Incidence and Impacts of Inflammatory Bowel Diseases among Kidney Transplant Recipients: A Meta-Analysis
Abstract
1. Introduction
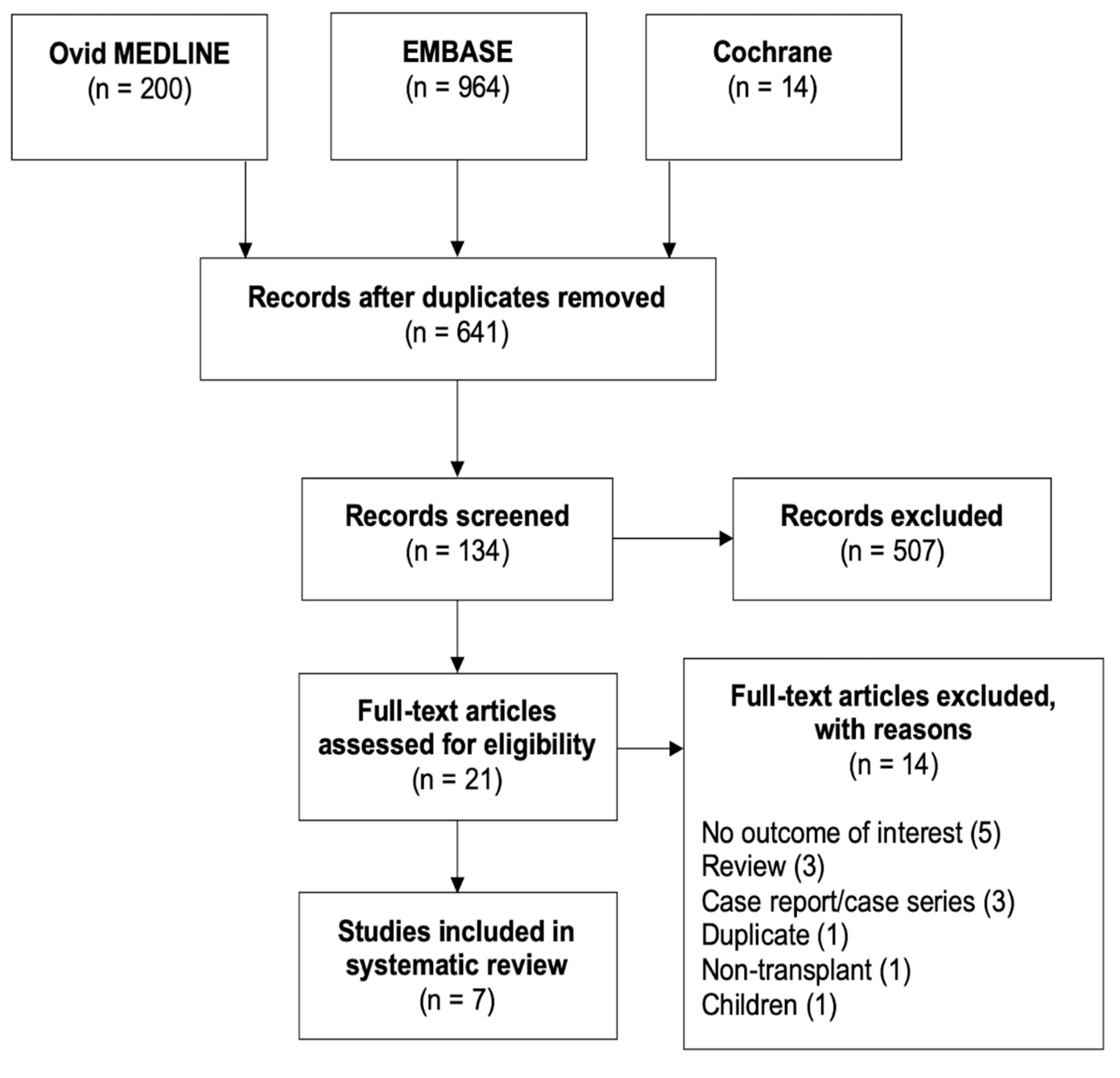
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Review Process and Data Extraction
2.4. Measurements
2.5. Subgroup Analysis, Meta-Regression Analysis, and Publication Bias
2.6. Statistical Analysis
3. Results
3.1. Study Characteristics
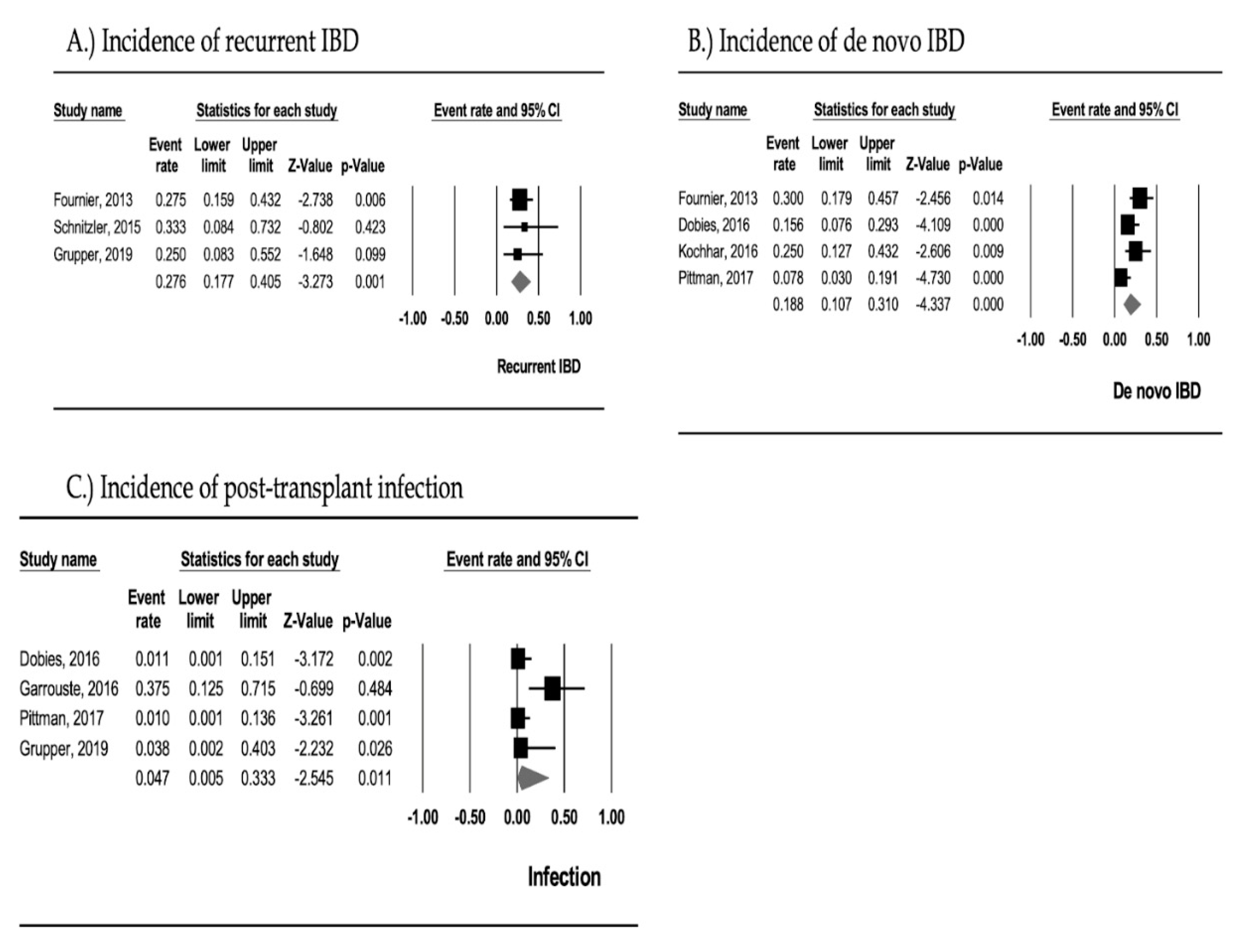
3.2. Recurrent IBD
3.3. De Novo IBD
3.4. Post-Transplant Infection
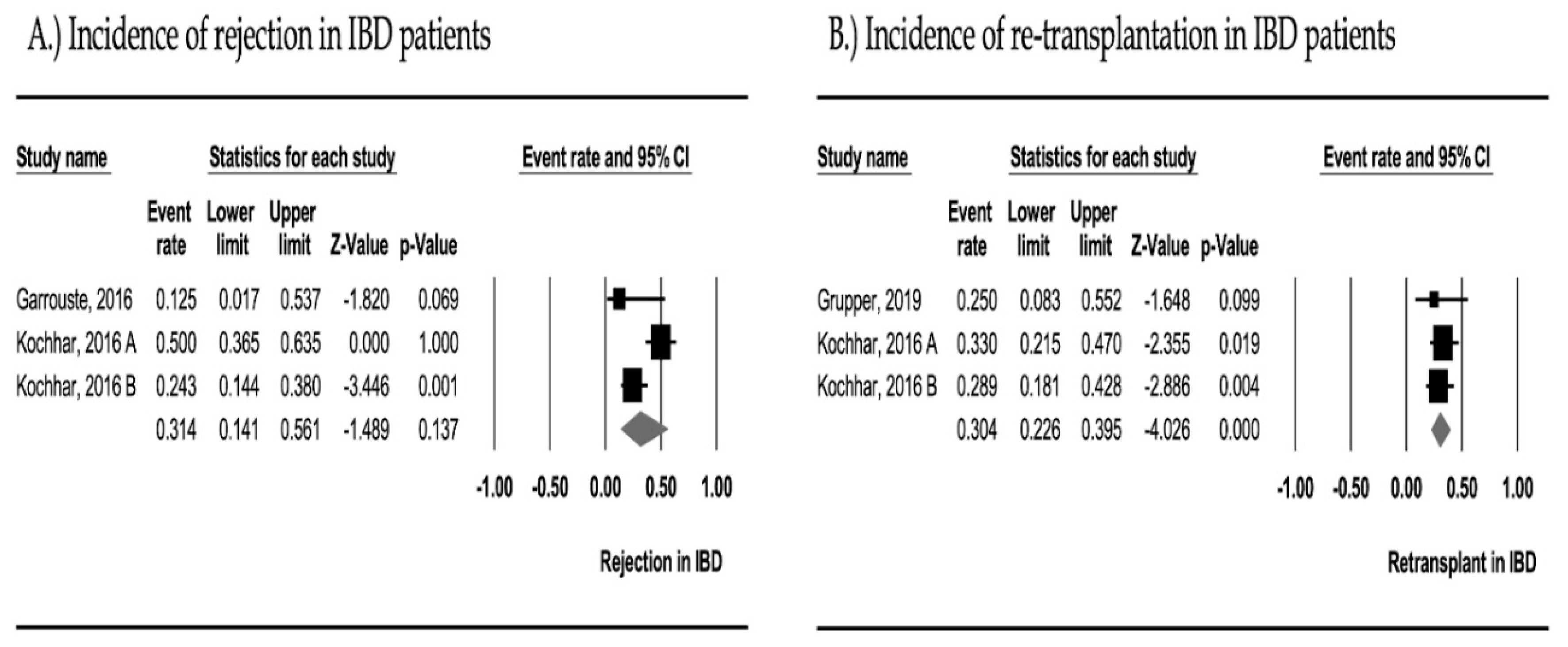
3.5. Graft Rejection
3.6. Re-Transplantation
3.7. Subgroup Analyses
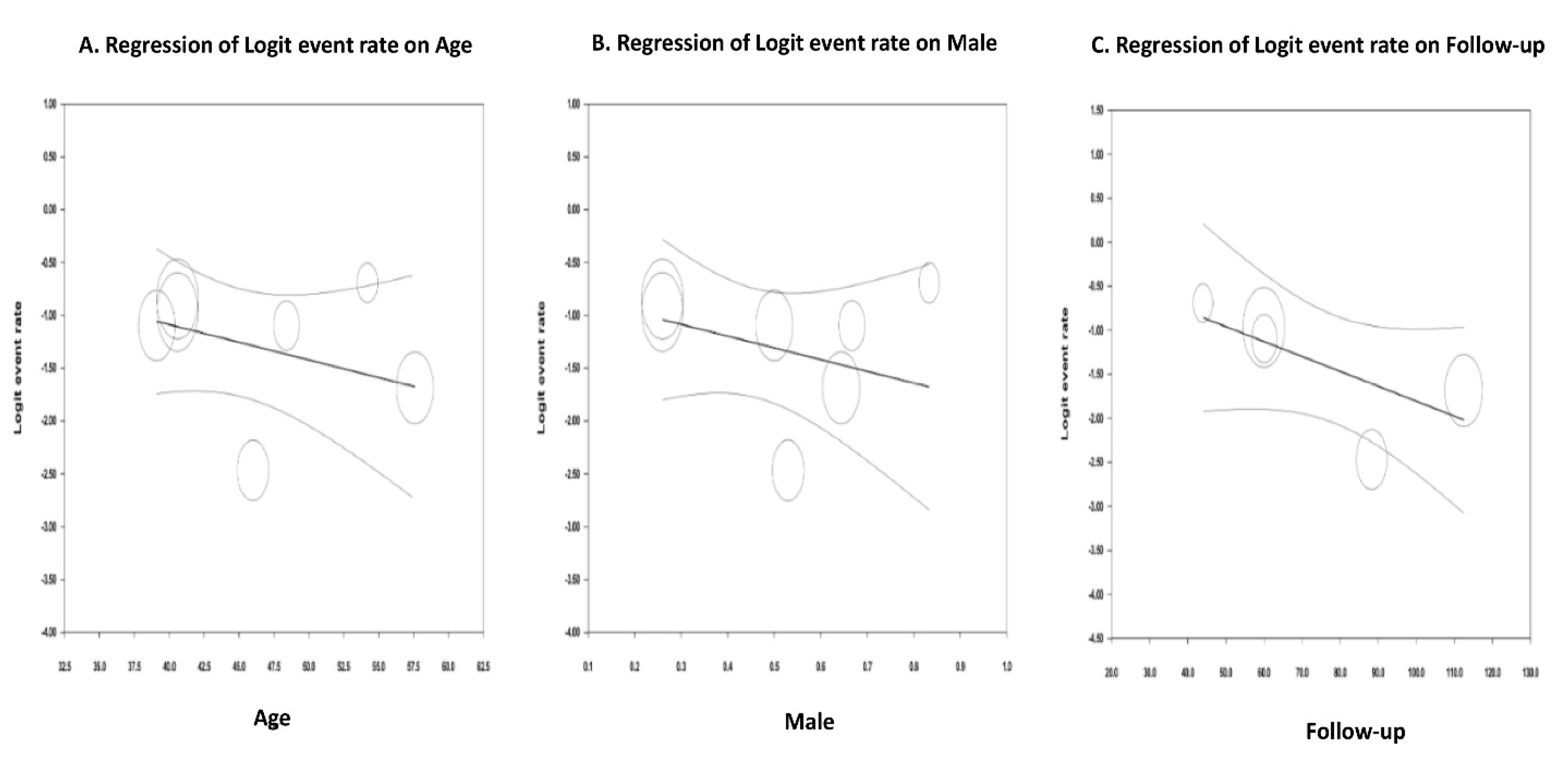
3.8. Meta-Regression Analyses
Evaluation for Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Verdonk, R.C.; Dijkstra, G.; Haagsma, E.B.; Shostrom, V.K.; Van den Berg, A.P.; Kleibeuker, J.H.; Langnas, A.N.; Sudan, D.L. Inflammatory bowel disease after liver transplantation: Risk factors for recurrence and de novo disease. Am. J. Transplant 2006, 6, 1422–1429. [Google Scholar] [CrossRef]
- Nannegari, V.; Roque, S.; Rubin, D.T.; Quera, R. A Review of Inflammatory Bowel Disease in the Setting of Liver Transplantation. Gastroenterol. Hepatol. 2014, 10, 626–630. [Google Scholar]
- Filipec Kanizaj, T.; Mijic, M. Inflammatory bowel disease in liver transplanted patients. World J. Gastroenterol. 2017, 23, 3214–3227. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.V.; Hardinger, K.L. Current state of renal transplant immunosuppression: Present and future. World J. Transplant. 2012, 2, 51–68. [Google Scholar] [CrossRef]
