Antidepressants and Risk of Sudden Cardiac Death: A Network Meta-Analysis and Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Literature Review and Search Strategy
2.2. Selection Criteria
2.3. Data Abstraction
2.4. Outcome of Interest
2.5. Ancillary Information
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| ECG | Electrocardiogram |
| NMA | Network meta-analysis |
| QTc | Corrected QT duration |
| SCD | Sudden cardiac death |
| SNRI | Serotonin-norepinephrine reuptake inhibitors |
| SSRI | selective serotonin reuptake inhibitors |
| TCA | Tricyclic antidepressants |
| TdP | Torsade de Pointes |
| VA | Ventricular arrhythmias |
References
- Ashok, A.H.; Marques, T.R.; Jauhar, S.; Nour, M.M.; Goodwin, G.M.; Young, A.H.; Howes, O.D. The dopamine hypothesis of bipolar affective disorder: The state of the art and implications for treatment. Mol. Psychiatry 2017, 22, 666–679. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; McIntyre, R.S.; Alves, G.S.; Fountoulakis, K.N.; Carvalho, A.F. Beyond Monoamines-Novel Targets for Treatment-Resistant Depression: A Comprehensive Review. Curr. Neuropharmacol. 2015, 13, 636–655. [Google Scholar] [CrossRef]
- Heninger, G.R.; Delgado, P.L.; Charney, D.S. The revised monoamine theory of depression: A modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 1996, 29, 2–11. [Google Scholar] [CrossRef]
- Hodgson, K.; Tansey, K.E.; Powell, T.R.; Coppola, G.; Uher, R.; Zvezdana Dernovsek, M.; Mors, O.; Hauser, J.; Souery, D.; Maier, W.; et al. Transcriptomics and the mechanisms of antidepressant efficacy. Eur. Neuropsychopharmacol. 2016, 26, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Arcos-Burgos, M.; Liu, S.; Velez, J.I.; Yu, C.; Baune, B.T.; Jawahar, M.C.; Arolt, V.; Dannlowski, U.; Chuah, A.; et al. The PHF21B gene is associated with major depression and modulates the stress response. Mol. Psychiatry 2017, 22, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, C.; Xia, L.; Yuan, Y.; Zhu, H.; Huang, X.; Li, C.; Tao, Y.; Qu, X.; Zhang, F.; et al. Targeted exome sequencing identifies five novel loci at genome-wide significance for modulating antidepressant response in patients with major depressive disorder. Transl. Psychiatry 2020, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, T.M.; Porter, J.H. A brief history of the development of antidepressant drugs: From monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015, 23, 1–21. [Google Scholar] [CrossRef]
