HDAC2 Inhibitor Valproic Acid Increases Radiation Sensitivity of Drug-Resistant Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Cell Culture
2.2. Chemicals, Radiation, and Treatment
2.3. MTT Assay
2.4. Cross-Resistance of the Drug-Resistant Cells to Inhibitors of Other Signaling Pathways and Valpronic Acid
2.5. Optimization of the Radiation Dose for Radiation Sensitization Experiment
2.6. Sensitization of Cells with Valpronic Acid and LDN193189
2.6.1. MTT Assay
2.6.2. Cell Viability by Trypan Blue Assay
2.6.3. Live/Dead Staining
2.6.4. Clonogenic Survival Assay
2.6.5. Melanin Determination
2.7. In Silico Docking of Valproic Acid on HDAC2
3. Results
3.1. Cross-Resistance with Inhibitors of Other Pathways and Valproic Acid
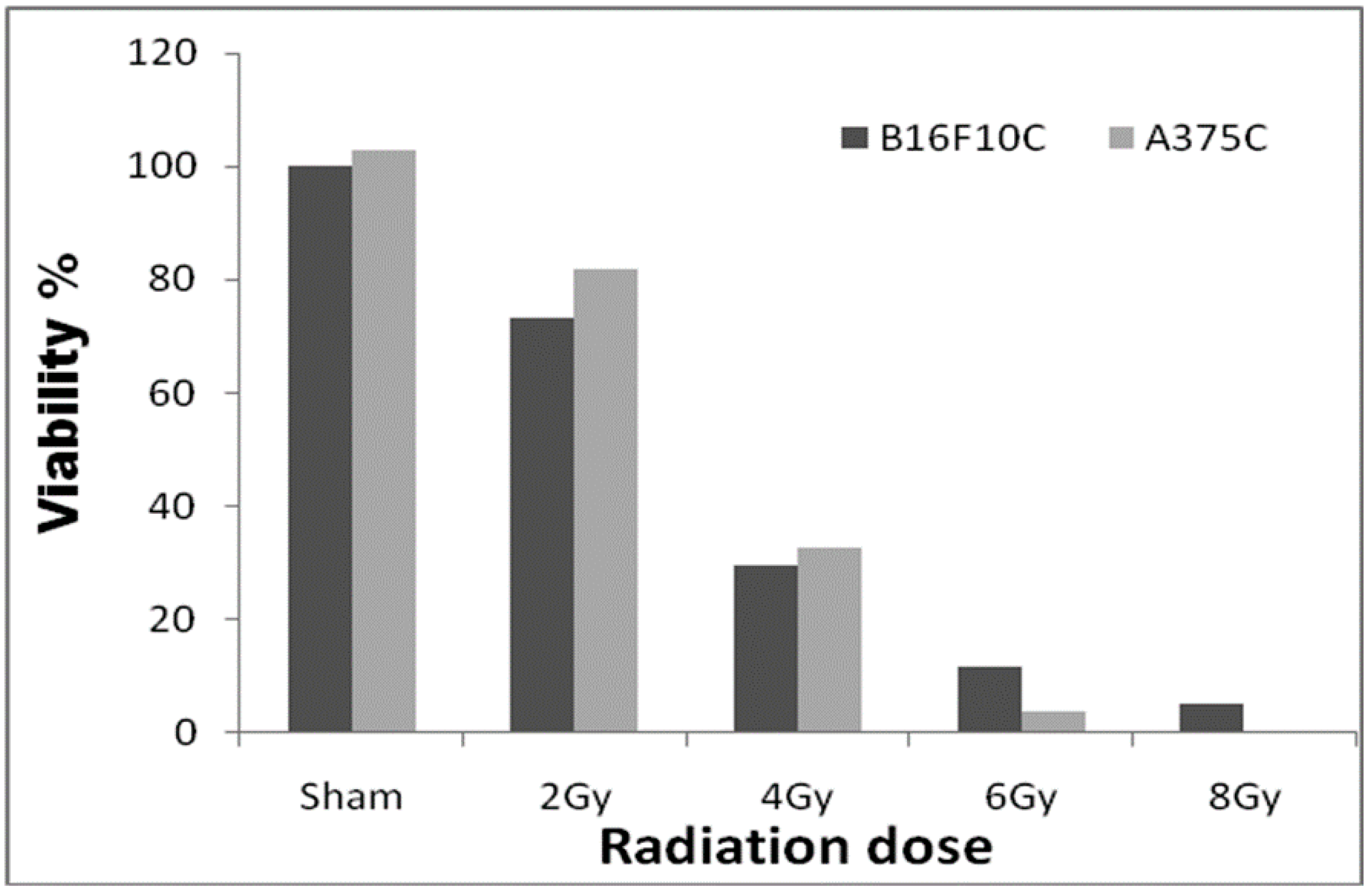
3.2. Radiation Dose Optimization with the Parental Cell Lines
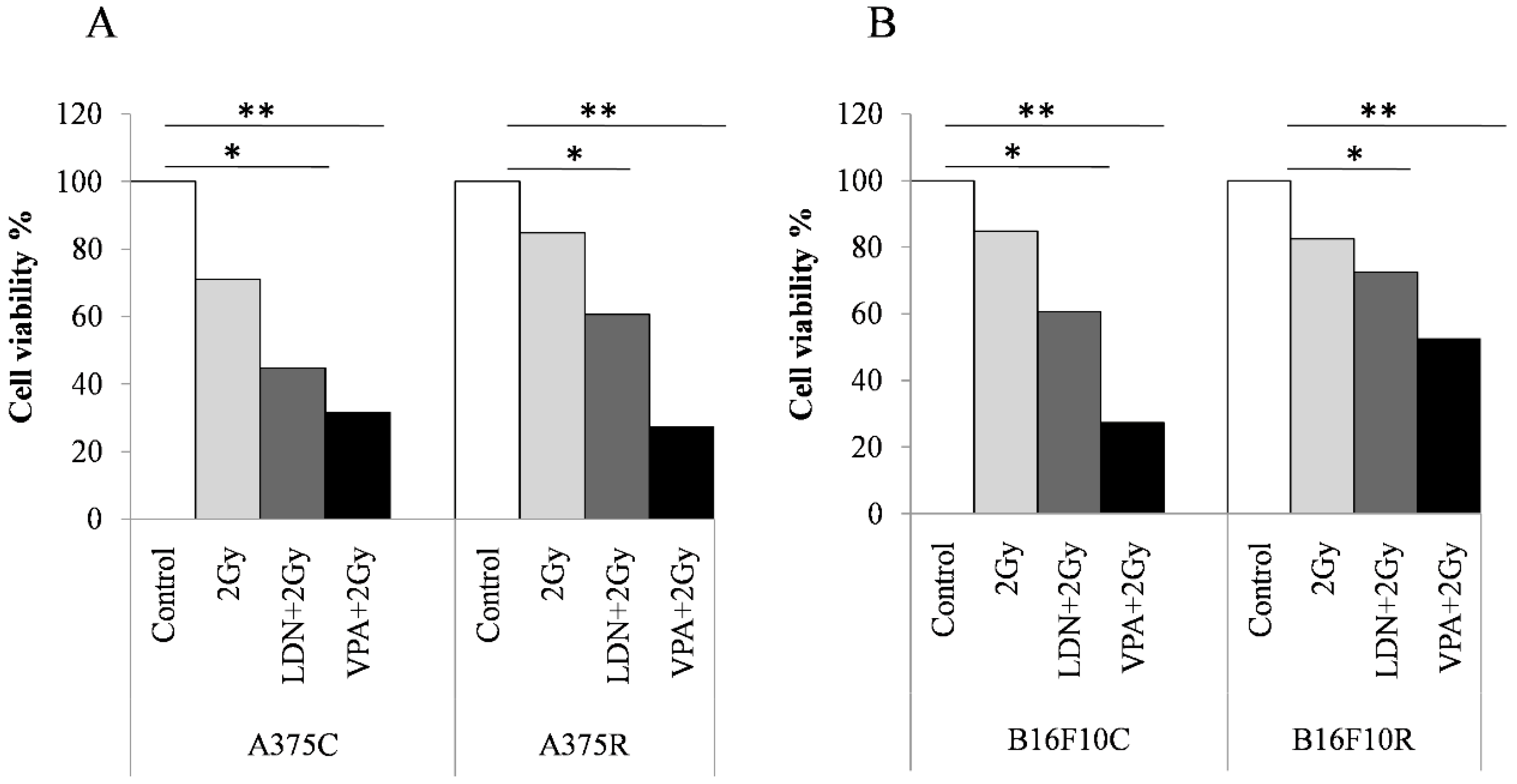
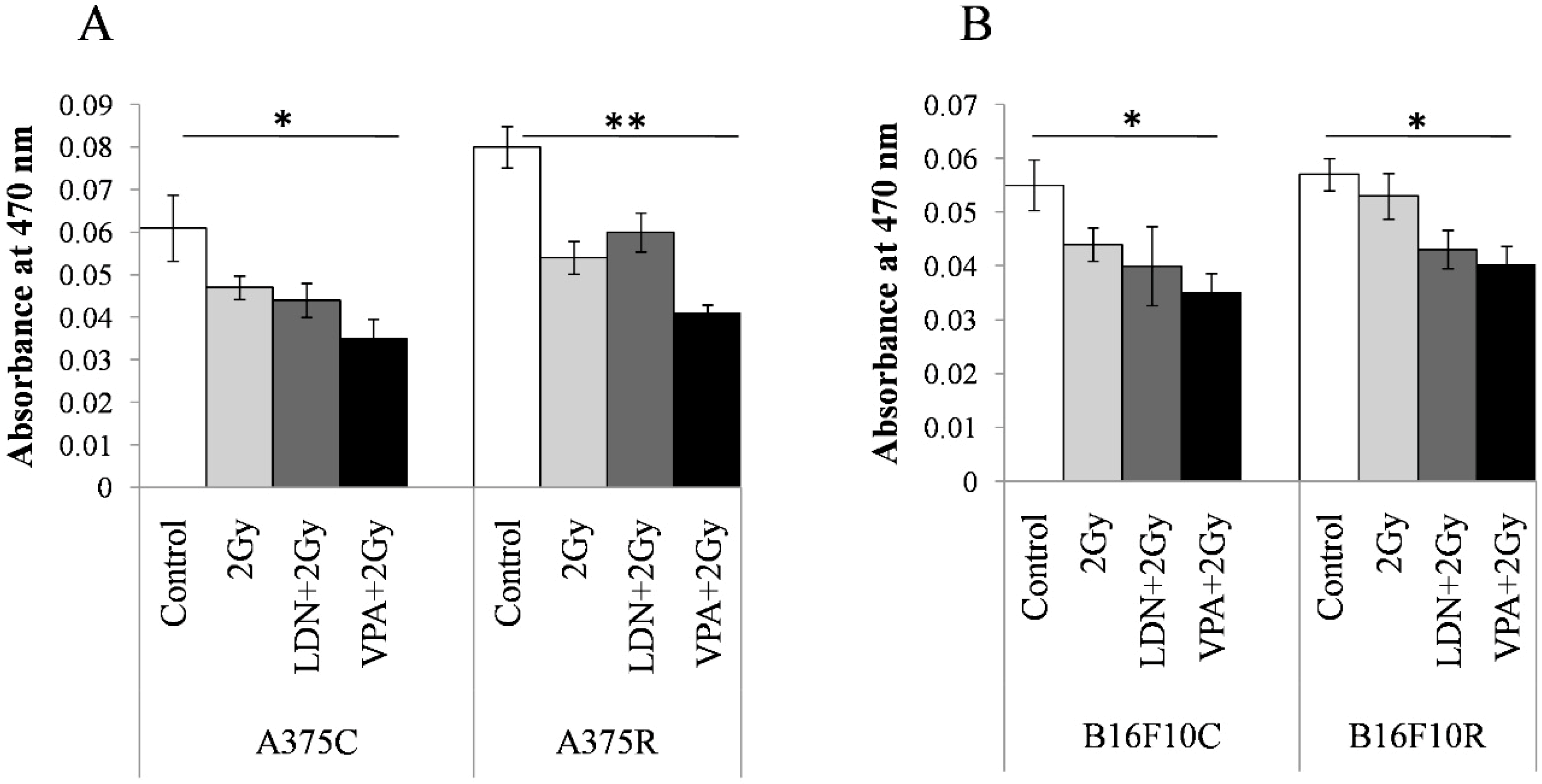
3.3. Synergistic Effect of Valproic Acid and 2Gy Radiation in Parental and Drug-Resistant Melanoma Cells
3.3.1. MTT Assay
3.3.2. Cell Viability by Trypan Blue Assay
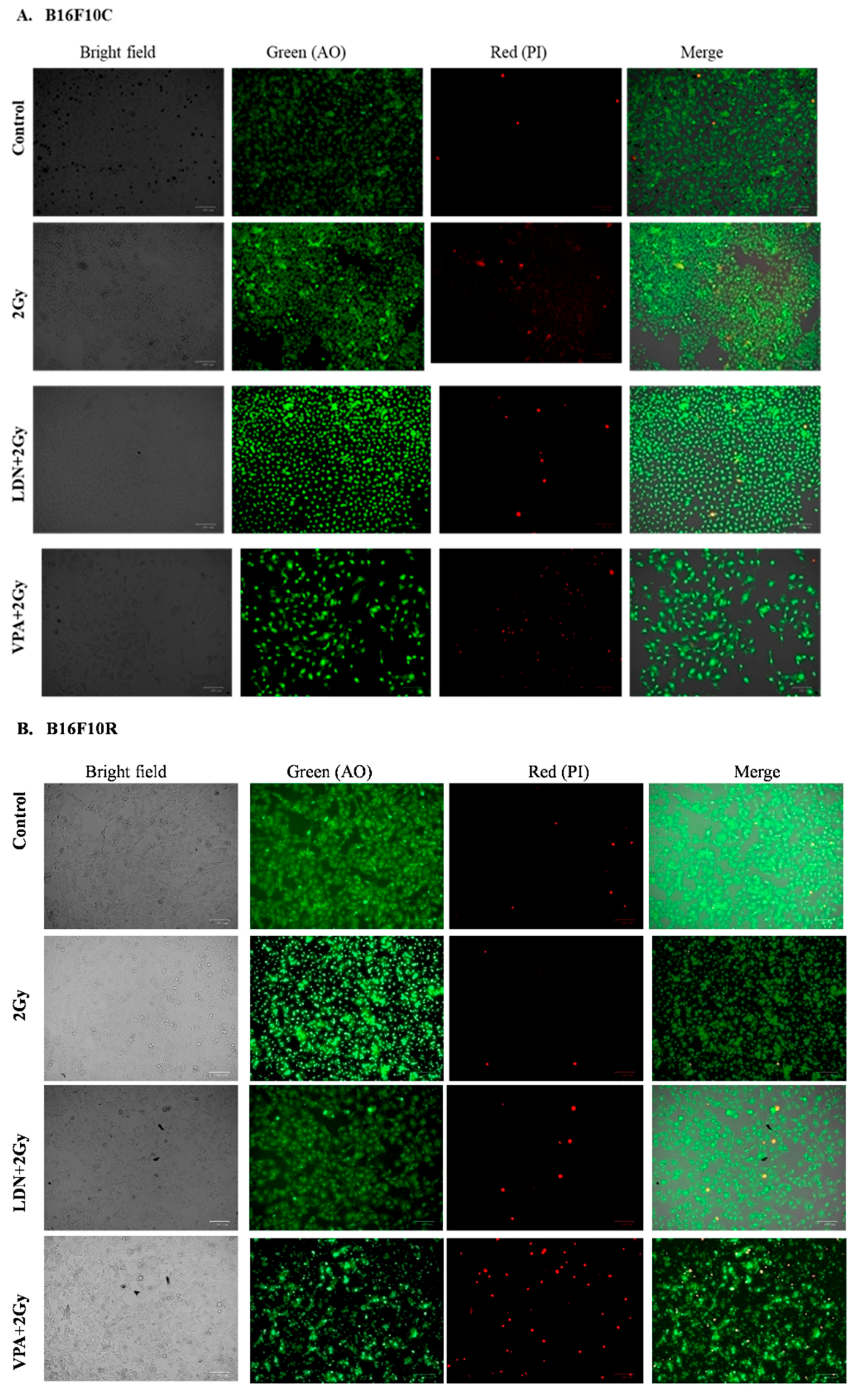
3.3.3. Live/Dead Assay
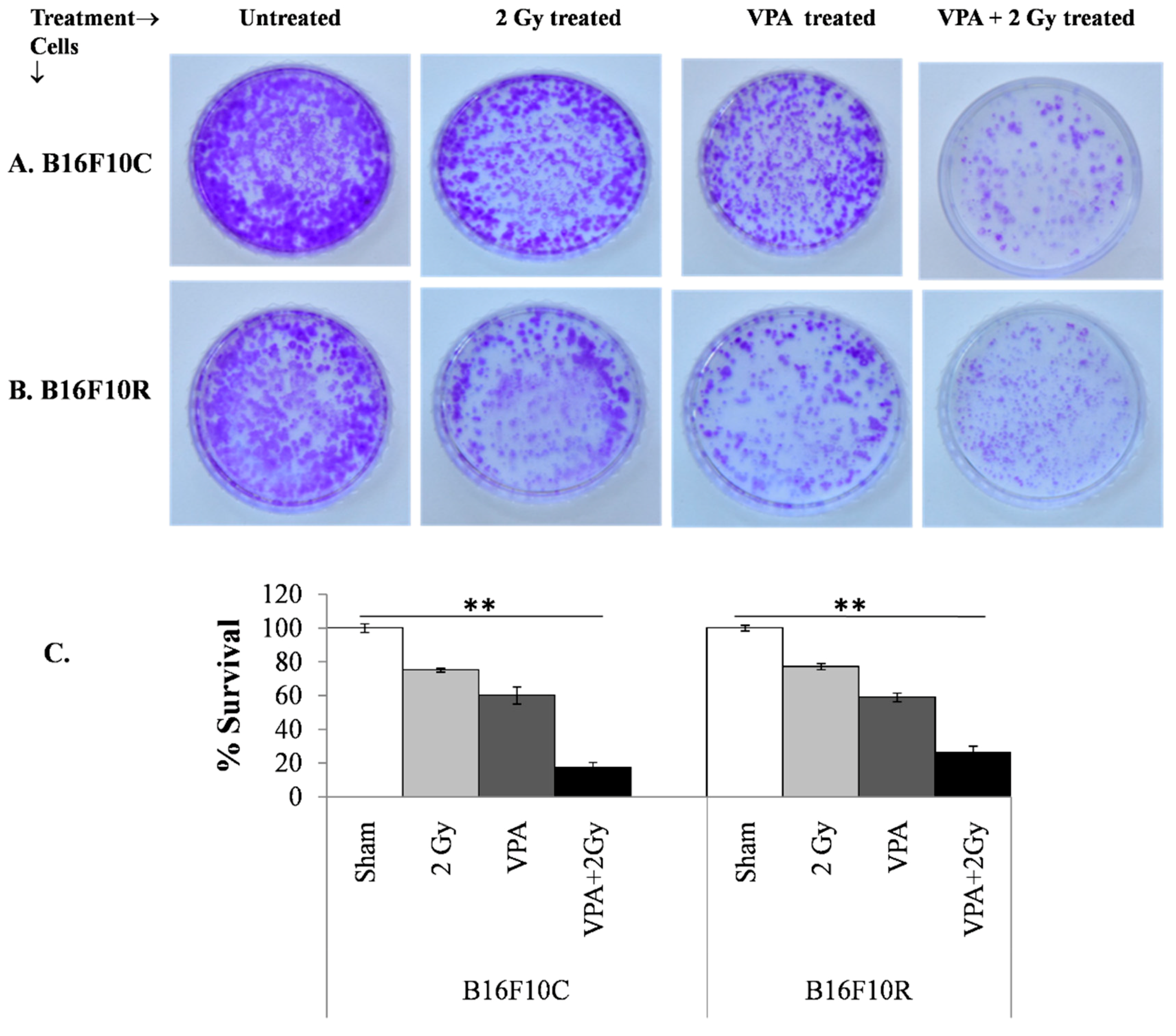
3.3.4. Clonogenic Assay
3.3.5. Melanin Concentration
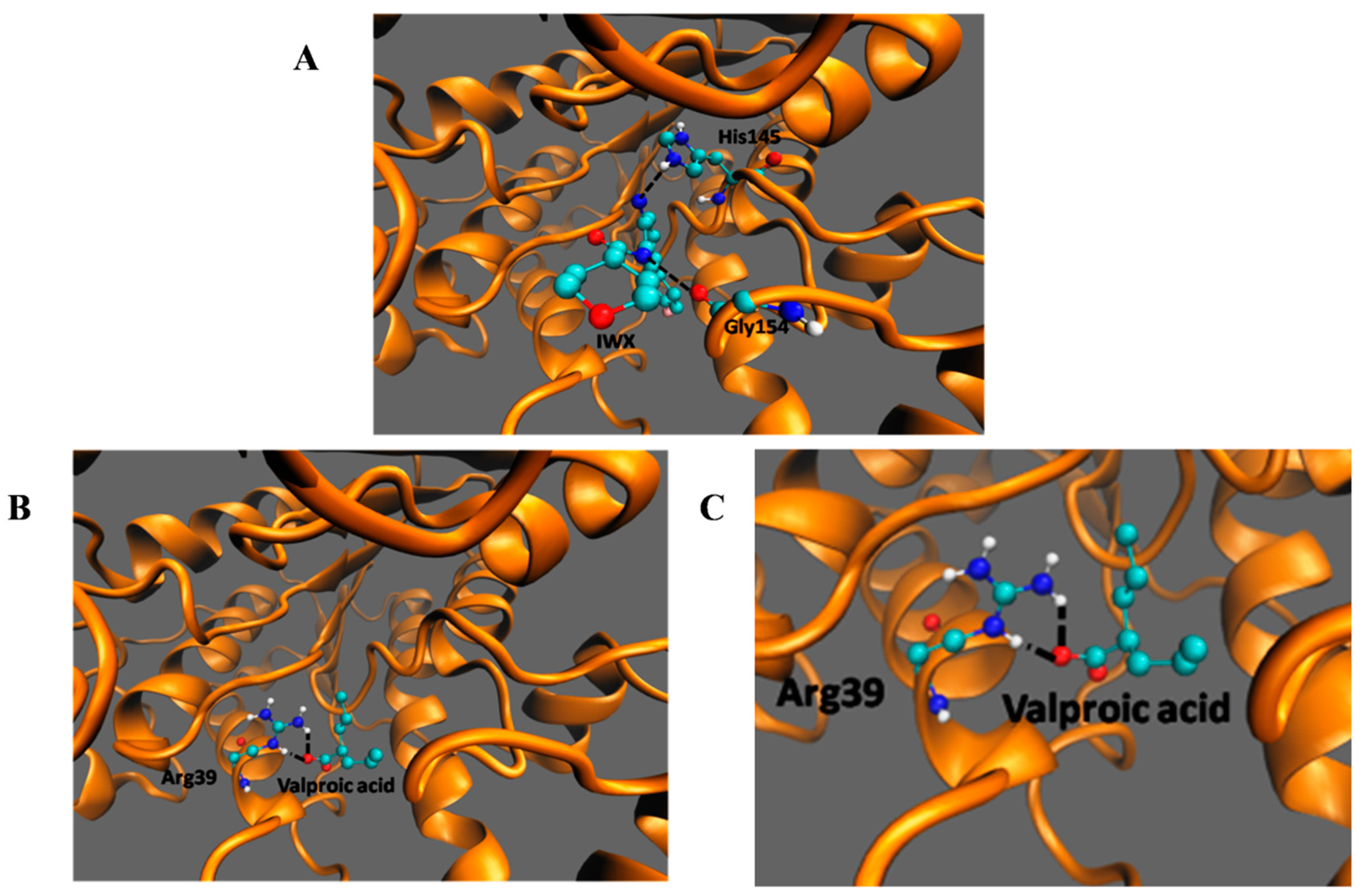
3.4. In SilicoDocking of Valproic Acid on HDAC2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jaffray, D.A.; Gospodarowicz, M.K. Radiation Therapy for Cancer. In Cancer: Disease Control Priorities, 3rd ed.; Gelband, H., Jha, P., Sankaranarayanan, R., Horton, S., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington DC, USA, 2015; Volume 3. [Google Scholar] [CrossRef]
- Owen, J.B.; Coia, L.R.; Hanks, G.E. Recent patterns of growth in radiation therapy facilities in the United States: A patterns of care study report. Int. J. Radiat. Oncol. Biol. Phys. 1992, 24, 983–986. [Google Scholar] [CrossRef]
