Patterns of Glycemic Variability During a Diabetes Self-Management Educational Program
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Participants
2.4. Procedures
2.5. Statistical Analysis
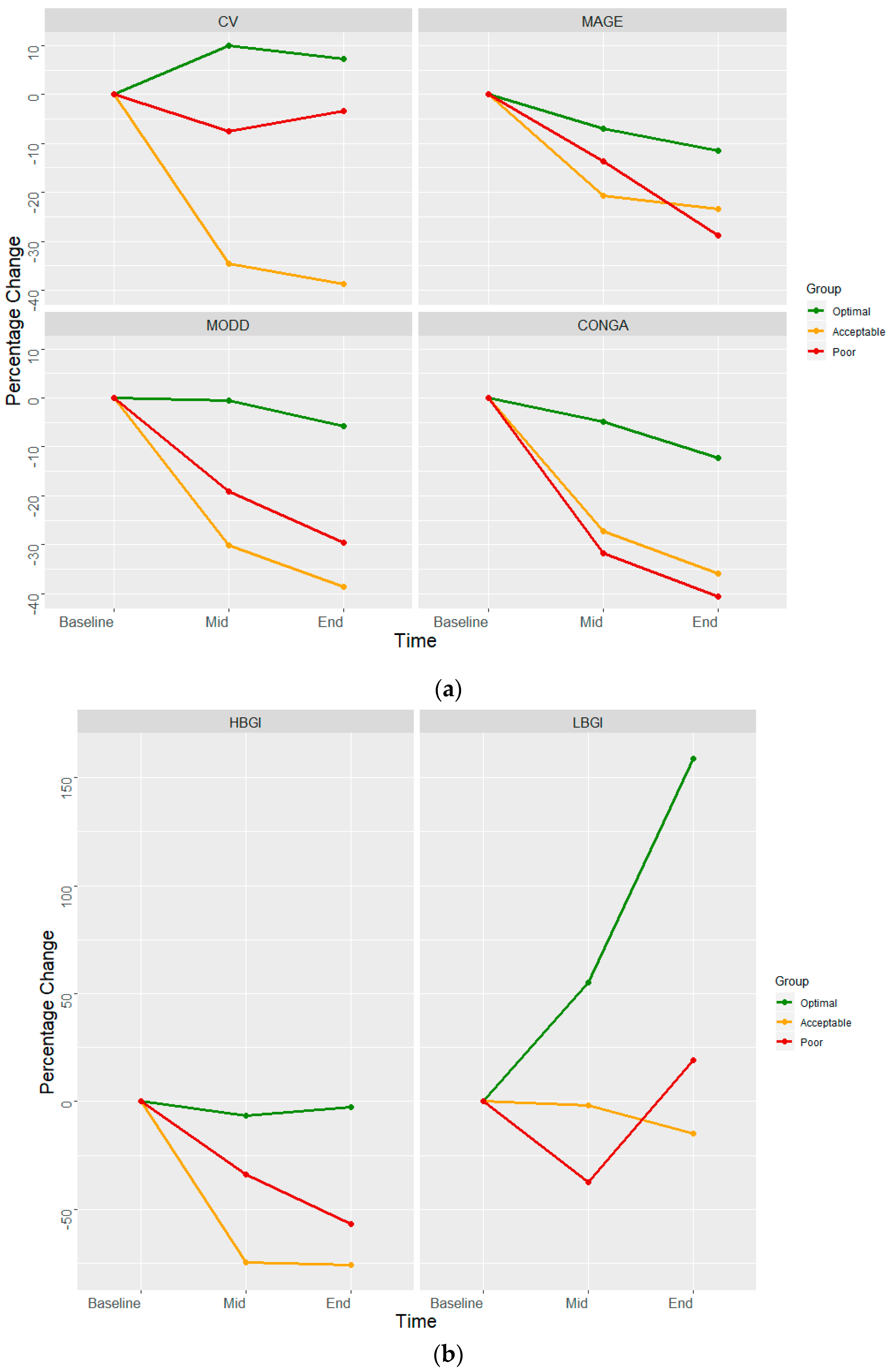
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet Lond. Engl. 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Association, A.D. 11. Older Adults: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41 (Suppl. 1), S119–S125. [Google Scholar] [CrossRef] [PubMed]
- Raccah, D.; Chou, E.; Colagiuri, S.; Gaàl, Z.; Lavalle, F.; Mkrtumyan, A.; Nikonova, E.; Tentolouris, N.; Vidal, J.; Davies, M. A Global Study of the Unmet Need for Glycemic Control and Predictor Factors among Patients with Type 2 Diabetes Mellitus Who Have Achieved Optimal Fasting Plasma Glucose Control on Basal Insulin. Diabetes Metab. Res. Rev. 2017, 33. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5347910/ (accessed on 11 September 2018). [CrossRef] [PubMed]
- Ceriello, A.; Monnier, L.; Owens, D. Glycaemic Variability in Diabetes: Clinical and Therapeutic Implications. Lancet Diabetes Endocrinol. 2019, 7, 221–230. Available online: https://www.thelancet.com/journals/landia/article/PIIS2213-8587(18)30136-0/abstract (accessed on 11 September 2018). [CrossRef]
- Dandona, P. Minimizing Glycemic Fluctuations in Patients with Type 2 Diabetes: Approaches and Importance. Diabetes Technol. Ther. 2017, 19, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mamas, M.A. Long-term Glycemic Variability and Risk of Adverse Outcomes: A Systematic Review and Meta-analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef]
- Lee, C.-L.; Sheu, W.H.-H.; Lee, I.-T.; Lin, S.-Y.; Liang, W.-M.; Wang, J.-S.; Li, Y.-F. Trajectories of fasting plasma glucose variability and mortality in type 2 diabetes. Diabetes Metab. 2018, 44, 121–128. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Service, F.J.; Molnar, G.D.; Rosevear, J.W.; Ackerman, E.; Gatewood, L.C.; Taylor, W.F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970, 19, 644–655. [Google Scholar] [CrossRef]
- Service, F.J.; Nelson, R.L. Characteristics of Glycemic Stability. Diabetes Care 1980, 3, 58–62. [Google Scholar] [CrossRef]
- Rodbard, D. Interpretation of continuous glucose monitoring data: Glycemic variability and quality of glycemic control. Diabetes Technol. Ther. 2009, 11 (Suppl. 1), S55–S67. [Google Scholar] [CrossRef]
- Service, F.J. Glucose Variability. Diabetes 2013, 62, 1398–1404. [Google Scholar] [CrossRef]
- Marling, C.R.; Shubrook, J.H.; Vernier, S.J.; Wiley, M.T.; Schwartz, F.L. Characterizing blood glucose variability using new metrics with continuous glucose monitoring data. J. Diabetes Sci. Technol. 2011, 5, 871–878. [Google Scholar] [CrossRef]