- McKenzie, M.S.; McFarland, B.H. Trends in antidepressant overdoses. Pharmacoepidemiol. Drug Saf. 2007, 16, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Anastasia, A.; Valchera, A.; Carano, A.; Orsolini, L.; Vellante, F.; Rapini, G.; Olivieri, L.; Di Natale, S.; Perna, G.; et al. The FDA “Black Box” Warning on Antidepressant Suicide Risk in Young Adults: More Harm Than Benefits? Front. Psychiatry 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Warrington, S.J.; Padgham, C.; Lader, M. The cardiovascular effects of antidepressants. Psychol. Med. Monogr. Suppl. 1989, 16, 1–40. [Google Scholar] [CrossRef]
- Sicouri, S.; Antzelevitch, C. Sudden cardiac death secondary to antidepressant and antipsychotic drugs. Expert. Opin. Drug Saf. 2008, 7, 181–194. [Google Scholar] [CrossRef]
- Leonard, C.E.; Bilker, W.B.; Newcomb, C.; Kimmel, S.E.; Hennessy, S. Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol. Drug Saf. 2011, 20, 903–913. [Google Scholar] [CrossRef]
- Jasiak, N.M.; Bostwick, J.R. Risk of QT/QTc prolongation among newer non-SSRI antidepressants. Ann. Pharmacother. 2014, 48, 1620–1628. [Google Scholar] [CrossRef]
- Li, M.; Ramos, L.G. Drug-Induced QT Prolongation And Torsades de Pointes. Pharm. Ther. 2017, 42, 473–477. [Google Scholar]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Alvarez-Pasquin, M.J.; Diaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ Clin. Res. Ed. 2011, 343, d5928. [Google Scholar] [CrossRef]
- Li, E.C.; Esterly, J.S.; Pohl, S.; Scott, S.D.; McBride, B.F. Drug-induced QT-interval prolongation: Considerations for clinicians. Pharmacotherapy 2010, 30, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Trinkley, K.E.; Page, R.L., 2nd; Lien, H.; Yamanouye, K.; Tisdale, J.E. QT interval prolongation and the risk of torsades de pointes: Essentials for clinicians. Curr. Med. Res. Opin. 2013, 29, 1719–1726. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Ades, A.E.; Welton, N.J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis Mak. 2013, 33, 607–617. [Google Scholar] [CrossRef]
- Higgins, J.P.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef]
