Neptunium Reactivity During Co-Precipitation and Oxidation of Fe(II)/Fe(III) (Oxyhydr)oxides
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Environmental Significance
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C. Neptunium: The Neglected Actinide: A Review of the Biological and Environmental Literature. Radiat. Res. 1982, 90, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Nenot, J.C. Metabolism and Toxcity of Neptunium. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:15061349 (accessed on 23 November 2018).
- Kaszuba, J.P.; Runde, W.H. The Aqueous Geochemistry of Neptunium: Dynamic Control of Soluble Concentrations with Applications to Nuclear Waste Disposal. Environ. Sci. Technol. 1999, 33, 4427–4433. [Google Scholar] [CrossRef]
- Law, G.T.W.; Geissler, A.; Lloyd, J.R.; Livens, F.R.; Boothman, C.; Begg, J.D.C.; Denecke, M.A.; Rothe, J.; Dardenne, K.; Burke, I.T.; et al. Geomicrobiological redox cycling of the transuranic element neptunium. Environ. Sci. Technol. 2010, 44, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.J.; Nitsche, H. Actinide environmental chemistry. Radiochim. Acta 1995, 70–71, 377–396. [Google Scholar] [CrossRef]
- Newsome, L.; Morris, K.; Lloyd, J.R. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Brookshaw, D.R.; Pattrick, R.A.D.; Bots, P.; Law, G.T.W.; Lloyd, J.R.; Mosselmans, J.F.W.; Vaughan, D.J.; Dardenne, K.; Morris, K. Redox Interactions of Tc(VII), U(VI), and Np(V) with Microbially Reduced Biotite and Chlorite. Environ. Sci. Technol. 2015, 49, 13139–13148. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.L.; Morris, K.; Lloyd, J.R.; Denecke, M.A.; Law, K.A.; Dardenne, K.; Boothman, C.; Bots, P.; Law, G.T.W. Neptunium and manganese biocycling in nuclear legacy sediment systems. Appl. Geochem. 2015, 63, 303–309. [Google Scholar] [CrossRef]