- Kovatchev, B.P.; Otto, E.; Cox, D.; Gonder-Frederick, L.; Clarke, W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006, 29, 2433–2438. [Google Scholar] [CrossRef]
- McDonnell, C.M.; Donath, S.M.; Vidmar, S.I.; Werther, G.A.; Cameron, F.J. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol. 2005, 7, 253–263. [Google Scholar] [CrossRef]
- Service, F.J.; O’Brien, P.C.; Rizza, R.A. Measurements of glucose control. Diabetes Care 1987, 10, 225–237. [Google Scholar] [CrossRef]
- Peyser, T.A.; Balo, A.K.; Buckingham, B.A.; Hirsch, I.B.; Garcia, A. Glycemic Variability Percentage: A Novel Method for Assessing Glycemic Variability from Continuous Glucose Monitor Data. Diabetes Technol. Ther. 2018, 20, 6–16. [Google Scholar] [CrossRef]
- Kohnert, K.-D.; Heinke, P.; Vogt, L.; Augstein, P.; Thomas, A.; Salzsieder, E. Associations of blood glucose dynamics with antihyperglycemic treatment and glycemic variability in type 1 and type 2 diabetes. J. Endocrinol. Investig. 2017, 40, 1201–1207. [Google Scholar] [CrossRef]
- Hirsch, I.B.; Balo, A.K.; Sayer, K.; Garcia, A.; Buckingham, B.A.; Peyser, T.A. A Simple Composite Metric for the Assessment of Glycemic Status from Continuous Glucose Monitoring Data: Implications for Clinical Practice and the Artificial Pancreas. Diabetes Technol. Ther. 2017, 19 (Suppl. 3), S38–S48. [Google Scholar] [CrossRef]
- Kohnert, K.-D.; Heinke, P.; Vogt, L.; Salzsieder, E. Utility of different glycemic control metrics for optimizing management of diabetes. World J. Diabetes 2015, 6, 17–29. [Google Scholar] [CrossRef]
- Murphy, M.E.; Byrne, M.; Galvin, R.; Boland, F.; Fahey, T.; Smith, S.M. Improving risk factor management for patients with poorly controlled type 2 diabetes: A systematic review of healthcare interventions in primary care and community settings. BMJ Open 2017, 7, e015135. [Google Scholar] [CrossRef]
- Health Quality Ontario. Behavioural interventions for type 2 diabetes: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2009, 9, 1–45. [Google Scholar]
- Cheng, L.; Sit, J.W.H.; Choi, K.-C.; Chair, S.-Y.; Li, X.; He, X. Effectiveness of Interactive Self-Management Interventions in Individuals with Poorly Controlled Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Worldviews Evid. Based Nurs. 2017, 14, 65–73. [Google Scholar] [CrossRef]
- American Diabetes Association. 6. Glycemic Targets: Standards of Medical Care in Diabetes. Diabetes Care 2018, 41 (Suppl. 1), S55–S64. [Google Scholar] [CrossRef]
- Meduru, P.; Helmer, D.; Rajan, M.; Tseng, C.L.; Pogach, L.; Sambamoorthi, U. Chronic illness with complexity: Implications for performance measurement of optimal glycemic control. J. Gen. Intern. Med. 2007, 22, 408–418. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 22 January 2019).