- Manz, M.; Vavricka, S.R.; Wanner, R.; Lakatos, P.L.; Rogler, G.; Frei, P.; Safroneeva, E.; Schoepfer, A.M. Therapy of steroid-resistant inflammatory bowel disease. Digestion 2012, 86 (Suppl. 10), 11–15. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Schroll, J.B.; Moustgaard, R.; Gøtzsche, P.C. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med. Res. Methodol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Dobies, A.; Renke, M.; Wołyniec, W.; Palenicek, L.; Januszczyk, J.; Król, E.; Lizakowski, S.; Rutkowski, P.; Tylicki, L.; Dębska-Ślizień, A.; et al. Gastrointestinal Pathologies in Patients After Successful Renal Transplantation—A Pilot Study. Transplant. Proc. 2016, 48, 1566–1569. [Google Scholar] [CrossRef] [PubMed]
- Pittman, M.E.; Jessurun, J.; Yantiss, R.K. Differentiating Posttransplant Inflammatory Bowel Disease and Other Colitides in Renal Transplant Patients. Am. J. Surg. Pathol. 2017, 41, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.; Barbet, C.; Toupance, O.; Etienne, I.; Hurault De Ligny, B.; Lemeur, Y.; Rerolle, J.; Ohlmann, S.; Tiple, A.; Anglicheau, D.; et al. Inflammatory bowel disease in renal transplant recipients: A retrospective multicenter study. Am. J. Transplant. 2013, 13, 437. [Google Scholar] [CrossRef]
- Schnitzler, F.; Friedrich, M.; Stallhofer, J.; Schönermarck, U.; Fischereder, M.; Habicht, A.; Karbalai, N.; Wolf, C.; Angelberger, M.; Olszak, T.; et al. Solid organ transplantation in patients with inflammatory bowel diseases (IBD): Analysis of transplantation outcome and IBD activity in a large single center cohort. PLoS ONE 2015, 10, e0135807. [Google Scholar] [CrossRef] [PubMed]