- Salanti, G.; Dias, S.; Welton, N.J.; Ades, A.E.; Golfinopoulos, V.; Kyrgiou, M.; Mauri, D.; Ioannidis, J.P. Evaluating novel agent effects in multiple-treatments meta-regression. Stat. Med. 2010, 29, 2369–2383. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, S.N.; Nemeroff, C.B.; Plott, S.J.; Rao, S.G.; Kranzler, J.; Owens, M.J. Milnacipran: A comparative analysis of human monoamine uptake and transporter binding affinity. Biol. Psychiatry 2004, 55, 320–322. [Google Scholar] [CrossRef]
- Machado, M.; Iskedjian, M.; Ruiz, I.; Einarson, T.R. Remission, dropouts, and adverse drug reaction rates in major depressive disorder: A meta-analysis of head-to-head trials. Curr. Med. Res. Opin. 2006, 22, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet Lond. Engl. 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Maslej, M.M.; Bolker, B.M.; Russell, M.J.; Eaton, K.; Durisko, Z.; Hollon, S.D.; Swanson, G.M.; Thomson, J.A., Jr.; Mulsant, B.H.; Andrews, P.W. The Mortality and Myocardial Effects of Antidepressants Are Moderated by Preexisting Cardiovascular Disease: A Meta-Analysis. Psychother. Psychosom. 2017, 86, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Anderson, I.M. SSRIS versus tricyclic antidepressants in depressed inpatients: A meta-analysis of efficacy and tolerability. Depress. Anxiety 1998, 7 (Suppl. 1), 11–17. [Google Scholar] [CrossRef]
- Jolly, K.; Gammage, M.D.; Cheng, K.K.; Bradburn, P.; Banting, M.V.; Langman, M.J. Sudden death in patients receiving drugs tending to prolong the QT interval. Br. J. Clin. Pharmacol. 2009, 68, 743–751. [Google Scholar] [CrossRef]
- Wu, C.S.; Tsai, Y.T.; Hsiung, C.A.; Tsai, H.J. Comparative Risk of Ventricular Arrhythmia and Sudden Cardiac Death Across Antidepressants in Patients With Depressive Disorders. J. Clin. Psychopharmacol. 2017, 37, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.A.; Meredith, S.; Thapa, P.B.; Hall, K.; Murray, K.T. Cyclic antidepressants and the risk of sudden cardiac death. Clin. Pharmacol. Ther. 2004, 75, 234–241. [Google Scholar] [CrossRef] [PubMed]
- White, N.; Litovitz, T.; Clancy, C. Suicidal antidepressant overdoses: A comparative analysis by antidepressant type. J. Med Toxicol. Off. J. Am. Coll. Med Toxicol. 2008, 4, 238–250. [Google Scholar] [CrossRef]
