Primary Producers and Anthropic Signs Related to the Flood Plain Soils of the Tablas de Daimiel Wetland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Sampling
2.3. Soil Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, H.S.; Bouma, J.; Pachepsky, Y. Revitalizing pedology through hydrology and connecting hydrology to pedology. Geoderma 2006, 131, 255–256. [Google Scholar] [CrossRef]
- Kutilek, M.; Nielsen, D.R. Interdisciplinarity of hydropedology. Geoderma 2007, 138, 252–260. [Google Scholar] [CrossRef]
- Rinklebe, J.; Langer, U. Microbial diversity in three floodplain soils at the Elbe River (Germany). Soil Biol. Biochem. 2006, 38, 2144–2151. [Google Scholar] [CrossRef]
- Rinklebe, J.; Langer, U. Floodplain soils at the Elbe River, Germany, and their diverse microbial biomass. Arch. Agron. Soil Sci. 2008, 54, 259–273. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands, 3rd ed.; Van Nostrand Reinhold: New York, NY, USA, 1993. [Google Scholar]
- Mitsch, W.J.; Gosselink, J.G. Wetlands; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- USEPA; USDA-NRCS. A Handbook of Constructed Wetlands; Volume 1: General Considerations; USEPA Region III with USDA-NRCS: Washington, DC, USA, 1995.
- Cooper, P.F.; Job, G.D.; Green, M.B.; Shutes, R.B.E. Reed Beds and Constructed Wetlands for Wastewater Treatment; WRc Publications: Medmenham, Marlow, UK, 1996. [Google Scholar]
- Vymazal, J.; Brix, H.; Cooper, P.F.; Green, M.B.; Haberl, R. Constructed Wetlands for Wastewater Treatment in Europe; Backhuys Publishers: Leiden, The Netherlands, 1998. [Google Scholar]
- United States Environmental Protection Agency (EPA). Wetland Functions and Values OF Wetlands; EPA 843-F-01-002c; EPA: Washington, DC, USA, 2001.
- Russi, D.; ten Brink, P.; Farmer, A.; Badura, T.; Coates, D.; Förster, J.; Kumar, R.; Davidson, N. The Economics of Ecosystems and Biodiversity for Water and Wetlands; Ramsar Secretariat, Gland; IEEP: London, UK; Brussels, Belgium, 2013. [Google Scholar]
- Whitton, B.A. Diversity, ecology and taxonomy of cyanobacteria. In Photosynthetic Procaryotes; Mann, N.H., Carr, N.G., Eds.; Biotechnology Handbooks; Plenum Press: London, UK, 1992; Volume 6, pp. 1–51. [Google Scholar]
- Scott, J.T.; Marcarelli, A.M. Cyanobacteria in freshwater benthic environments. In The Ecology of Cyanobacteria; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Komárek, J. Cyanobacterial taxonomy: Current problems and prospects for the integration of traditional and molecular approaches. Algae 2006, 21, 349–375. [Google Scholar] [CrossRef]
- Sejnohova, L.; Marsalek, B. Microcystis. In Ecology of Cyanobacteria II; Whitton, B.A., Ed.; Springer: Berlin, Germany, 2012; pp. 126–154. [Google Scholar]
- Kennedy, N.; Brodie, E.; Connolloy, J.; Clipson, N. Impact of lime, nitrogen and plant species on bacterial community structure in grassland microcosms. Environ. Microbiol. 2004, 6, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, E.S.; Kamst-Van Agterveld, M.P.; Zwart, G. Distribution of typical freshwater bacterial groups is associated with pH, temperature, and lake water retention time. Appl. Environ. Microbiol. 2005, 71, 8201–8206. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, B.D. Algalization of rice in salt-affected soils. Ann. Agric. Res. 1994, 14, 105–106. [Google Scholar]
- Jiménez-Ballesta, R.; García-Navarro, F.J.; Bravo Martín-Consuegra, S.; Pérez-de-los-Reyes, C.; Amorós Ortíz-Villajos, J.A.; Fernández San Miguel, M. The impact of the Storage on Nutrients and Other Trace Elements on the Degradation of a Wetland. Int. J. Environ. Res. 2018. [Google Scholar] [CrossRef]
- Perona, E.; Bonilla, I.; Mateo, P. Spatial and temporal changes in wáter in a Spanish river. Sci. Total Environ. 1999, 241, 75–90. [Google Scholar] [CrossRef]
- Dodds, W.; Castenholz, R. Effects of grazing and light on the growth of Nostoc pruniforme (Cyanobacteria). Br. Phycol. J. 1988, 23, 219–227. [Google Scholar] [CrossRef]
- Mejías, M. Las Tablas y Los Ojos del Guadiana: Agua, Paisaje y Gente; Instituto Geológico y Minero, Organismo Autónomo Parques Naturales: Madrid, Spain, 2014; 360p. [Google Scholar]