- Garrouste, C.; Anglicheau, D.; Kamar, N.; Bachelier, C.; Rivalan, J.; Pereira, B.; Caillard, S.; Aniort, J.; Gatault, P.; Soubrier, M.; et al. Anti-TNFα therapy for chronic inflammatory disease in kidney transplant recipients Clinical outcomes. Medicine 2016, 95, e5108. [Google Scholar] [CrossRef]
- Kochhar, G.; Chua, T.; Alsuleiman, B.; Lopez, R.; Gordon, I.; Shen, B. De novo inflammatory bowel diseases after renal transplantation. Am. J. Gastroenterol. 2016, 111, S321–S322. [Google Scholar] [CrossRef]
- Grupper, A.; Schwartz, D.; Baruch, R.; Schwartz, I.F.; Nakache, R.; Goykhman, Y.; Katz, P.; Lebedinsky, A.; Nachmany, I.; Lubezky, N.; et al. Kidney transplantation in patients with inflammatory bowel diseases (IBD): Analysis of transplantation outcome and IBD activity. Transplant. Int. 2019, 32, 730–738. [Google Scholar] [CrossRef]
- Easterbrook, P.J.; Berlin, J.A.; Gopalan, R.; Matthews, D.R. Publication bias in clinical research. Lancet 1991, 337, 867–872. [Google Scholar] [CrossRef]
- Sattianayagam, P.T.; Gillmore, J.D.; Pinney, J.H.; Gibbs, S.D.; Wechalekar, A.D.; Gilbertson, J.A.; Rowczenio, D.; Hawkins, P.N.; Lachmann, H.J. Inflammatory bowel disease and systemic AA amyloidosis. Dig. Dis. Sci. 2013, 58, 1689–1697. [Google Scholar] [CrossRef]
- Bots, S.J.; Kuin, S.; Ponsioen, C.Y.; Gecse, K.B.; Duijvestein, M.; D’Haens, G.R.; Löwenberg, M. Relapse rates and predictors for relapse in a real-life cohort of IBD patients after discontinuation of anti-TNF therapy. Scand. J. Gastroenterol. 2019, 54, 281–288. [Google Scholar] [CrossRef]
