Pharmacoeconomic Analysis of Drugs Used in the Treatment of Pneumonia in Paediatric Population in a Tertiary Care Hospital in India—A Cost-of-Illness Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Outcome Measures
Economic Outcomes
2.3. Statistics
3. Results
3.1. Demographic Characteristics
3.2. Clinical Outcomes
3.3. Economic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Health Expenditure Database (GHED); World Health Organization: Geneva, Switzerland, 2015. Available online: http://www.who.int/ health-accounts/ghed/en/ (accessed on 11 November 2017).
- Jakovljevic, M.B. BRIC’s growing share of global health spending and their diverging pathways. Front. Public Health 2015, 3, 135. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, M.; Potapchik, E.; Popovich, L.; Barik, D.; Getzen, T. Evolving health expenditure landscape of the BRICS nations and projections to 2025. Health Econ. 2017, 26, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national under-5 mortality, adult mortality, age—Specific mortality, and life expectancy, 1970–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1084–1150.
- World Health Organization. Pneumonia; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Rudan, I.; Tomaskovic, L.; Boschi-Pinto, C.; Campbell, H. WHO Child Health Epidemiology Reference Group. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull. World Health Organ. 2004, 356, 895–903. [Google Scholar]
- Cupurdija, V.; Lazic, Z.; Petrovic, M.; Mojsilovic, S.; Cekerevac, I.; Rancic, N.; Jakovljevic, M. Community-acquired pneumonia: Economics of inpatient medical care vis-à-vis clinical severity. J. Bras. Pneumol. 2015, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, T.; Salama, P.; Johansson, E.W.; Mason, E. Pneumonia: The leading killer of children. Lancet 2006, 368, 1048–1050. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Groot, W.; Souliotis, K. Health care financing and affordability in the emerging global markets. Front. Public Health 2016. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, M.; Almirall, J.; Morera, J.; Pera, G.; Ortun, V.; Bassa, J.; Bolibar, I.; Balanzo, X.; Verdaguer, A. A population-based study of the costs of care for community-acquired pneumonia. Eur. Respir. J. 2004, 23, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Rubenfeld, G.D.; Park, D.R.; Sherbin, V.L.; Goodman, M.S.; Root, R.K. Cost and incidence of social co morbidities in low-risk patients with community acquired pneumonia admitted to a public hospital. Chest 2003, 124, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Shoham, Y.; Dagan, R.; Givon-Lavi, N.; Liss, Z.; Shagan, T.; Zamir, O.; Greenberg, D. Community-acquired pneumonia in children: Quantifying the burden on patients and their families including decrease in quality of life. Pediatrics 2005, 115, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Winston, L.G.; Moore, D.H.; Bent, S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: A meta-analysis. Am. J. Med. 2007, 120, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Scalera, N.M.; File, T.M., Jr. How long should we treat community-acquired pneumonia? Curr. Opin. Infect. Dis. 2007, 20, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Gupta, U.; Agarwal, A.K. Cost of medicines and their affordability in private pharmacies in Delhi (India). Indian J. Med. Res. 2012, 136, 827. [Google Scholar]
- Sariano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K.; et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global burden of Disease study 2015. Lancet Respir Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Lorgelly, P.K.; Atkinson, M.; Lakhanpaul, M.; Smyth, A.R.; Vyas, H.; Weston, V.; Stephenson, T. Oral versus IV antibiotics for CAP in children: A cost minimisation analysis. ERJ Express 2009. [Google Scholar] [CrossRef]
- Jakovljevic, M.; Lazic, Z.; Verhaeghe, N.; Jankovic, S.; Gajovic, O.; Annemans, L. Direct medical costs of COPD diagnosis and treatment, Eastern vs. Western European country—Examples of Serbia and Belgium. Farmeconomia Health Econ. Ther. Pathw. 2013, 14, 161–168. [Google Scholar] [CrossRef]
- Ciommo, V.D.; Russo, P.; Attanasio, D.E.; Liso, G.D.; Graziani, C.; Caprino, L. Clinical and economic outcomes of pneumonia in children: A longitudinal observational study in an Italian paediatric hospital. J. Eval. Clin. Pract. 2002, 8, 341–348. [Google Scholar] [CrossRef] [PubMed]

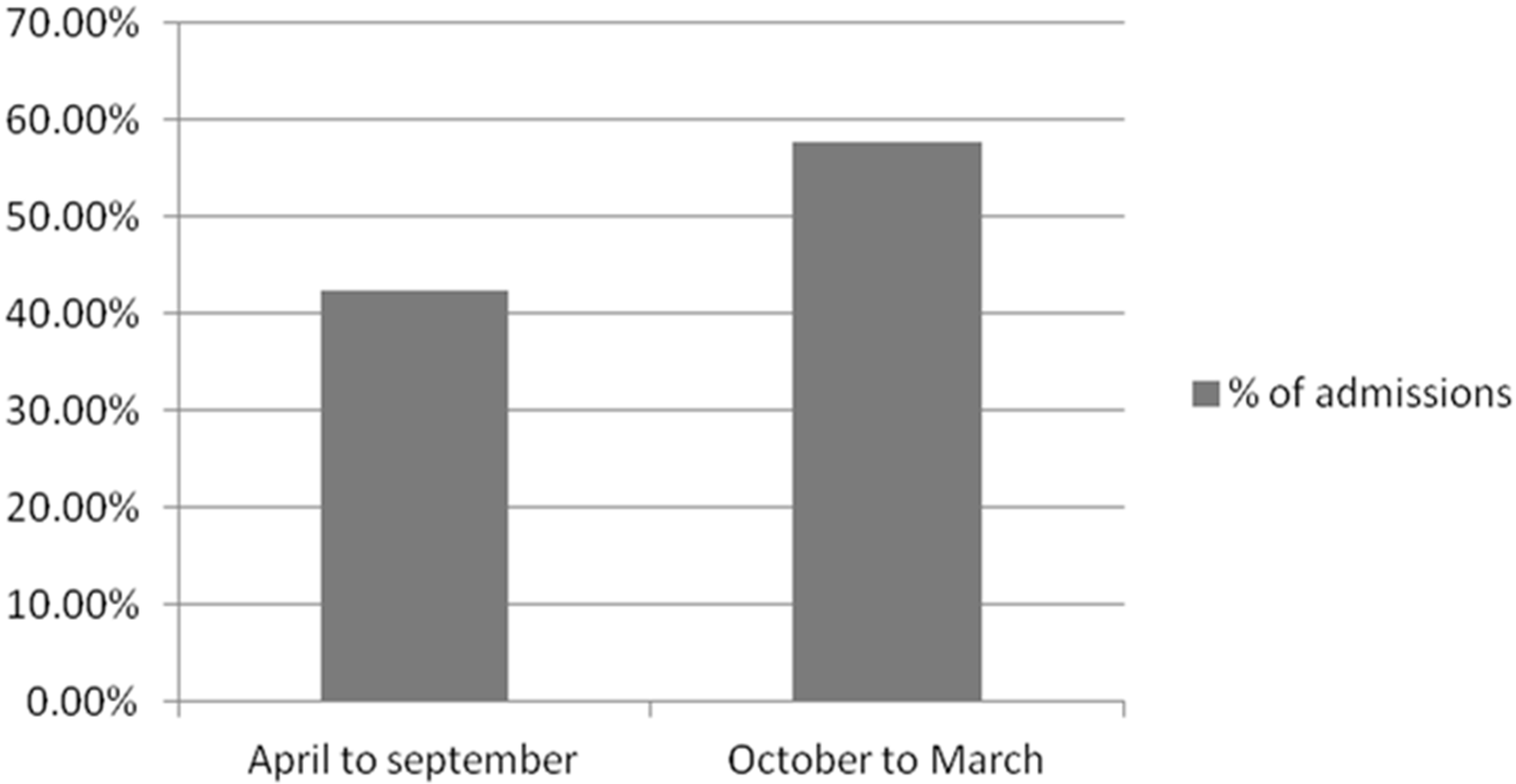
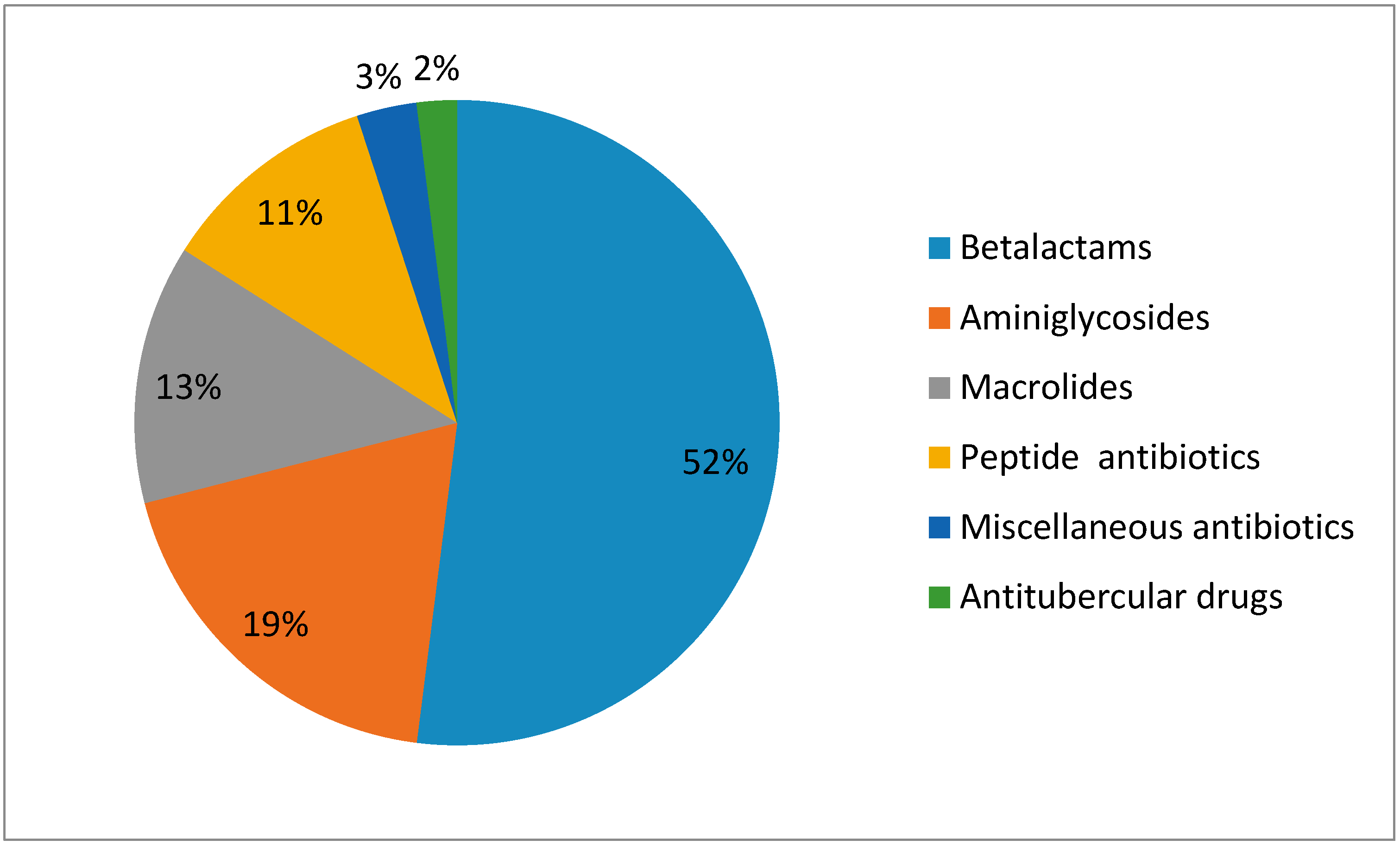
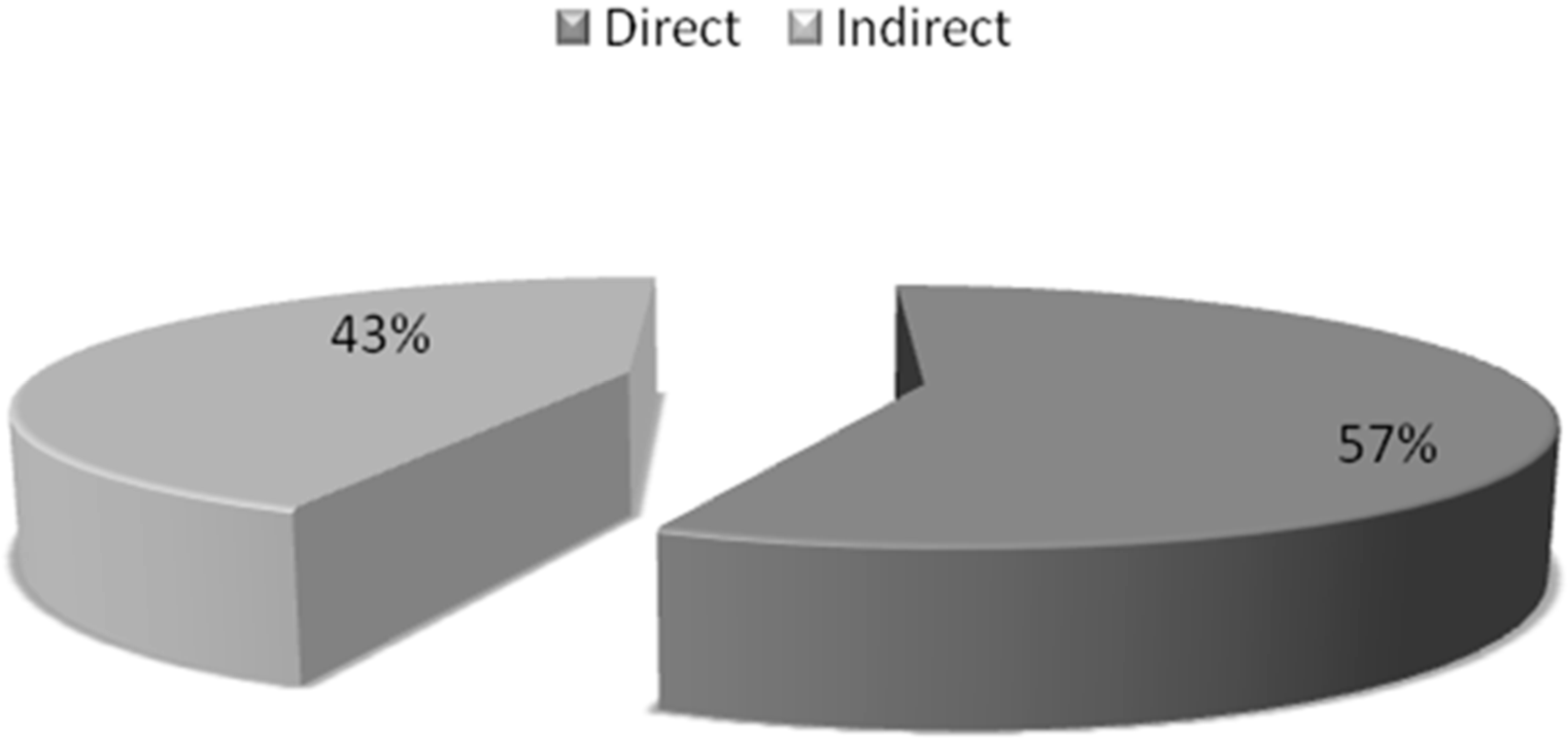
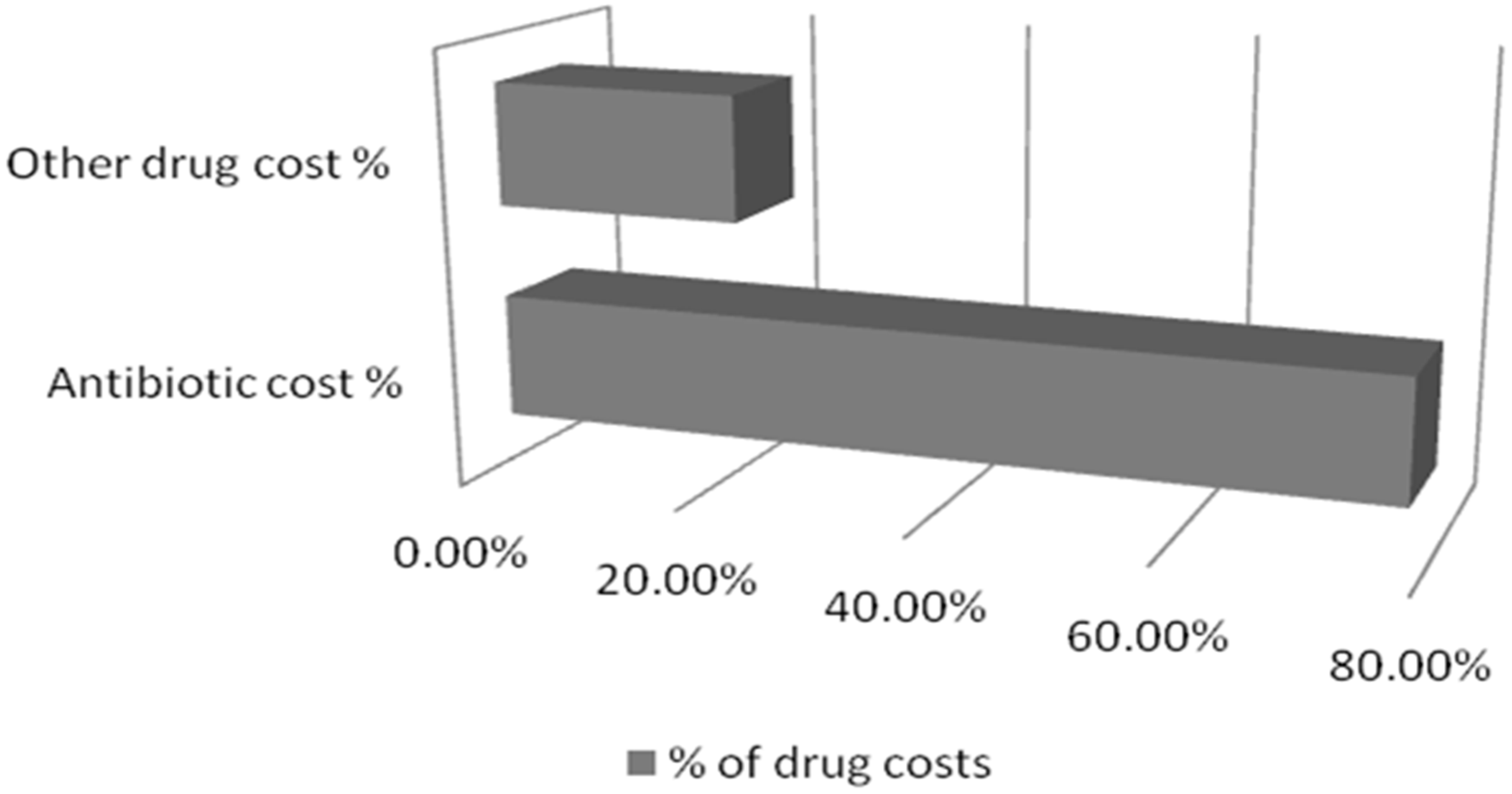
| Diagnosis | % of Patients |
|---|---|
| Mild-to-moderate pneumonia | 76.8 |
| Severe pneumonia | 23.2 |
| No. | System Involved | Percentage (%) |
|---|---|---|
| 1 | Congenital diseases | 17.6 |
| 2 | Liver disease | 2.4 |
| 3 | Renal disease | 0.8 |
| 4 | Protein energy malnutrition | 5.6 |
| 5 | Neurological disorders | 4.8 |
| 6 | Infectious disorders | 19.2 |
| 7 | Hematological disorders | 2.4 |
| 8 | Oncological disorders | 2.4 |
| 9 | Respiratory disorders | 16.8 |
| 10 | Diabetes mellitus | 0.8 |
| 11 | Cardiovascular disorders | 4.8 |
| 12 | Miscellaneous | 4.8 |
