical Sciences 9, no. 2: 44. https://doi.org/10.3390/medsci9020044
APA StyleSagar, N. A., Tarafdar, S., Agarwal, S., Tarafdar, A., & Sharma, S. (2021). Polyamines: Functions, Metabolism, and Role in Human Disease Management. Medical Sciences, 9(2), 44. https://doi.org/10.3390/medsci9020044