- Jørgensen, K.K.; Lindström, L.; Cvancarova, M.; Karlsen, T.H.; Castedal, M.; Friman, S.; Schrumpf, E.; Foss, A.; Isoniemi, H.; Nordin, A.; et al. Immunosuppression after liver transplantation for primary sclerosing cholangitis influences activity of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2013, 11, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Cholongitas, E.; Papatheodoridis, G.V.; Zappoli, P.; Giannakopoulos, A.; Patch, D.; Marelli, L.; Shusang, V.; Kalambokis, G.; Shirling, G.; Rolando, N.; et al. Combined HLA-DR and -DQ disparity is associated with a stable course of ulcerative colitis after liver transplantation for primary sclerosing cholangitis. Liver Transpl. 2007, 13, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.; Woodroffe, R.C.; Taylor, R.S.; Chapman, J.R.; Craig, J.C. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Alameel, T.; Dale, C.H.; Levstik, M.; Chande, N. The safety and efficacy of antitumour necrosis factor-alpha therapy for inflammatory bowel disease in patients post liver transplantation: A case series. Aliment. Pharmacol. Ther. 2012, 36, 159–165. [Google Scholar] [CrossRef]
- El-Nachef, N.; Terdiman, J.; Mahadevan, U. Anti-tumor necrosis factor therapy for inflammatory bowel disease in the setting of immunosuppression for solid organ transplantation. Am. J. Gastroenterol. 2010, 105, 1210–1211. [Google Scholar] [CrossRef]
- Lal, S.; Steinhart, A.H. Infliximab for ulcerative colitis following liver transplantation. Eur. J. Gastroenterol. Hepatol. 2007, 19, 277–280. [Google Scholar] [CrossRef]