- Troya, A.; Bernués, M. Humedales Españoles en la Lista del Convenio de Ramsar: ICONA Ministerio de Agricultura; Alimentación y Pesca: Madrid, Spain, 1990. [Google Scholar]
- Ortega-Mayagoitia, E.; Armengol, X.; Rojo, C. Structure and dynamics of zooplankton in a semi-arid wetland, the National Park Las Tablas de Daimiel (Spain). Wetlands 2000, 20, 629–638. [Google Scholar] [CrossRef]
- Rojo, C.; Ortega-Mayagoitia, E.; Rodrigo, M.A.; Alvarez-Cobelas, M. Phytoplankton structure and dynamics in a semiarid wetland, the National Park ‘Las Tablas de Daimiel’ (Spain). Arch. Hydrobiol. 2000, 148, 397–419. [Google Scholar] [CrossRef]
- Jiménez Ballesta, R. Los suelos del Parque Natural de Las Tablas de Daimiel; Universidad Autónoma de Madrid: Madrid, Spain, 2014; 104p. [Google Scholar]
- Sanchez-Ramos, D.; Sanchez-Emeterio, G.; Florin, M. Changes in water quality of treated effluents by their receiving environments in Tablas de Daimiel National Park, Spain. Environ. Sci. Pollut. Res. 2016, 23, 6082–6090. [Google Scholar] [CrossRef] [PubMed]
- Toluna, L.G.; Okaya, O.S.; Gainesb, A.F.; Tolayc, M.; Tuefekceia, H.; Koratlod, N. The pollution status and the toxicity of surface sediments in Izmit Bay (Marmara Sea), Turkey. Environ. Int. 2001, 26, 63–168. [Google Scholar]
- Laguna, C.; López-Perea, J.J.; Viñuela, J.; Florín, M.; Feliu, J.; Chicote, A.; Cirujano, S.; Mateo, R. Effects of invasive fish and quality of water and sediment on macrophytes biomass, and their consequences of a waterbird community of a Mediterranean floodplain. Sci. Total Environ. 2016, 551–552, 312–521. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff. Keys to Soil Taxonomy, 7th ed.; USDA-NCRS: Washington, DC, USA, 2006.
- FAO-ISRIC-ISSS. World Reference Base for Soil Resources. A Framework for International Classification, Correlation and Communication; World Soil Resources Reports 103; FAO: Rome, Italy, 2006; 132p. [Google Scholar]
- Anne, A. Sur le dosage rapid du carbone organique de sols. Ann. Agron. 1945, 2, 161–172. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Methods of soil analysis, part 2 chemical and microbiological properties. Soil Sci. Soc. Am. 1982, 1159, 595–624. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954; Volume 939. [Google Scholar]
- Douterelo, I.; Perona, E.; Mateo, P. Use of cyanobacteria to assess water quality in running water. Environ. Pollut. 2004, 127, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Marker, A.F.H. The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin. Freshw. Boil. 1972, 2, 361–385. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Nomenclatural novelties in Chroococcalean cyanoprokaryotes. Preslia 1995, 67, 15–23. [Google Scholar]
- Komárek, J.; Kaštovský, J. Coincidences of structural and molecular characters in evolutionary lines of cyanobacteria. Algol. Stud. 2003, 109, 305–325. [Google Scholar] [CrossRef]
- Komárek, J.; Komárkova-Legnerová, J. Taxonomic evaluation of cyanobacterial microflora from alkaline marshes of northern Belize. 1. Phenotypic diversity of coccoid morphotypes. Nova Hedwig. 2007, 84, 65–111. [Google Scholar] [CrossRef]
- Munsell Color Company. Munsell Soil Color Charts; Munsell Color Company: Baltimore, MD, USA, 1954. [Google Scholar]
- Rinklebe, J.; Heinrich, K.; Neue, H.U. Der umsetzbare Kohlenstoff als Indikator für die potentielle bodenmikrobielle Aktivit€at in Auenb€oden. In Projektbereich Naturnahe Landschaften und L€andliche R€aume; Scholz, M., Stab, S., Henle, K., Eds.; UFZ Bericht Nr: Leipzig, Germany, 2001; Volume 8, pp. 74–83. [Google Scholar]
- Jokic, A.; Cutler, J.N.; Ponomarenko, E.; van der Kamp, G.; Anderson, D.W. Organic carbon and sulphur compounds in wetland soils: Insights on structure and transformation processes using K-edge XANES and NMR spectroscopy. Geochim. Cosmochim. Acta 2003, 67, 2585–2597. [Google Scholar] [CrossRef]