- Cantrell, K.J.; Um, W.; Williams, B.D.; Bowden, M.E.; Gartman, B.; Lukens, W.W.; Buck, E.C.; Mausolf, E.J. Chemical stabilization of Hanford tank residual waste. J. Nucl. Mater. 2014, 446, 246–256. [Google Scholar] [CrossRef]
- Morris, K.; Law, G.T.W.; Bryan, N.D. Geodisposal of Higher Activity Wastes. In Nuclear Power and the Environment; Harrison, R.M., Hester, R.E., Eds.; Royal Society of Chemistry: Cambridge, UK, 2011; pp. 129–151. [Google Scholar]
- Dodge, C.J.; Francis, A.J.; Gillow, J.B.; Halada, G.P.; Eng, C.; Clayton, C.R. Association of uranium with iron oxides typically formed on corroding steel surfaces. Environ. Sci. Technol. 2002, 36, 3504–3511. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Nagasaki, S.; Tanaka, S.; Sakamoto, Y.; Tanaka, T.; Ogawa, H. Sorption and reduction of neptunium(V) on the surface of iron oxides. Radiochim. Acta 2002, 90, 665–669. [Google Scholar] [CrossRef]
- Christiansen, B.C.; Geckeis, H.; Marquardt, C.M.; Bauer, A.; Römer, J.; Wiss, T.; Schild, D.; Stipp, S.L.S. Neptunyl (Np) interaction with green rust. Geochim. Cosmochim. Acta 2011, 75, 1216–1226. [Google Scholar] [CrossRef]
- Kirsch, R.; Fellhauer, D.; Altmaier, M.; Neck, V.; Rossberg, A.; Fanghanel, T.; Charlet, L.; Scheinost, A.C. Oxidation state and local structure of plutonium reacted with magnetite, mackinawite, and chukanovite. Environ. Sci. Technol. 2011, 45, 7267–7274. [Google Scholar] [CrossRef]
- Latta, D.E.; Mishra, B.; Cook, R.E.; Kemner, K.M.; Boyanov, M.I. Stable U(IV) complexes form at high-affinity mineral surface sites. Environ. Sci. Technol. 2014, 48, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Wylie, E.M.; Olive, D.T.; Powell, B.A. Effects of Titanium Doping in Titanomagnetite on Neptunium Sorption and Speciation. Environ. Sci. Technol. 2016, 50, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.E.; Morris, K.; Law, G.T.L.; Mosselmans, J.F.W.; Bots, P.; Kvashnina, K.O.; Shaw, S. Uranium(V) incorporation mechanisms and stabilityin Fe(II)/Fe(III) iron (oxyhydr)oxides. Environ. Sci. Technol. Lett. 2017, 4, 421–426. [Google Scholar] [CrossRef]