- Hawton, K.; Bergen, H.; Simkin, S.; Cooper, J.; Waters, K.; Gunnell, D.; Kapur, N. Toxicity of antidepressants: Rates of suicide relative to prescribing and non-fatal overdose. Br. J. Psychiatry 2010, 196, 354–358. [Google Scholar] [CrossRef]
- Buckley, N.A.; McManus, P.R. Fatal toxicity of serotoninergic and other antidepressant drugs: Analysis of United Kingdom mortality data. BMJ Clin. Res. Ed. 2002, 325, 1332–1333. [Google Scholar] [CrossRef]
- Bauer, M.; Monz, B.U.; Montejo, A.L.; Quail, D.; Dantchev, N.; Demyttenaere, K.; Garcia-Cebrian, A.; Grassi, L.; Perahia, D.G.; Reed, C.; et al. Prescribing patterns of antidepressants in Europe: Results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur. Psychiatry 2008, 23, 66–73. [Google Scholar] [CrossRef]
- Gautam, S.; Jain, A.; Gautam, M.; Vahia, V.N.; Grover, S. Clinical Practice Guidelines for the management of Depression. Indian J. Psychiatry 2017, 59, S34–S50. [Google Scholar] [CrossRef]
- Avasthi, A.; Grover, S. Clinical Practice Guidelines for Management of Depression in Elderly. Indian J. Psychiatry 2018, 60, S341–S362. [Google Scholar] [CrossRef]
- Coupland, C.; Dhiman, P.; Morriss, R.; Arthur, A.; Barton, G.; Hippisley-Cox, J. Antidepressant use and risk of adverse outcomes in older people: Population based cohort study. BMJ Clin. Res. Ed. 2011, 343, d4551. [Google Scholar] [CrossRef]
- Best, J.; Nijhout, H.F.; Reed, M. Serotonin synthesis, release and reuptake in terminals: A mathematical model. Biol. Med. Model. 2010, 7, 34. [Google Scholar] [CrossRef]
- Tripathi, A.; Avasthi, A.; Desousa, A.; Bhagabati, D.; Shah, N.; Kallivayalil, R.A.; Grover, S.; Trivedi, J.K.; Shinfuku, N. Prescription pattern of antidepressants in five tertiary care psychiatric centres of India. Indian J. Med. Res. 2016, 143, 507–513. [Google Scholar] [CrossRef]
- Lockhart, P.; Guthrie, B. Trends in primary care antidepressant prescribing 1995-2007: A longitudinal population database analysis. Br. J. Gen. Pr. 2011, 61, e565–e572. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.L.; Medina-Inojosa, J.R.; Thomas, R.J.; Krause, H.; Vickers-Douglas, K.S.; Palmer, B.A.; Lopez-Jimenez, F. Antidepressant Use by Class: Association with Major Adverse Cardiac Events in Patients with Coronary Artery Disease. Psychother. Psychosom. 2018, 87, 85–94. [Google Scholar] [CrossRef]