- Imagawa, D.K.; Millis, J.M.; Seu, P.; Olthoff, K.M.; Hart, J.; Wasef, E.; Dempsey, R.A.; Stephens, S.; Busuttil, R.W. The role of tumor necrosis factor in allograft rejection. III. Evidence that anti-TNF antibody therapy prolongs allograft survival in rats with acute rejection. Transplantation 1991, 51, 57–62. [Google Scholar] [CrossRef]
- Gabe, S.M.; Bjarnason, I.; Tolou-Ghamari, Z.; Tredger, J.M.; Johnson, P.G.; Barclay, G.R.; Williams, R.; Silk, D.B. The effect of tacrolimus (FK506) on intestinal barrier function and cellular energy production in humans. Gastroenterology 1998, 115, 67–74. [Google Scholar] [CrossRef]
- Shibutani, S.; Inoue, F.; Aramaki, O.; Akiyama, Y.; Matsumoto, K.; Shimazu, M.; Kitajima, M.; Ikeda, Y.; Shirasugi, N.; Niimi, M. Effects of immunosuppressants on induction of regulatory cells after intratracheal delivery of alloantigen. Transplantation 2005, 79, 904–913. [Google Scholar] [CrossRef]
- Demirkiran, A.; Kok, A.; Kwekkeboom, J.; Metselaar, H.J.; Tilanus, H.W.; van der Laan, L.J. Decrease of CD4+CD25+ T cells in peripheral blood after liver transplantation: Association with immunosuppression. Transplant. Proc. 2005, 37, 1194–1196. [Google Scholar] [CrossRef]
- Baan, C.C.; van der Mast, B.J.; Klepper, M.; Mol, W.M.; Peeters, A.M.; Korevaar, S.S.; Balk, A.H.; Weimar, W. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation 2005, 80, 110–117. [Google Scholar] [CrossRef]
- Takahashi, K.; Reynolds, M.; Ogawa, N.; Longo, D.L.; Burdick, J. Augmentation of T-cell apoptosis by immunosuppressive agents. Clin. Transplant. 2004, 18 (Suppl. 12), 72–75. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.; Osen, W.; Debatin, K.M. Induction of apoptosis and modulation of activation and effector function in T cells by immunosuppressive drugs. Clin. Exp. Immunol. 