- Girvin, D.C.; Ames, L.L.; Schwab, A.P.; McGarrah, J.E. Neptunium adsorption on synthetic amorphous iron oxyhydroxide. J. Colloid Interface Sci. 1991, 141, 67–78. [Google Scholar] [CrossRef]
- Bots, P.; Shaw, S.; Law, G.T.W.; Marshall, T.A.; Mosselmans, J.F.W.; Morris, K. Controls on the Fate and Speciation of Np(V) during Iron (Oxyhydr)oxide Crystallization. Environ. Sci. Technol. 2016, 50, 3382–3390. [Google Scholar] [CrossRef]
- Li, D.; Kaplan, D.I. Sorption coefficients and molecular mechanisms of Pu, U, Np, Am and Tc to Fe (hydr)oxides: A review. J. Hazard. Mater. 2012, 243, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Powell, B.A.; Zhang, S.; Rao, L. Surface complexation modeling of neptunium(V) sorption to lepidocrocite (γ-FeOOH). Radiochim. Acta 2015, 103, 707–717. [Google Scholar] [CrossRef]
- Combes, J.M.; Chisholm-Brause, C.J.; Brown, G.E.; Parks, G.A.; Conradson, S.D.; Eller, P.G.; Triay, I.R.; Hobart, D.E.; Meijer, A.; Chisholm-Brause, C.J.; et al. EXAFS Spectroscopic Study of Neptunium(V) Sorption at the alpha-FeOOH/Water Interface. Environ. Sci. Technol. 1992, 26, 376–382. [Google Scholar] [CrossRef]
- Müller, K.; Gröschel, A.; Rossberg, A.; Bok, F.; Franzen, C.; Brendler, V.; Foerstendorf, H. In situ spectroscopic identification of neptunium(V) inner-sphere complexes on the hematite-water interface. Environ. Sci. Technol. 2015, 49, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Nagasaki, S.; Tanaka, S.; Sakamoto, Y.; Tanaka, T.; Ogawa, H. Reduction rate of neptunium(V) in heterogeneous solution with magnetite. Radiochim. Acta 2004, 92, 145–149. [Google Scholar] [CrossRef]
- Arai, Y.; Moran, P.B.; Honeyman, B.D.; Davis, J.A. In situ spectroscopic evidence for neptunium(V)-carbonate inner-sphere and outer-sphere ternary surface complexes on hematite surfaces. Environ. Sci. Technol. 2007, 41, 3940–3944. [Google Scholar] [CrossRef] [PubMed]