| Antibiotics | Parenteral | Oral |
|---|---|---|
| Beta lactams | 50% | 6.8% |
| Macrolides | - | 3.6% |
| Aminoglycosides | 21.2% | - |
| Peptide antibiotics | 12.04% | - |
| Antituberculosis antibiotics | - | 2.8% |
| Fluoroquinolones | 0.5% | - |
| Misc. | 1.83% | 1.04% |
| Category | Parameters | Mean Cost ± SD (INR) | % |
|---|---|---|---|
| Direct Costs | Costs of drug, Investigations costs, Hospitalization costs | 6918 ± 104 ($105.84 ± 1.59) | 57% of total costs |
| Indirect Costs | Costs of food, costs of stay, travelling costs, telephone bill and salary curtailed of the parents or relatives | 5326 ± 977 ($81.48 ± 14.94) | 43% of total costs |
| Total Costs | Direct Costs + Indirect Costs | 12,245 ± 593 ($187.34 ± 9.07) | 100% |
| Antibiotic Costs | 3823.602 ± 456 ($58.50 ± 6.97) | 78.13% of total drug costs | |
| Other Drug Costs | 1069.742 ± 561 ($16.36 ± 8.58) | 21.86% of total drug costs | |
| Total Drug Costs | 4993.344 ± 467 ($76.39 ± 7.14) | 100% |
| Standardized Coefficients | |||
|---|---|---|---|
| Model | Beta | t | Significance |
| Age | 0.055 | 1.013 | 0.313 |
| Duration | 0.203 | 3.563 | 0.001 b |
| Severity | 0.067 | 1.291 | 0.199 |
| System | 0.074 | 1.377 | 0.171 |
| Antibiotics | 0.003 | 0.057 | 0.955 |
| Cost of antibiotics | 0.735 | 13.675 | 0.000 b |