- Mohammed, R.; Norton, J.; Geraci, S.A.; Newman, D.B.; Koch, C.A. Prolonged QTc interval due to escitalopram overdose. J. Miss. State Med Assoc. 2010, 51, 350–353. [Google Scholar] [PubMed]
- Tarabar, A.F.; Hoffman, R.S.; Nelson, L. Citalopram overdose: Late presentation of torsades de pointes (TdP) with cardiac arrest. J. Med Toxicol. Off. J. Am. Coll. Med Toxicol. 2008, 4, 101–105. [Google Scholar] [CrossRef]
- Sala, M.; Lazzaretti, M.; De Vidovich, G.; Caverzasi, E.; Barale, F.; d’Allio, G.; Brambilla, P. Electrophysiological changes of cardiac function during antidepressant treatment. Adv. Cardiovasc. Dis. 2009, 3, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Fricchione, G.L.; Woznicki, R.M.; Klesmer, J.; Vlay, S.C. Vasoconstrictive effects and SSRIs. J. Clin. Psychiatry 1993, 54, 71–72. [Google Scholar]
- Pacher, P.; Ungvari, Z.; Nanasi, P.P.; Furst, S.; Kecskemeti, V. Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Curr. Med. Chem. 1999, 6, 469–480. [Google Scholar] [PubMed]
- Yekehtaz, H.; Farokhnia, M.; Akhondzadeh, S. Cardiovascular considerations in antidepressant therapy: An evidence-based review. J. Tehran. Heart Cent. 2013, 8, 169–176. [Google Scholar] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.L.; Reim, E.K.; Lanctot, K.L. Efficacy and tolerability of antidepressants for treatment of depression in coronary artery disease: A meta-analysis. Can. J. Psychiatry. Rev. Can. Psychiatr. 2010, 55, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Rajeswaran, T.; Plymen, C.M.; Doherty, A.M. The effect of antidepressant medications in the management of heart failure on outcomes: Mortality, cardiovascular function and depression-a systematic review. Int. J. Psychiatry Clin. Pr. 2018, 22, 164–169. [Google Scholar] [CrossRef]
- Bradley, S.M.; Rumsfeld, J.S. Depression and cardiovascular disease. Trends Cardiovasc. Med. 2015, 25, 614–622. [Google Scholar] [CrossRef]
- Stahl, S.M.; Grady, M.M.; Moret, C.; Briley, M. SNRIs: Their pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. Cns Spectr. 2005, 10, 732–747. [Google Scholar] [CrossRef]
- Martinez, C.; Assimes, T.L.; Mines, D.; Dell’aniello, S.; Suissa, S. Use of venlafaxine compared with other antidepressants and the risk of sudden cardiac death or near death: A nested case-control study. BMJ Clin. Res. Ed. 2010, 340, c249. [Google Scholar] [CrossRef]