- Khan, M.K.; Khan, N.; Almasan, A.; Macklis, R. Future of radiation therapy for malignant melanoma in an era of newer, more effective biological agents. OncoTargets Ther. 2011, 4, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, R.; Villanueva, J.; Herlyn, M. Intratumoral heterogeneity as a therapy resistance mechanism: Role of melanoma subpopulations. Adv. Pharmacol. 2012, 65, 335–359. [Google Scholar] [CrossRef]
- Sambade, M.J.; Peters, E.C.; Thomas, N.E.; Kaufmann, W.K.; Kimple, R.J.; Shields, J.M. Melanoma cells show a heterogeneous range of sensitivity to ionizing radiation and are radiosensitized by inhibition of B-RAF with PLX-4032. Radiother. Oncol. 2011, 98, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Pak, B.J.; Lee, J.; Thai, B.L.; Fuchs, S.Y.; Shaked, Y.; Ronai, Z.; Kerbel, R.S.; Ben-David, Y. Radiation resistance of human melanoma analysed by retroviral insertional mutagenesis reveals a possible role for dopachrome tautomerase. Oncogene 2004, 23, 30–38. [Google Scholar] [CrossRef]
- Petrovic, I.; Ristic-Fira, A.; Todorovic, D.; Koricanac, L.; Valastro, L.; Cirrone, P.; Cuttone, G. Response of a radioresistant human melanoma cell line along the proton spread-out Bragg peak. Int. J. Radiat. Biol. 2010, 86, 742–751. [Google Scholar] [CrossRef]
- Ristic-Fira, A.M.; Todorovic, D.V.; Koricanac, L.B.; Petrovic, I.M.; Valastro, L.M.; Cirrone, P.G.; Raffaele, L.; Cuttone, G. Response of a human melanoma cell line to low and high ionizing radiation. Ann. N. Y. Acad. Sci. 2007, 1095, 165–174. [Google Scholar] [CrossRef]