2002, 128, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Sandalova, E.; Wei, C.H.; Masucci, M.G.; Levitsky, V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc. Natl. Acad. Sci. USA 2004, 101, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Liverani, E.; Scaioli, E.; Digby, R.J.; Bellanova, M.; Belluzzi, A. How to predict clinical relapse in inflammatory bowel disease patients. World J. Gastroenterol. 2016, 22, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Ferraresso, M.; Berardinelli, L. Nosocomial infection in kidney transplant recipients: A retrospective analysis of a single-center experience. Transplant Proc. 2005, 37, 2495–2496. [Google Scholar] [CrossRef]
- Saran, R.; Li, Y.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Ayanian, J.; Bragg-Gresham, J.; Balkrishnan, R.; Chen, J.L.; Cope, E.; et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2016, 67, S1–S305. [Google Scholar] [CrossRef]
- Marcén, R.; Fernández-Rodriguez, A.; Rodríguez-Mendiola, N.; Ponte, B.; Galeano, C.; Villafruela, J.J.; Teruel, J.L.; Burgos, F.J.; Ortuño, J. Evolution of rejection rates and kidney graft survival: A historical analysis. Transplant. Proc. 2009, 41, 2357–2359. [Google Scholar] [CrossRef]
| Study | Country | Design | N | Subjects | Age, yr | Male, % | Regimen | Anti-TNF Pre-KTx | Diarrhea, % | Abdominal Pain, % | Anemia or Bleeding, % | Outcomes | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fournier, 2013 [13] | France | R | 40 | IBD + KTx | 40.6 | 26 | - | - | - | - | - | Recurrent IBD: 27.5% De novo IBD: 30.0% Median delay of flare-up: 17 mo in recurrent IBD, 91 mo in de novo IBD CMV: 36.0% in recurrent IBD, 5.9% in no recurrent IBD | - |