| Sex | 0.096 | −1.840 | 0.068 |
| Indirect cost | 0.004 | 0.074 | 0.941 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, L.; Kumari, S.; Khosla, P.; Rani, A.; Kaur, S. Pharmacoeconomic Analysis of Drugs Used in the Treatment of Pneumonia in Paediatric Population in a Tertiary Care Hospital in India—A Cost-of-Illness Study. Med. Sci. 2017, 5, 33. https://doi.org/10.3390/medsci5040033
Saha L, Kumari S, Khosla P, Rani A, Kaur S. Pharmacoeconomic Analysis of Drugs Used in the Treatment of Pneumonia in Paediatric Population in a Tertiary Care Hospital in India—A Cost-of-Illness Study. Medical Sciences. 2017; 5(4):33. https://doi.org/10.3390/medsci5040033
Chicago/Turabian StyleSaha, Lekha, Sweta Kumari, Pratibha Khosla, Alka Rani, and Sharonjeet Kaur. 2017. "Pharmacoeconomic Analysis of Drugs Used in the Treatment of Pneumonia in Paediatric Population in a Tertiary Care Hospital in India—A Cost-of-Illness Study" Medical Sciences 5, no. 4: 33. https://doi.org/10.3390/medsci5040033
APA StyleSaha, L., Kumari, S., Khosla, P., Rani, A., & Kaur, S. (2017). Pharmacoeconomic Analysis of Drugs Used in the Treatment of Pneumonia in Paediatric Population in a Tertiary Care Hospital in India—A Cost-of-Illness Study. Medical Sciences, 5(4), 33. https://doi.org/10.3390/medsci5040033