- Shinsato, Y.; Hanada, T.; Kisanuki, T.; Yonezawa, H.; Yunoue, S.; Yoshioka, T.; Hanaya, R.; Tokimura, H.; Hirano, H.; Arita, K. Primary malignant melanoma in the pineal region treated without chemotherapy. Surg. Neurol. Int. 2012, 3, 123. [Google Scholar] [CrossRef]
- Khan, N.; Khan, M.K.; Almasan, A.; Singh, A.D.; Macklis, R. The evolving role of radiation therapy in the management of malignant melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 645–654. [Google Scholar] [CrossRef]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy Resistance Mechanisms in Advanced Skin Cancer. Oncol. Rev. 2017, 11, 326. [Google Scholar] [CrossRef]
- Hu, Q.; Baeg, G.H. Role of epigenome in tumorigenesis and drug resistance. Food Chem. Toxicol. 2017, 109, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Rastgoo, N.; Abdi, J.; Hou, J.; Chang, H. Role of epigenetics-microRNA axis in drug resistance of multiple myeloma. J. Hematol. Oncol. 2017, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hao, D.; Wang, L.; Wang, H.; Wang, Y.; Zhao, Z.; Li, P.; Deng, C.; Di, L.J. Epigenetic targeting drugs potentiate chemotherapeutic effects in solid tumor therapy. Sci. Rep. 2017, 7, 4035. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkolchai, P.; Owens, P.; Hong, C.C. Emerging roles of the bone morphogenetic protein pathway in cancer: Potential therapeutic target for kinase inhibition. Biochem. Soc. Trans. 2016, 44, 1117–1134. [Google Scholar] [CrossRef] [PubMed]
- Madhav, A.; Andres, A.; Duong, F.; Mishra, R.; Haldar, S.; Liu, Z.; Angara, B.; Gottlieb, R.; Zumsteg, Z.S.; Bhowmick, N.A. Antagonizing CD105 enhances radiation sensitivity in prostate cancer. Oncogene 2018, 37, 4385–4397. [Google Scholar] [CrossRef]
- Hardee, M.E.; Marciscano, A.E.; Medina-Ramirez, C.M.; Zagzag, D.; Narayana, A.; Lonning, S.M.; Barcellos-Hoff, M.H. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res. 2012, 72, 4119–4129. [Google Scholar] [CrossRef]