- Guo, S.H. Assessing quality of glycemic control: Hypo-and hyperglycemia, and glycemic variability using mobile self-monitoring of blood glucose system. Health Inform. J. 2019. [Google Scholar] [CrossRef]
- Taylor, P.J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Wittert, G.; Brinkworth, G.D. Efficacy of Real-Time Continuous Glucose Monitoring to Improve Effects of a Prescriptive Lifestyle Intervention in Type 2 Diabetes: A Pilot Study. Diabetes Ther. 2019, 1–14. [Google Scholar] [CrossRef]
- Thompson, S.V.; Winham, D.M.; Hutchins, A.M. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr. J. 2012, 11, 23. [Google Scholar] [CrossRef]
| GV Measure | Formula | Interpretation | |
|---|---|---|---|
| SD | where: xi = individual observation = mean of observation k = number of observations | Traditional measure of dispersion; Measures short-term, within-day variability; Easy to compute, used very often | |
| % CV | × 100 | where: s = standard deviation = mean of observation | Traditional measure of dispersion, standardized for mean; Measures short-term, within-day variability; Easy to compute using mean and standard deviation |
| MAGE | if: λ > υ where: λ = each blood glucose increase or decrease n = number of observations υ = 1 SD of mean glucose for 24 hour period | Average of all glycemic excursions (except excursion having value <1 SD from mean glucose) in a 24 h time period; Captures short-term, within-day variability; Most commonly used | |
| CONGA | where: k* = No. of observations where, there is an observation m mins ago GRt = glucose reading at time t m = n × 60 Dt = GRt − GRt−m | Standard deviation of summated difference between current observation and previous observation; Captures short-term, within-day variability; Complex calculation, specifically developed for CGM | |
| MODD | where: = 1440 (60 × 24); if reading taken every 1 min 96 (4 × 24); if reading taken every 15 min 24 (1 × 24); if reading taken every 60 min | 24 h mean absolute differences between two values measured at the same timepoint; short-term, inter-day variation; Needs additional computation | |
| HBGI | where: | Log transformation of glucose values; Captures risk for predicting severe glycaemia; Complex calculation, easy to interpret | |
| LBGI | where: | Log transformation of glucose values; Captures risk for predicting severe hyperglycaemia (HGBI); Complex calculation, easy to interpret | |
| Domain | Activity | Days | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| Knowledge | Understanding diabetes | ✓ | ✓ | ✓ | |||||||||||
| Understanding therapies | ✓ | ✓ | |||||||||||||
| Improving self-care | ✓ | ✓ | |||||||||||||
| Foot care | ✓ | ||||||||||||||
| Physical activity | A 30-min brisk walk | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Using activity trackers | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Yoga/meditation | ✓ | ✓ | ✓ | ||||||||||||
| Physical activity rewards | ✓ | ✓ | |||||||||||||
| Nutrition | Meal planning | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Low-GI breakfast | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Food diary feedback | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Meal planning rewards | ✓ | ✓ | |||||||||||||
| Behavior | Stress reduction | ✓ | ✓ | ||||||||||||
| Coping skills | ✓ | ✓ | |||||||||||||
| Tobacco cessation | ✓ | ✓ | |||||||||||||
| Disease management | CGM insertion/removal | ✓ | ✓ | ||||||||||||
| CGM readings and feedback | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Screening for complications | ✓ | ||||||||||||||
| Drug prescription review | ✓ | ✓ | |||||||||||||