| Schnitzler, 2015 [14] | Germany | P | 6 | IBD + KTx | 54.2 | 83.3 | 66.7% CsA, 33.3% Tac | 0% | - | - | - | Recurrent IBD: 33.3% | 112.5 mo |
| Dobies, 2016 [11] | Poland | P | 45 | KTx | 57.6 | 64.4 | - | - | 40 | 37.8 | 15.6 | De novo IBD: 15.6% Infection: 0% | - |
| Garrouste, 2016 [15] | France | P | 8 | IBD + KTx + anti-TNF | 46.5 | 50 | - | 100% | - | - | - | Infection: 37.5% Cancer: 37.5% Acute rejection: 12.5% | 88.3 mo |
| Kochhar, 2016 [16] | USA | R | 50 | KTx | 39.1 | 50 | - | - | - | - | - | De novo IBD: 25% Graft rejection in de novo IBD (50.0%) vs. non-de novo IBD (24.3%) Re-transplantation in de novo IBD (33%) vs. non-de novo IBD groups (28.9%), p = 0.32 | 60 mo |
| Pittman, 2017 [12] | USA | R | 51 | KTx | 46 | 53 | - | - | 57 | 12 | 27 | De novo IBD: 7.8% (25% CD, 75% UC) Infection: 0% | 44 mo |
| Grupper, 2019 [17] | Israel | R | 12 | IBD + KTx (41.7% UC, 58.3% CD) | 48.4 | 66.7 | 91.7% CNI, MMF, Pred | 50% | - | - | - | Recurrent IBD: 25% (33.3% UC, 66.7% CD) Re-transplantation: 16.7% Infection: 0% | 60.1 mo |
| Subgroup Analyses | ||||
|---|---|---|---|---|
| Subgroup | N | Incidence | 95% CI | |
| Year | ||||
| ≤2015 | 3 | 29.1% | 20.5–39.5 | |
| >2015 | 4 | 16.9% | 10.0–27.1 | Q = 3.267, p = 0.071 |
| Country | ||||
| USA | 2 | 14.8% | 4.3–39.9 | |
| Others | 5 | 25.1% | 18.6–33.0 | Q = 0.854, p = 0.356 |
| Study design | ||||
| Prospective | 2 | 18.5% | 9.4–33.2 | |
| Retrospective | 5 | 22.6% | 14.8–33.1 | Q = 0.281, p = 0.596 |