- Kalal, B.S.; Pai, V.R.; Upadhya, D. Valproic acid reduces tumor cell survival and proliferation with inhibitors of downstream molecules of epidermal growth factor receptor pathway. J. Pharmacol. Pharmacother. 2018, 9, 11–16. [Google Scholar] [CrossRef]
- Saito, K.; Funayama, T.; Yokota, Y.; Murakami, T.; Kobayashi, Y. Histone Deacetylase Inhibitors Sensitize Murine B16F10 Melanoma Cells to Carbon Ion Irradiation by Inducing G1 Phase Arrest. Biol. Pharm. Bull. 2017, 40, 844–851. [Google Scholar] [CrossRef]
- Castro-Galache, M.D.; Ferragut, J.A.; Barbera, V.M.; Martin-Orozco, E.; Gonzalez-Ros, J.M.; Garcia-Morales, P.; Saceda, M. Susceptibility of multidrug resistance tumor cells to apoptosis induction by histone deacetylase inhibitors. Int. J. Cancer 2003, 104, 579–586. [Google Scholar] [CrossRef]
- Kramer, O.H.; Zhu, P.; Ostendorff, H.P.; Golebiewski, M.; Tiefenbach, J.; Peters, M.A.; Brill, B.; Groner, B.; Bach, I.; Heinzel, T.; et al. The histone deacetylase inhibitor valproic acid selectively induces proteasomal degradation of HDAC2. EMBO J. 2003, 22, 3411–3420. [Google Scholar] [CrossRef]
- Yang, C.; Choy, E.; Hornicek, F.J.; Wood, K.B.; Schwab, J.H.; Liu, X.; Mankin, H.; Duan, Z. Histone deacetylase inhibitor PCI-24781 enhances chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. Anticancer Res. 2011, 31, 1115–1123. [Google Scholar] [PubMed]
- To, K.K.; Tong, W.S.; Fu, L.W. Reversal of platinum drug resistance by the histone deacetylase inhibitor belinostat. Lung Cancer 2017, 103, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Cerna, T.; Hrabeta, J.; Eckschlager, T.; Frei, E.; Schmeiser, H.H.; Arlt, V.M.; Stiborova, M. The Histone Deacetylase Inhibitor Valproic Acid Exerts a Synergistic