- Bach, D.; Christiansen, B.C.; Schild, D.; Geckeis, H. Tem study of green rust sodium sulphate (GRNa,SO4) Interacted with Neptunyl Ions (NpO2+). Radiochim. Acta 2014, 102, 279–289. [Google Scholar] [CrossRef]
- Latta, D.E.; Pearce, C.I.; Rosso, K.M.; Kemner, K.M.; Boyanov, M.I. Reaction of UVI with Titanium-Substituted Magnetite: Influence of Ti on UIV Speciation. Environ. Sci. Technol. 2013, 47, 4121–4130. [Google Scholar] [CrossRef]
- Harris, W.E.; Kolthoff, I.M. The Polarography of Uranium. III. Polarography in Very Weakly Acid, Neutral or Basic Solution. J. Am. Chem. Soc. 1946, 62, 446–451. [Google Scholar]
- Ekstrom, A. Kinetics and mechanism of the Disproportionation of Uranium (V). Inorg. Chem. 1974, 13, 2237–2241. [Google Scholar] [CrossRef]
- Pidchenko, I.; Kvashnina, K.O.; Yokosawa, T.; Finck, N.; Bahl, S.; Schild, D.; Polly, R.; Bohnert, E.; Rossberg, A.; Göttlicher, J.; et al. Uranium Redox Transformations after U(VI) Coprecipitation with Magnetite Nanoparticles. Environ. Sci. Technol. 2017, 51, 2217–2225. [Google Scholar] [CrossRef]
- Skomurski, F.N.; Ilton, E.S.; Engelhard, M.H.; Arey, B.W.; Rosso, K.M. Heterogeneous reduction of U6+ by structural Fe2+ from theory and experiment. Geochim. Cosmochim. Acta 2011, 75, 7277–7290. [Google Scholar] [CrossRef]
- Ilton, E.S.; Boily, J.F.; Buck, E.C.; Skomurski, F.N.; Rosso, K.M.; Cahill, C.L.; Bargar, J.R.; Felmy, A.R. Influence of Dynamical Conditions on the Reduction of UVI at the Magnetite-Solution Interface. Environ. Sci. Technol. 2010, 44, 170–176. [Google Scholar] [CrossRef]
- Pearce, C.I.; Qafoku, O.; Liu, J.; Arenholz, E.; Heald, S.M.; Kukkadapu, R.K.; Gorski, C.A.; Henderson, C.M.B.; Rosso, K.M. Synthesis and properties of titanomagnetite (Fe3-xTixO4) nanoparticles: A tunable solid-state Fe(II/III) redox system. J. Colloid Interface Sci. 2012, 387, 24–38. [Google Scholar] [CrossRef]