| Disease | ||||
| Recurrent IBD | 3 | 27.6% | 17.7–40.5 | |
| De novo IBD | 4 | 18.8% | 10.7–31.0 | Q = 1.239, p = 0.096 |
| Ethnicity | ||||
| White | 4 | 25.1% | 18.3–33.5 | |
| Others | 3 | 17.3% | 7.7–34.4 | Q = 0.849, p = 0.357 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansrivijit, P.; Puthenpura, M.M.; Thongprayoon, C.; Brar, H.S.; Bathini, T.; Kovvuru, K.; Kanduri, S.R.; Wijarnpreecha, K.; Cheungpasitporn, W. Incidence and Impacts of Inflammatory Bowel Diseases among Kidney Transplant Recipients: A Meta-Analysis. Med. Sci. 2020, 8, 39. https://doi.org/10.3390/medsci8030039
Hansrivijit P, Puthenpura MM, Thongprayoon C, Brar HS, Bathini T, Kovvuru K, Kanduri SR, Wijarnpreecha K, Cheungpasitporn W. Incidence and Impacts of Inflammatory Bowel Diseases among Kidney Transplant Recipients: A Meta-Analysis. Medical Sciences. 2020; 8(3):39. https://doi.org/10.3390/medsci8030039
Chicago/Turabian StyleHansrivijit, Panupong, Max M. Puthenpura, Charat Thongprayoon, Himmat S. Brar, Tarun Bathini, Karthik Kovvuru, Swetha R. Kanduri, Karn Wijarnpreecha, and Wisit Cheungpasitporn. 2020. "Incidence and Impacts of Inflammatory Bowel Diseases among Kidney Transplant Recipients: A Meta-Analysis" Medical Sciences 8, no. 3: 39. https://doi.org/10.3390/medsci8030039
APA StyleHansrivijit, P., Puthenpura, M. M., Thongprayoon, C., Brar, H. S., Bathini, T., Kovvuru, K., Kanduri, S. R., Wijarnpreecha, K., & Cheungpasitporn, W. (2020). Incidence and Impacts of Inflammatory Bowel Diseases among Kidney Transplant Recipients: A Meta-Analysis. Medical Sciences, 8(3), 39. https://doi.org/10.3390/medsci8030039