Cytotoxicity with the DNA-Damaging Drug Ellipticine in Neuroblastoma Cells. Int. J. Mol. Sci. 2018, 19, 164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Wang, H.; Niu, J.; Hou, H.; Jiang, Y. Histone deacetylase inhibitor, valproic acid, radiosensitizes the C6 glioma cell line in vitro. Oncol. Lett. 2014, 7, 203–208. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.; Eustace, A.J.; Busschots, S.; Breen, L.; Crown, J.; Clynes, M.; O’Donovan, N.; Stordal, B. In vitro Development of Chemotherapy and Targeted Therapy Drug-Resistant Cancer Cell Lines: A Practical Guide with Case Studies. Front. Oncol. 2014, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kalal, B.S.; Fathima, F.; Pai, V.R.; Sanjeev, G.; Krishna, C.M.; Upadhya, D. Inhibition of ERK1/2 or AKT Activity Equally Enhances Radiation Sensitization in B16F10 Cells. World J. Oncol. 2018, 9, 21–28. [Google Scholar] [CrossRef]
- Van Nifterik, K.A.; Van den Berg, J.; Slotman, B.J.; Lafleur, M.V.; Sminia, P.; Stalpers, L.J. Valproic acid sensitizes human glioma cells for temozolomide and gamma-radiation. J. Neurooncol. 2012, 107, 61–67. [Google Scholar] [CrossRef]
- Yarmohamadi, A.; Asadi, J.; Gharaei, R.; Mir, M.; Khoshnazar, A.K. Valproic acid, a histone deacetylase inhibitor, enhances radiosensitivity in breast cancer cell line. J. Radiat. Cancer. Res. 2018, 9, 86–92. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Bank, H.L. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cell. Dev. Biol. 1988, 24, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Rafehi, H.; Orlowski, C.; Georgiadis, G.T.; Ververis, K.; El-Osta, A.; Karagiannis, T.C. Clonogenic assay: Adherent cells. J. Vis. Exp. JoVE 2011. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chen, H.; Kolev, V.; Aull, K.H.; Jung, I.; Wang, J.; Miyamoto, S.; Hosoi, J.; Mandinova, A.; Fisher, D.E. High-throughput, high-content screening for novel pigmentation regulators using a keratinocyte/melanocyte co-culture system. Exp. Dermatol. 2014, 23, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Vriend, G. WHAT IF: A molecular modeling and drug design program. J. Mol. Graph. 1990, 8, 52–56. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Chistyakov, V.V.; Thornton, J.M. PDBsum more: New summaries and analyses of the known 3D structures of proteins and nucleic acids. Nucleic Acids Res. 2005, 33, D266–D268. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Saito, M.; Nakano, A.; Iwashita, S.; Ishizaka, A.; Ueda, K.; Iwakawa, S. Acquired resistance to decitabine and cross-resistance to gemcitabine during the long-term treatment of human HCT116 colorectal cancer cells with decitabine. Oncol. Lett. 2015, 10, 761–767. [Google Scholar] [CrossRef]
- Jung, K.H.; Noh, J.H.; Kim, J.K.; Eun, J.W.; Bae, H.J.; Xie, H.J.; Chang, Y.G.; Kim, M.G.; Park, H.; Lee, J.Y.; et al. HDAC2 overexpression confers oncogenic potential to human lung cancer cells by deregulating expression of apoptosis and cell cycle proteins. J. Cell. Biochem. 2012, 113, 2167–2177. [Google Scholar] [CrossRef]
- Huang, W.T.; Tsai, Y.H.; Chen, S.H.; Kuo, C.W.; Kuo, Y.L.; Lee, K.T.; Chen, W.C.; Wu, P.C.; Chuang, C.Y.; Cheng, S.M.; et al. HDAC2 and HDAC5 Up-Regulations Modulate Survivin and miR-125a-5p Expressions and Promote Hormone Therapy Resistance in Estrogen Receptor Positive Breast Cancer Cells. Front. Pharmacol. 2017, 8, 902. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, Z.; Zhao, L.; He, M.; Ren, J.; Wu, H.; Chen, Q.; Yao, W.; Wei, M. HDAC2 overexpression is a poor prognostic factor of breast cancer patients with increased multidrug resistance-associated protein expression who received anthracyclines therapy. Jpn. J. Clin. Oncol. 2016, 46, 893–902. [Google Scholar] [CrossRef]