- Pacher, P.; Kecskemeti, V. Cardiovascular side effects of new antidepressants and antipsychotics: New drugs, old concerns? Curr. Pharm Des. 2004, 10, 2463–2475. [Google Scholar] [CrossRef]
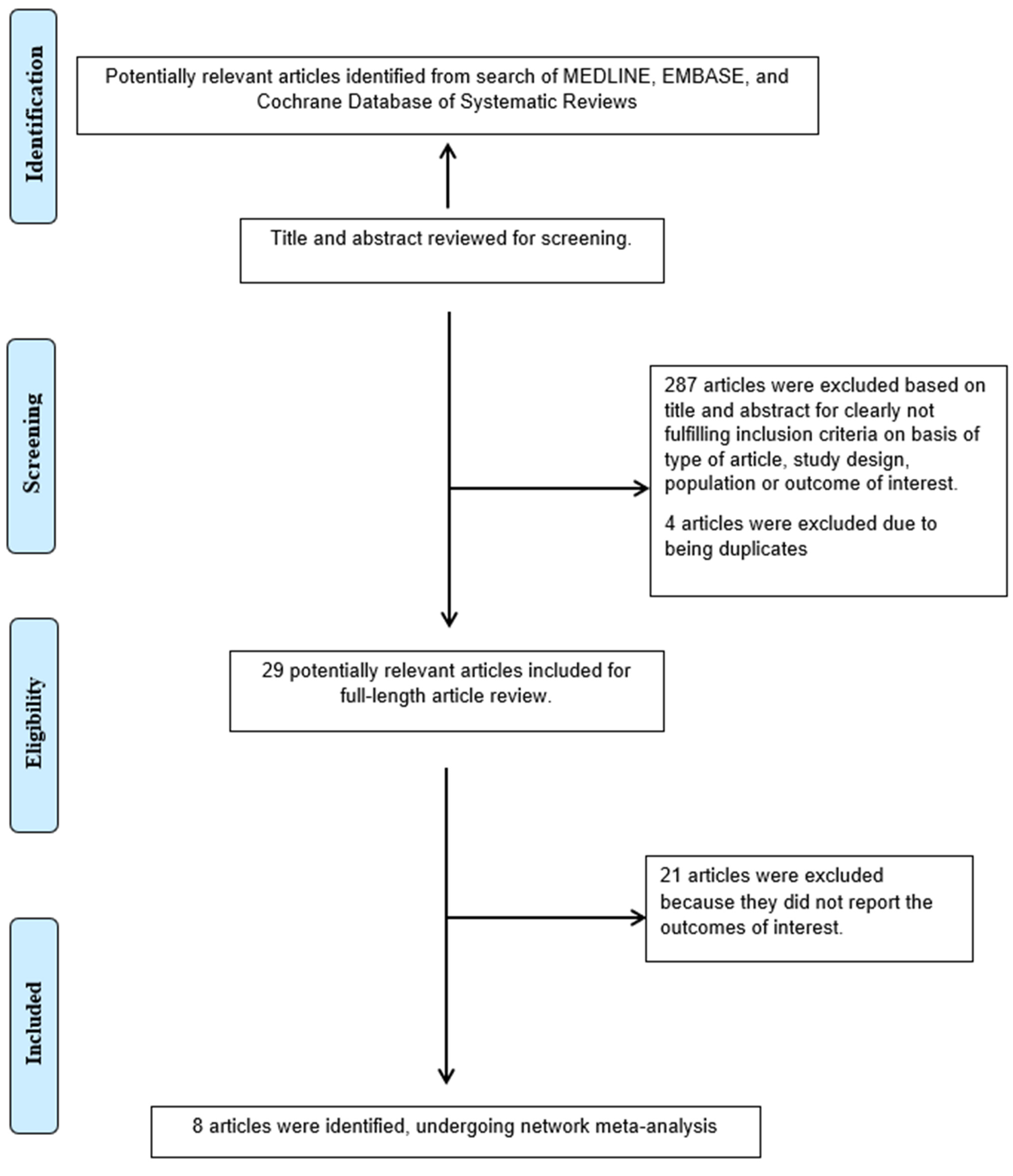
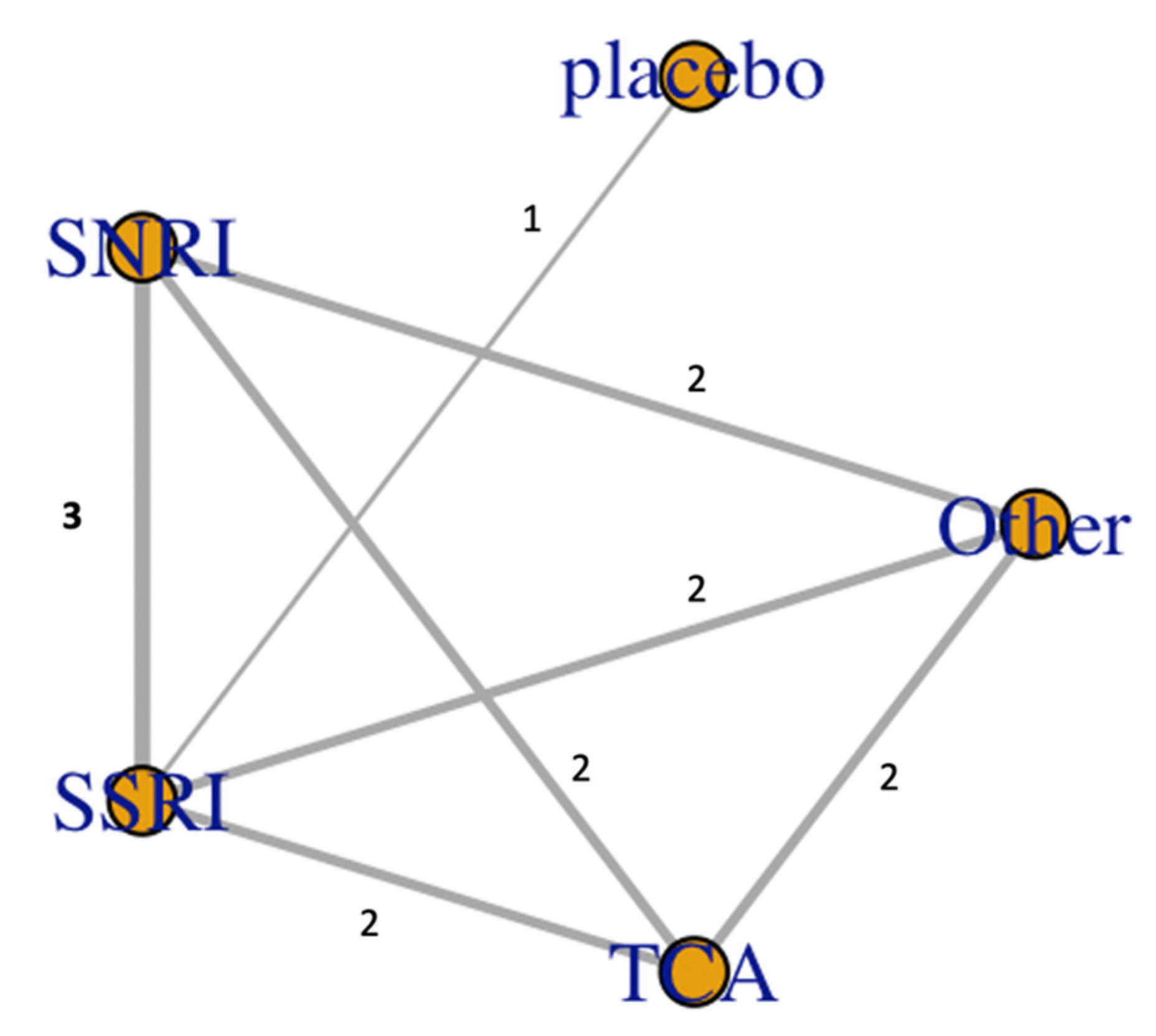

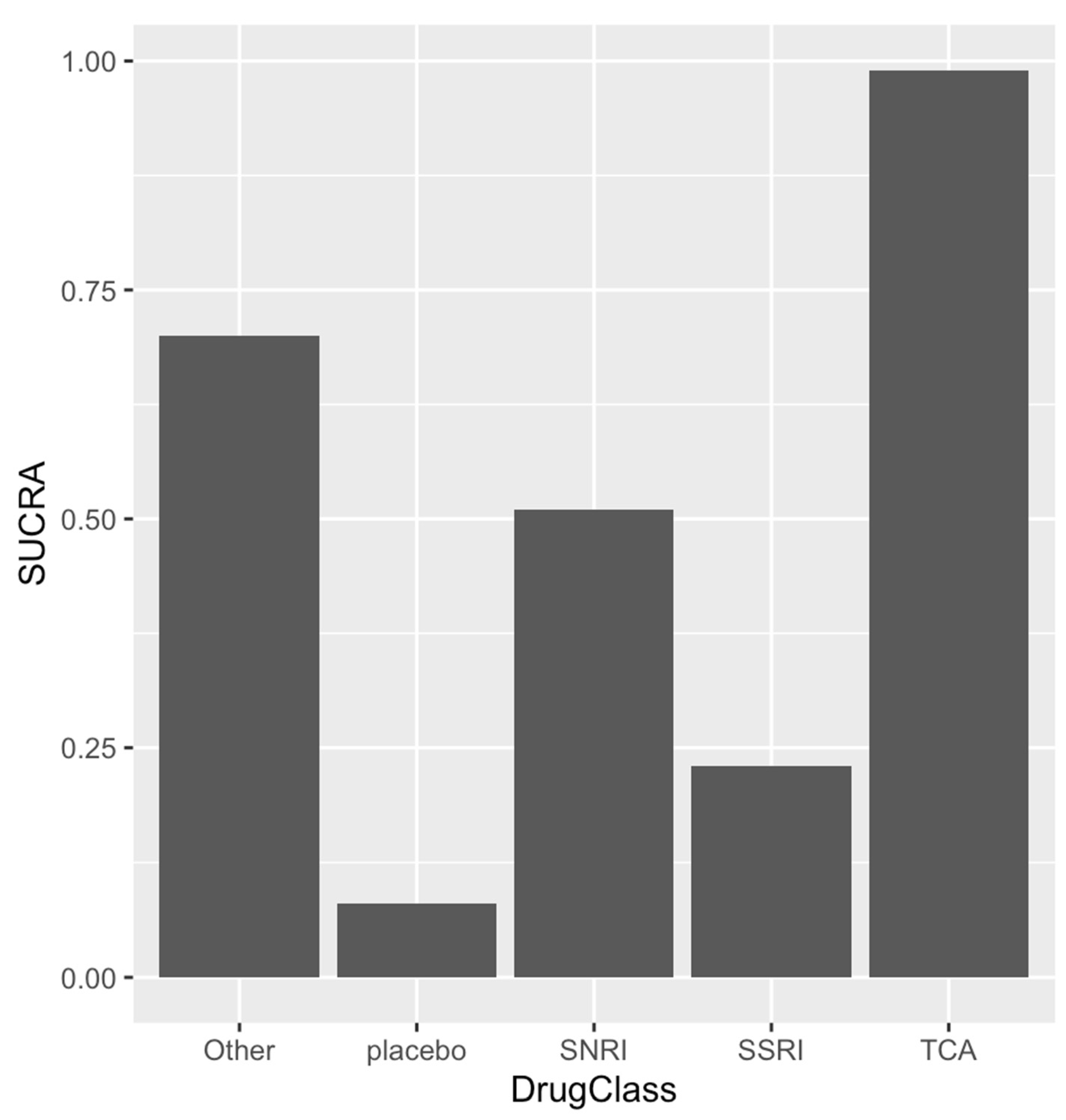
| Author | Country | Type of Study | Sex (Female%) | Participants | Mean Age (Years) | Mean Follow Up (Months) | Medications Ascertainment | Outcome Ascertainment | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Angerman 2016 | USA | RCT | 24 | HF patients with depression | 62 | 18.5 | Data and safety committee | Chart review adjudicated by steering committee | Referred to supplementary |
| Leonerd 2011 | USA | Prospective cohort study | N/A | Antidepressant users from Medicaid data from 5 large states | N/A | N/A | Mapping national drug code to Lexicom | ICD-9 | 8 |
| Martinez 2010 | Australia | Case-control | 56.7 | New antidepressant users from UK database | 72.9 | 39.6 | Prescription records from UK-GPRD | Read/OXMIS codes | 9 |
| Qirjazi 2016 | Canada | Retrospective cohort study | 66 | Participants who took SSRI | 76 | 3 | Data from the Ontario Drug Benefit database | ICD-10 | 6 |
| Ray 2017 | USA | Retrospective cohort study | 76 | Participants who took high-dose SSRI | 47 | 8.4 | Tennessee Medicaid files | death certificate-Medicaid enrollment and ICD 9/10 | 8 |
| Lin 2019 | Taiwan | Retrospective cohort study | 60 | Participants with depression who took medications | N/A | N/A | Longitudinal Health Insurance Databases | ICD-9 | 7 |
| Wu 2017 | Taiwan | Retrospective cohort study | 63.9 | Participants with depression who took medications | N/A | 2.5 | Taiwan’s National Health Insurance Research Database | ICD-9CM | 7 |
| Zivin 2013 | USA | Retrospective cohort study | 9.6 | VA Participants with depression who took medications | N/A | N/A | VHA National Registry for Depression | ICD-9 | 8 |
| Conditions | Countermeasures | |
|---|---|---|
| Metabolic derangements | Hypokalemia, Hypomagnesemia, Hypocalcemia | Correct electrolytes |
| Bradyarrhythmias | Sick sinus syndrome, AV block | Correct reversible causes, Pacemaker implantation |