- Diaz-Moreno, S.; Hayama, S.; Amboage, M.; Freeman, A.; Sutter, J.; Duller, G. I20; The Versatile X-ray Absorption spectroscopy beamline at Diamond Light Source. J. Phys. Conf. Ser. 2009, 190, 12038. [Google Scholar] [CrossRef]
- Diaz-Moreno, S.; Amboage, M.; Basham, M.; Boada, R.; Bricknell, N.E.; Cibin, G.; Cobb, T.M.; Filik, J.; Freeman, A.; Geraki, K.; et al. The Spectroscopy Village at Diamond Light Source. J. Synchrotron Radiat. 2018, 25, 998–1009. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Downward, L.; Booth, C.H.; Lukens, W.W.; Bridges, F. A variation of the F-test for determining statistical relevance of particular parameters in EXAFS fits. AIP Conf. Proc. 2007, 882, 129–131. [Google Scholar] [CrossRef]
- Marshall, T.A.; Morris, K.; Law, G.T.W.; Mosselmans, J.F.W.; Bots, P.; Parry, S.A.; Shaw, S. Incorporation and retention of 99-Tc(IV) in magnetite under high pH conditions. Environ. Sci. Technol. 2014, 48, 11853–11862. [Google Scholar] [CrossRef] [PubMed]
- Romanchuk, A.Y.; Kalmykov, S.N. Actinides sorption onto hematite: Experimental data, surface complexation modeling and linear free energy relationship. Radiochim. Acta 2014, 102, 303–310. [Google Scholar] [CrossRef]
- Hennig, C.; Ikeda-Ohno, A.; Tsushima, S.; Seheinost, A.C. The sulfate coordination of Np(IV), Np(V), and Np(VI) in aqueous solution. Inorg. Chem. 2009, 48, 5350–5360. [Google Scholar] [CrossRef]
- Husar, R.; Weiss, S.; Hennig, C.; Hübner, R.; Ikeda-Ohno, A.; Zänker, H. Formation of neptunium(IV)-silica colloids at near-neutral and slightly alkaline pH. Environ. Sci. Technol. 2014, 49, 665–671. [Google Scholar] [CrossRef]
- Williamson, A.J.; Morris, K.; Boothman, C.; Dardenne, K.; Law, G.T.W.; Lloyd, J.R. Microbially mediated reduction of Np(V) by a consortium of alkaline tolerant Fe(III)-reducing bacteria. Mineral. Mag. 2015, 79, 1287–1295. [Google Scholar] [CrossRef]