| Activity count | 4 | 4 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 7 | |
| Baseline Characteristic | Optimal Control (n = 12) | Acceptable Control (n = 12) | Poor Control (n = 22) | Overall (n = 46) |
|---|---|---|---|---|
| Mean (SD) or N (%) | ||||
| Male | 8 (66.7%) | 6 (50%) | 10 (45.5%) | 24 (52.2%) |
| Female | 4 (33.3%) | 6 (50%) | 12 (54.5%) | 22 (47.8%) |
| Age | 56.7 (13.2) | 54.5 (10.6) | 52.2 (11.2) | 54.0 (11.5) |
| BMI | 26.5 (2.6) | 25.8 (4.4) | 26.5 (5.1) | 26.3 (4.3) |
| Waist Circumference (cm) | 97.8 (7.8) | 93.4 (7.8) | 97.7 (10.2) | 96.59 (9.1) |
| Hip Circumference (cm) | 100 (7.3) | 99.3 (7.7) | 103 (11.5) | 101.4 (9.6) |
| WHR | 0.98 (0.1) | 0.94 (0.1) | 0.95 (0.1) | 0.96 (0.1) |
| SBP | 126 (22) | 130 (9.9) | 132 (17.8) | 129.8 (17.2) |
| DBP | 82.5 (10.4) | 77.8 (10.3) | 83.4 (10.5) | 81.7 (10.45) |
| Body Fat Percentage ‡ | 26 (4.1) | 24.8 (5.1) | 28.1 (4.2) | 26.6 (4.6) |
| HbA1c | 6.6 (0.3) | 7.5 (0.3) | 9.5 (1.2) | 8.21 (1.6) |
| Duration of diabetes (years) | 6.8 (8.8) | 10.1 (6.9) | 8.4 (7.8) | 8.4 (7.8) |
| Hypertension | 5 (41.7%) | 7 (58.3%) | 8 (36.4%) | 20 (43.5%) |
| IHD | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypothyroidism | 3 (25.0%) | 2 (16.7%) | 1 (4.5%) | 6 (13.0%) |
| Stroke | 1 (8.3%) | 0 (0%) | 0 (0%) | 1 (2.2%) |
| PVD | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Retinopathy | 0 (0%) | 0 (0%) | 1 (4.5%) | 1 (2.2%) |
| Neuropathy | 1 (8.3%) | 2 (16.7%) | 3 (13.6%) | 6 (13.0%) |
| Nephropathy | 1 (8.3%) | 2 (16.7%) | 0 (0%) | 3 (6.5%) |
| Measure | Baseline (Day 2) | Mid (Day 7) | End (Day 13) |
|---|---|---|---|
| Optimal Control (n = 11) | |||
| Mean Glucose | 115.90 | 107.96 | 98.21 |
| SD Glucose | 34.15 | 34.15 | 34.86 |
| Coefficient of variation | 29.46 | 31.64 | 35.49 |
| MODD | 24.56 | 24.44 | 23.14 |
| MAGE | 126.15 | 117.27 | 111.55 |
| CONGA | 8.67 | 8.24 | 7.61 |
| HBGI | 3.13 | 2.93 | 3.05 |
| LBGI | 2.30 | 3.57 | 5.95 |
| Acceptable Control (n = 10) | |||
| Mean Glucose | 127.22 | 104.29 | 102.21 |
| SD Glucose | 50.02 | 27.62 | 25.50 |
| Coefficient of variation | 39.32 | 26.48 | 24.94 |
| MODD | 30.79 | 21.53 | 18.90 |
| MAGE | 138.01 | 109.43 | 105.65 |
| CONGA | 8.87 | 6.46 | 5.69 |
| HBGI | 5.56 | 1.41 | 1.34 |
| LBGI | 4.08 | 4.00 | 3.47 |
| Poor Control (n = 20) | |||
| Mean Glucose | 203.67 | 176.81 | 144.62 |
| SD Glucose | 77.43 | 62.22 | 53.26 |
| Coefficient of variation | 38.02 | 35.19 | 36.83 |
| MODD | 45.82 | 37.04 | 32.28 |
| MAGE | 216.63 | 186.93 | 154.13 |
| CONGA | 14.10 | 9.62 | 8.38 |
| HBGI | 16.62 | 10.99 | 7.16 |
| LBGI | 3.41 | 2.14 | 4.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, A.; Mitra, A.; Anjum, N.; Shrivastava, N.; Khadanga, S.; Pakhare, A.; Joshi, R. Patterns of Glycemic Variability During a Diabetes Self-Management Educational Program. Med. Sci. 2019, 7, 52. https://doi.org/10.3390/medsci7030052
Joshi A, Mitra A, Anjum N, Shrivastava N, Khadanga S, Pakhare A, Joshi R. Patterns of Glycemic Variability During a Diabetes Self-Management Educational Program. Medical Sciences. 2019; 7(3):52. https://doi.org/10.3390/medsci7030052
Chicago/Turabian StyleJoshi, Ankur, Arun Mitra, Nikhat Anjum, Neelesh Shrivastava, Sagar Khadanga, Abhijit Pakhare, and Rajnish Joshi. 2019. "Patterns of Glycemic Variability During a Diabetes Self-Management Educational Program" Medical Sciences 7, no. 3: 52. https://doi.org/10.3390/medsci7030052
APA StyleJoshi, A., Mitra, A., Anjum, N., Shrivastava, N., Khadanga, S., Pakhare, A., & Joshi, R. (2019). Patterns of Glycemic Variability During a Diabetes Self-Management Educational Program. Medical Sciences, 7(3), 52. https://doi.org/10.3390/medsci7030052