- Gerelchuluun, A.; Maeda, J.; Manabe, E.; Brents, C.A.; Sakae, T.; Fujimori, A.; Chen, D.J.; Tsuboi, K.; Kato, T.A. Histone Deacetylase Inhibitor Induced Radiation Sensitization Effects on Human Cancer Cells after Photon and Hadron Radiation Exposure. Int. J. Mol. Sci. 2018, 19, 496. [Google Scholar] [CrossRef] [PubMed]
- Thotala, D.; Karvas, R.M.; Engelbach, J.A.; Garbow, J.R.; Hallahan, A.N.; DeWees, T.A.; Laszlo, A.; Hallahan, D.E. Valproic acid enhances the efficacy of radiation therapy by protecting normal hippocampal neurons and sensitizing malignant glioblastoma cells. Oncotarget 2015, 6, 35004–35022. [Google Scholar] [CrossRef]
- Kandakatla, N.; Ramakrishnan, G. Ligand Based Pharmacophore Modeling and Virtual Screening Studies to Design Novel HDAC2 Inhibitors. Adv. Bioinform. 2014, 2014, 812148. [Google Scholar] [CrossRef] [PubMed]
- Ganai, S.A.; Abdullah, E.; Rashid, R.; Altaf, M. Combinatorial In Silico Strategy towards Identifying Potential Hotspots during Inhibition of Structurally Identical HDAC1 and HDAC2 Enzymes for Effective Chemotherapy against Neurological Disorders. Front. Mol. Neurosci. 2017, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, H.; Zhang, F.; Tian, Y.; Tian, Z.; Cai, Z.; Lim, D.; Feng, Z. The Effect of VPA on Increasing Radiosensitivity in Osteosarcoma Cells and Primary-Culture Cells from Chemical Carcinogen-Induced Breast Cancer in Rats. Int. J. Mol. Sci. 2017, 18, 1027. [Google Scholar] [CrossRef] [PubMed]
| Sl. No. | Inhibitor | Human Melanoma Cells (A375) | Mouse Melanoma Cells (B16F10) | ||||
|---|---|---|---|---|---|---|---|