- Missana, T.; García-Gutiérrez, M.; Fernńdez, V. Uranium (VI) sorption on colloidal magnetite under anoxic environment: Experimental study and surface complexation modelling. Geochim. Cosmochim. Acta 2003, 67, 2543–2550. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, G.; Zhao, D.; He, G. XAFS study of starch-stabilized magnetite nanoparticles and surface speciation of arsenate. Environ. Pollut. 2011, 159, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Pinakidou, F.; Katsikini, M.; Simeonidis, K.; Kaprara, E.; Paloura, E.C.; Mitrakas, M. On the passivation mechanism of Fe3O4 nanoparticles during Cr(VI) removal from water: A XAFS study. Appl. Surf. Sci. 2016, 360, 1080–1086. [Google Scholar] [CrossRef]
- Husar, R.; Hübner, R.; Hennig, C.; Martin, P.M.; Chollet, M.; Weiss, S.; Stumpf, T.; Zänker, H.; Ikeda-Ohno, A. Intrinsic formation of nanocrystalline neptunium dioxide under neutral aqueous conditions relevant to deep geological repositories. Chem. Commun. 2015, 51, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Farges, F.; Ponader, C.W.; Calas, G.; Brown, G.E. Structural environments of incompatible elements in silicate glass/melt systems: II. UIV, UV, and UVI. Geochim. Cosmochim. Acta 1992, 56, 4205–4220. [Google Scholar] [CrossRef]
- Denecke, M.A.; Dardenne, K.; Marquardt, C.M. Np(IV)/Np(V) valence determinations from Np L3 edge XANES/EXAFS. Talanta 2005, 65, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Den Auwer, C.; Simoni, E.; Conradson, S.; Madic, C. Investigating Actinyl Oxo Cations by X-ray Absorption Spectroscopy. Eur. J. Inorg. Chem. 2003, 3843–3859. [Google Scholar] [CrossRef]
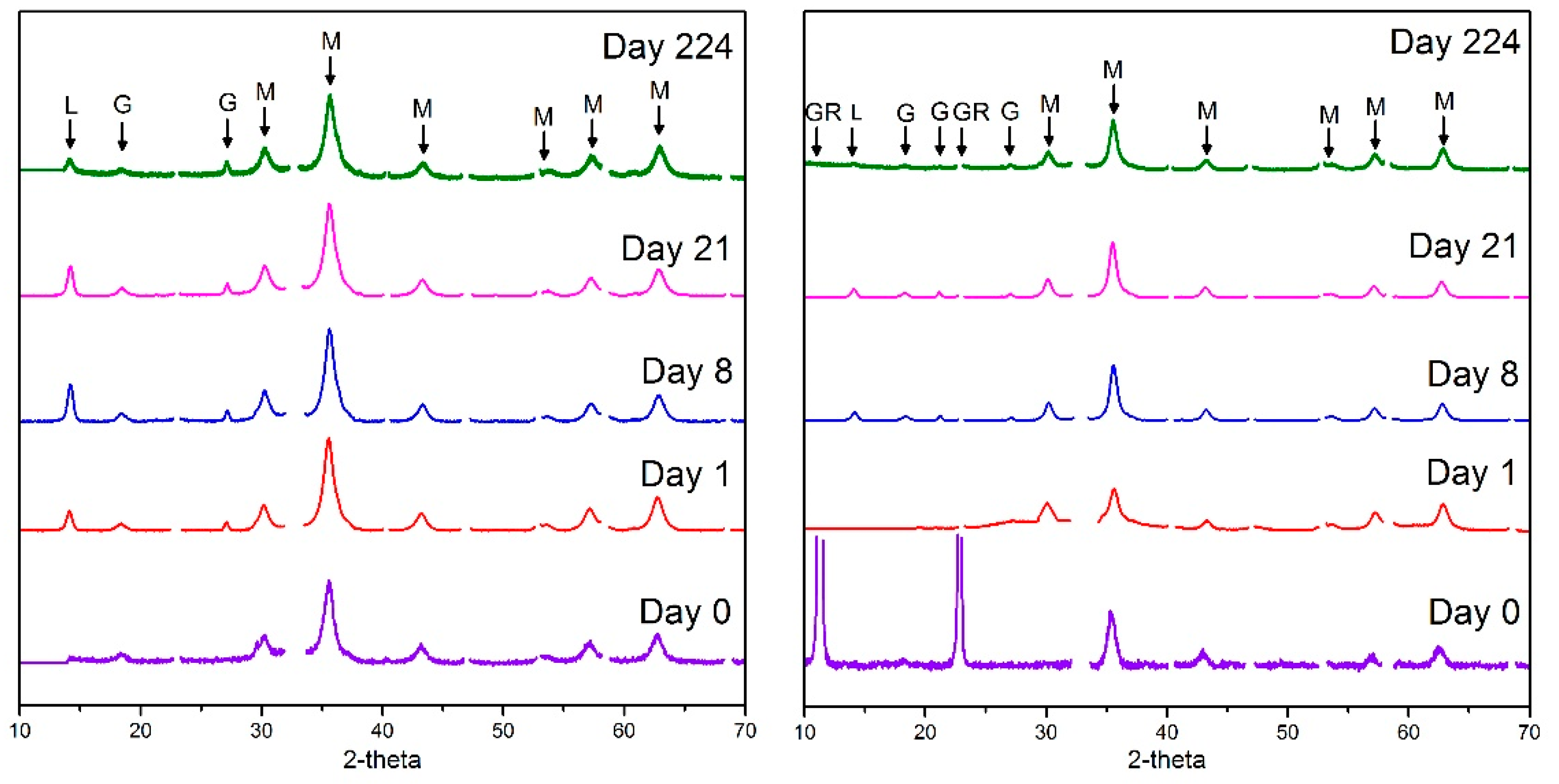
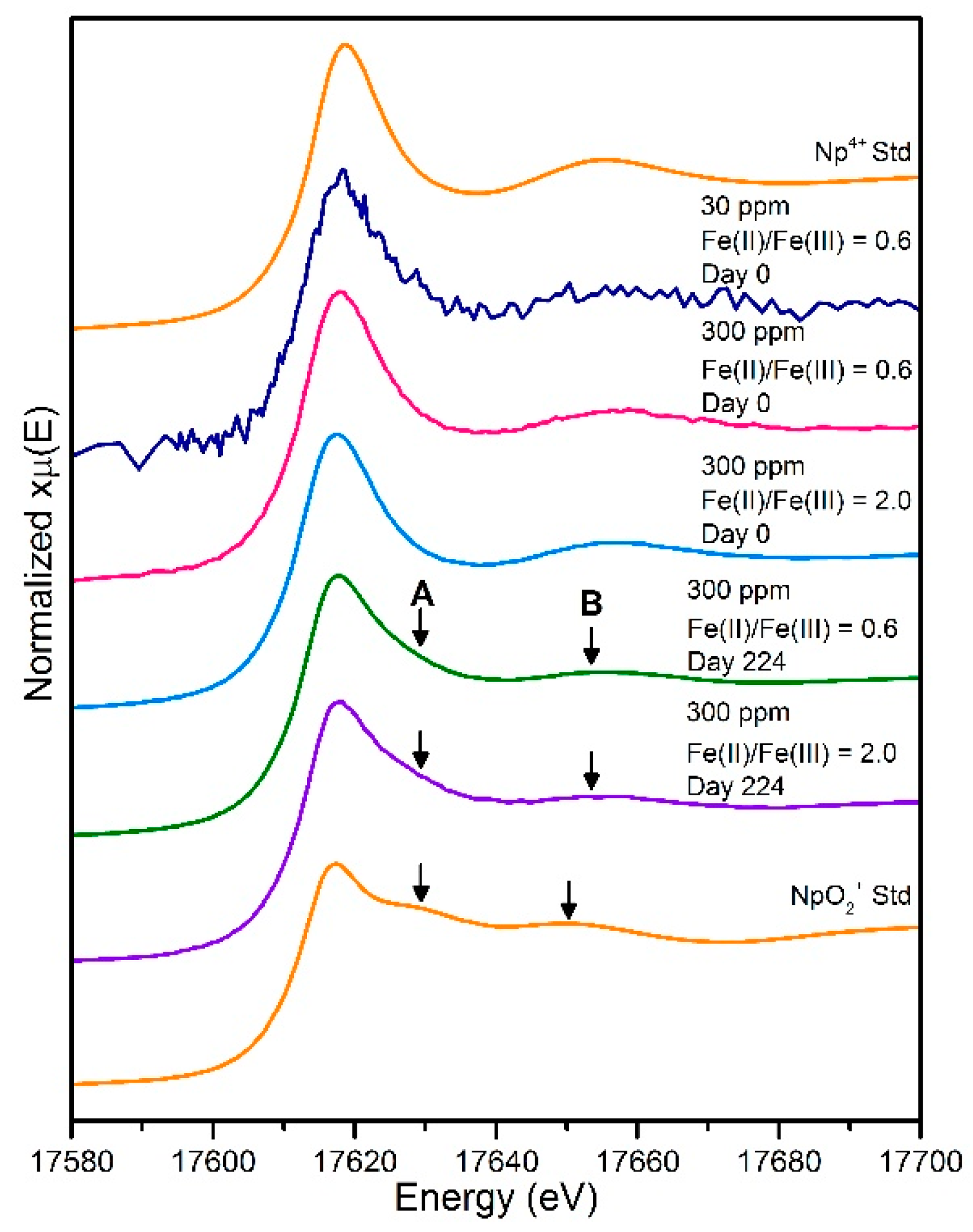
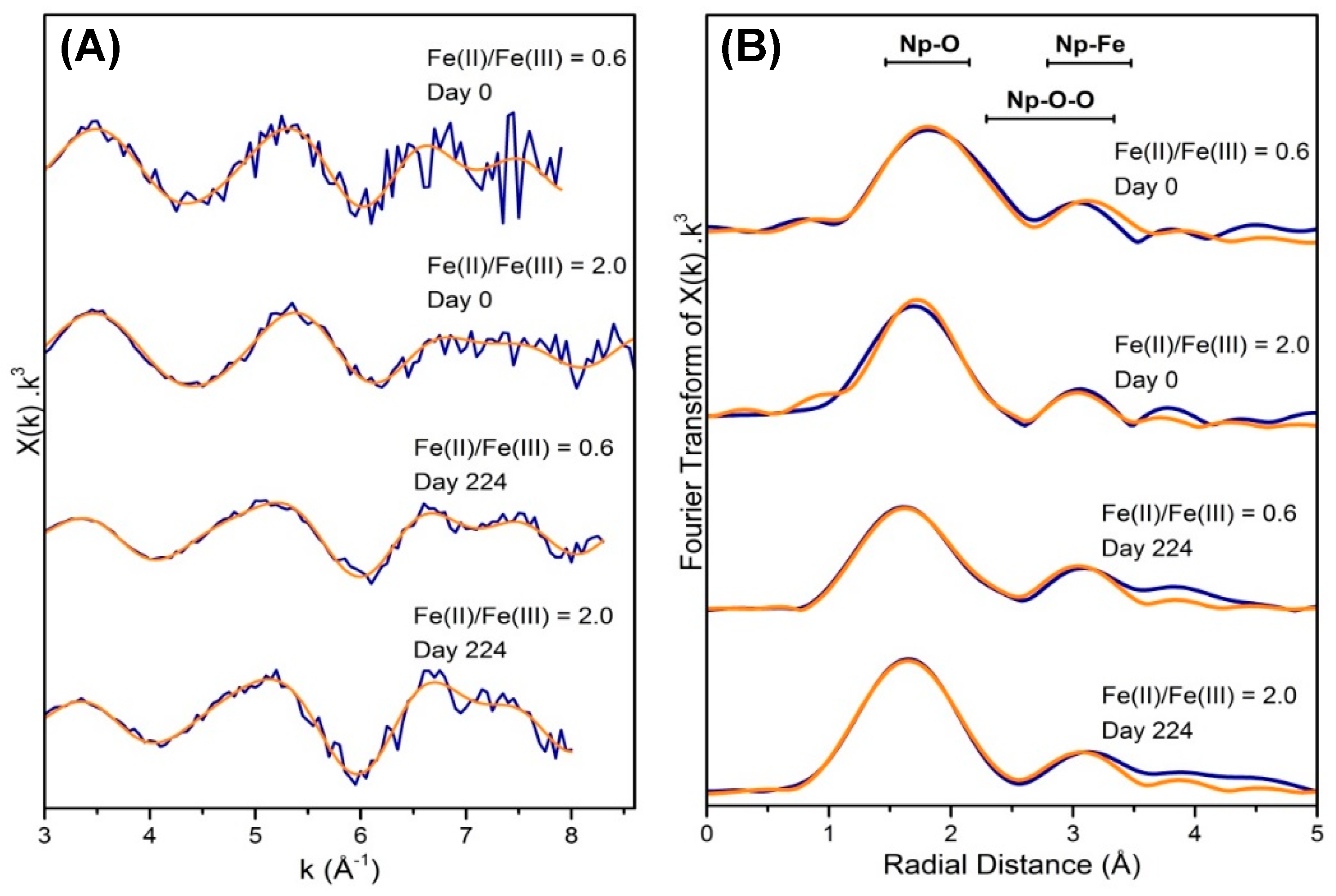
| Component 1: Np(V) (%) | Component 2: Np(IV) (%) | |
|---|---|---|
| Fe(II)/Fe(III) = 0.6, magnetite: 300 ppm Day 224 | 53 | 47 |