| Antidepressants | TCA, SSRI, SRNI, atypical antidepressants such as Trazodone, Atomoxetine | Avoidance, removal, serial ECG monitoring |
| Antipsychotics | Haloperidol, Clozapine, Chlorpromazine, Risperidone, thioridazine | Avoidance, removal, serial ECG monitoring, |
| Antiarrhythmic drugs | Class Ia, Ic and class III | Avoidance, removal, serial ECG monitoring, |
| Antibiotics | Macrolides, Fluroquinolones, Azoles, Quinines, Quinidine, Antiretroviral drugs | Avoidance, removal, serial ECG monitoring, |
| Gastrointestinal drugs | Cisapride, Metoclopramide, Domperidone, Ondansetron | Avoidance, removal, serial ECG monitoring, |
| Others | Myocardial infarction, increased intracranial pressures, hypothermia, organophosphate poisoning, cocaine intoxication, | Address underlying, Avoidance offending agents, antidotes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasitlumkum, N.; Cheungpasitporn, W.; Tokavanich, N.; Ding, K.R.; Kewcharoen, J.; Thongprayoon, C.; Kaewput, W.; Bathini, T.; Vallabhajosyula, S.; Chokesuwattanaskul, R. Antidepressants and Risk of Sudden Cardiac Death: A Network Meta-Analysis and Systematic Review. Med. Sci. 2021, 9, 26. https://doi.org/10.3390/medsci9020026
Prasitlumkum N, Cheungpasitporn W, Tokavanich N, Ding KR, Kewcharoen J, Thongprayoon C, Kaewput W, Bathini T, Vallabhajosyula S, Chokesuwattanaskul R. Antidepressants and Risk of Sudden Cardiac Death: A Network Meta-Analysis and Systematic Review. Medical Sciences. 2021; 9(2):26. https://doi.org/10.3390/medsci9020026
Chicago/Turabian StylePrasitlumkum, Narut, Wisit Cheungpasitporn, Nithi Tokavanich, Kimberly R. Ding, Jakrin Kewcharoen, Charat Thongprayoon, Wisit Kaewput, Tarun Bathini, Saraschandra Vallabhajosyula, and Ronpichai Chokesuwattanaskul. 2021. "Antidepressants and Risk of Sudden Cardiac Death: A Network Meta-Analysis and Systematic Review" Medical Sciences 9, no. 2: 26. https://doi.org/10.3390/medsci9020026
APA StylePrasitlumkum, N., Cheungpasitporn, W., Tokavanich, N., Ding, K. R., Kewcharoen, J., Thongprayoon, C., Kaewput, W., Bathini, T., Vallabhajosyula, S., & Chokesuwattanaskul, R. (2021). Antidepressants and Risk of Sudden Cardiac Death: A Network Meta-Analysis and Systematic Review. Medical Sciences, 9(2), 26. https://doi.org/10.3390/medsci9020026