| IC50 | Fold Resistance | IC50 | Fold Resistance | ||||
| Control | Resistant | Control | Resistant | ||||
| 1. | LDN193189 (μM) | 1.5 ± 0.4 | 3.1 ± 0.8 | 2.03 | 2.8 ± 0.3 | 4.6 ± 0.5 | 1.65 |
| 2. | SP600125 (μM) | 5.6 ± 1.3 | 8.0 ± 1.0 | 1.42 | 5.2 ± 0.9 | 8.3 ± 1.5 | 1.59 |
| 3. | IWP-2 (μM) | 12.8 ± 1.9 | 12.1 ± 1.1 | 0.95 | 73.3 ± 2.5 | 99.1 ± 2.7 | 1.35 |
| 4. | VPA (mM) | 2.59 ± 0.1 | 3.56 ± 0.4 | 1.37 | 1.44 ± 0.1 | 1.91 ± 0.1 | 1.32 |
| Sl. No. | Cells | Treatment | Viability (%) | ||||
|---|---|---|---|---|---|---|---|
| 2 Gy | LDN | VPA | 2Gy + LDN | 2Gy + VPA | |||
| 1. | A375 | Control | 80 | 77 | 78 | 67 | 46 ** |
| Resistant | 81 | 75 | 76 | 71 | 57 * | ||
| 2. | B16F10 | Control | 92 | 77 | 76 | 65 ** | 43 ** |
| Resistant | 97 | 79 | 80 | 75 * | 61 ** | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalal, B.S.; Pai, V.R.; Behera, S.K.; Somashekarappa, H.M. HDAC2 Inhibitor Valproic Acid Increases Radiation Sensitivity of Drug-Resistant Melanoma Cells. Med. Sci. 2019, 7, 51. https://doi.org/10.3390/medsci7030051
Kalal BS, Pai VR, Behera SK, Somashekarappa HM. HDAC2 Inhibitor Valproic Acid Increases Radiation Sensitivity of Drug-Resistant Melanoma Cells. Medical Sciences. 2019; 7(3):51. https://doi.org/10.3390/medsci7030051
Chicago/Turabian StyleKalal, Bhuvanesh Sukhlal, Vinitha Ramanath Pai, Santosh Kumar Behera, and Hiriyur Mallaiah Somashekarappa. 2019. "HDAC2 Inhibitor Valproic Acid Increases Radiation Sensitivity of Drug-Resistant Melanoma Cells" Medical Sciences 7, no. 3: 51. https://doi.org/10.3390/medsci7030051
APA StyleKalal, B. S., Pai, V. R., Behera, S. K., & Somashekarappa, H. M. (2019). HDAC2 Inhibitor Valproic Acid Increases Radiation Sensitivity of Drug-Resistant Melanoma Cells. Medical Sciences, 7(3), 51. https://doi.org/10.3390/medsci7030051