| Fe(II)/Fe(III) = 2.0, green rust: 300 ppm Day 224 | 60 | 40 |
| Sample | Path | CN | R (Å) | σ2(Å2) | Confidence (%) | ∆E0 (eV) | S02 | R | |
|---|---|---|---|---|---|---|---|---|---|
| 0.6, Day 0 | Np-O | 7 | 2.38(1) | 0.016(1) | 9.0 | 1 ** | 0.006 | ||
| Np-Fe | 2 | 3.49(2) | 0.005(2) | 99 | |||||
| 2.0, Day 0 | Np-O | 7 | 2.31(2) | 0.016(1) | 3.9 | 1 ** | 0.009 | ||
| Np-Fe | 2 | 3.41(3) | 0.012(4) | 97 | |||||
| 0.6,Day 224 | Np-O | 1.06 * | 1.86(1) | 0.005(1) | 9.5 | 1 ** | 0.012 | ||
| Np-O | 5.94 * | 2.36(1) | 0.023(1) | ||||||
| Np-Fe | 1.9(5) | 3.47(3) | 0.008(2) | 94 | |||||
| Np-O-O | 1.06 * | 3.72(2) | 0.009(2) | ||||||
| 2.0, Day 224 | Np-O | 1.2 * | 1.88(1) | 0.001(1) | 9.4 | 1 ** | 0.004 | ||
| Np-O | 5.8 * | 2.39(4) | 0.017(2) | ||||||
| Np-Fe | 1.5(5) | 3.45(2) | 0.002(2) | 88 | |||||
| Np-O-O | 1.2 * | 3.76(2) | 0.002(2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberts, H.E.; Morris, K.; Mosselmans, J.F.W.; Law, G.T.W.; Shaw, S. Neptunium Reactivity During Co-Precipitation and Oxidation of Fe(II)/Fe(III) (Oxyhydr)oxides. Geosciences 2019, 9, 27. https://doi.org/10.3390/geosciences9010027
Roberts HE, Morris K, Mosselmans JFW, Law GTW, Shaw S. Neptunium Reactivity During Co-Precipitation and Oxidation of Fe(II)/Fe(III) (Oxyhydr)oxides. Geosciences. 2019; 9(1):27. https://doi.org/10.3390/geosciences9010027
Chicago/Turabian StyleRoberts, Hannah E., Katherine Morris, J. Frederick W. Mosselmans, Gareth T. W. Law, and Samuel Shaw. 2019. "Neptunium Reactivity During Co-Precipitation and Oxidation of Fe(II)/Fe(III) (Oxyhydr)oxides" Geosciences 9, no. 1: 27. https://doi.org/10.3390/geosciences9010027
APA StyleRoberts, H. E., Morris, K., Mosselmans, J. F. W., Law, G. T. W., & Shaw, S. (2019). Neptunium Reactivity During Co-Precipitation and Oxidation of Fe(II)/Fe(III) (Oxyhydr)oxides. Geosciences, 9(1), 27. https://doi.org/10.3390/geosciences9010027