- Hill, J.R.; O’Driscoll, N.J.; Lean, D.R. Size distribution of methylmercury associated with particulate and dissolved organic matter in freshwaters. Sci. Total Environ. 2009, 408, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.L.; Reddy, K.R. Heterotrophic microbial activity in northern Everglades wetlands soils. Soil Sci. Soc. Am. J. 2001, 65, 1856–1864. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impact of carbon and flooding on the metabolic diversity of microbial communities in soils. Appl. Environ. Microbiol. 1995, 61, 4043–4050. [Google Scholar] [PubMed]
- Rinklebe, J.; Langer, U. Relationship between soil microbial biomass determined by SIR and PLFA analysis in floodplain soils. J. Soils Sediments 2010, 10, 4–8. [Google Scholar] [CrossRef]
- Frohne, T.; Rinklebe, J.; Diaz-Bone, R.A. Contamination of floodplain soils along the Wupper River, Germany, with As, Co, Cu, Ni, Sb, and Zn and the impact of pre-definite redox variations on the mobility of these elements. Soil Sediment Contam. 2014, 23, 779–799. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J.; Rupp, H.; Meissner, R. Lysimeter trials to assess the impact of different flood-dry-cycles on the dynamics of pore water concentrations of As, Cr, Mo and V in a contaminated floodplain soil. Geoderma 2014, 228–229, 5–13. [Google Scholar] [CrossRef]
- Otero, X.L.; Macías, F. Biogeochemistry and Pedogenetic Process in Saltmarsh and Mangrove Systems; New Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Patnaik, K.C.; Misra, P.M. Seasonal variation in the physiochemical properties of Rushikulya estuary and its effect on the occurrence of chanos fry. J. Indian Fish. 1990, 20, 69–71. [Google Scholar]
- Rani Deep, P.; Bhattacharyya, S.; Nayak, B. Cyanobacteria in wetlands of the industrialized Sambalpur District of India. Aquat. Biosyst. 2013, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Stancheva, R.; Sheath, R.G.; Read, B.A.; McArthur, K.D.; Schroepfer, C.; Kociolek, J.P.; Fetscher, A.E. Nitrogen-fixing cyanobacteria (free-living and diatom endosymbionts): Their use in southern California stream bioassessment. Hydrobiologia 2013, 720, 111–127. [Google Scholar] [CrossRef]
- Moss, B. Ecology of Freshwaters, Man and Medium, Past to Future; Blackwell Science Ltd.: Oxford, UK, 1998. [Google Scholar]
- Mbonde, A.S.E.; Shayo, S.; Sekadende, B.C.; Lyimo, T.J. Phytoplankton species diversity and abundance in the near shore waters of Tanzanian side of Lake Victoria. Tanzan. J. Sci. 2004, 30, 71–81. [Google Scholar] [CrossRef]
- Loza, V.; Perona, E.; Mateo, P. Specific responses to nitrogen and phosphorus enrichment in cyanobacteria: Factors influencing changes in species dominance along eutrophic gradients. Water Res. 2014, 48, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Moche, M.; Gutknecht, J.; Schulz, E.; Langer, U.; Rinklebe, J. Monthly dynamics of microbial community structure and their controlling factors in three floodplain soils. Soil Biol. Biochem. 2015, 90, 169–178. [Google Scholar] [CrossRef]
- Roger, P.A.; Reynaud, P.A. Free Living Blue Green Algae in Tropical Soils. In Microbiology of Tropical Soils and Plant Productivity; Dommer-Gues, Y., Diem, H., Eds.; Martinus Nijhoff Publisher: La Hague, France, 1982; pp. 147–168. [Google Scholar]
- Zancan, S.; Trevisan, R. and Paoletti, M.G. Soil Algae Composition under Different Agro-Ecosystem in NorthEastern Italy. Agric. Ecosyst. Environ. 2006, 112, 1–12. [Google Scholar] [CrossRef]
- Lukesova, A. Soil Algae in Brown coal and Lignite Post mining areas in Central Europe (Czech Republic and Germany). Res. Ecol. 2001, 9, 341–350. [Google Scholar] [CrossRef]
- Boominathan, M. Bioremediation Studies on Dairy Effluent Using Cyanobacteria. Ph.D. Thesis, Bharathidasan University, Tiruchirapalli, Tamil Nadu, India, 2005. [Google Scholar]
- Vijayakumar, S.; Thajuddin, N.; Manoharan, C. Role of cyanobacteria in the treatment of dye industry effluent. Pollut. Res. 2005, 24, 69–74. [Google Scholar]
- Nayak, S.; Prasanna, R. Soil pH and Its Role in Cyanobacterial Abundance and Diversity in Rice Field Soils. Appl. Ecol. Environ. Res. 2007, 5, 103–113. [Google Scholar] [CrossRef]
- Selvi, T.K.; Sivakumar, K. Cynobacterial Diversity and Related Physico-Chemical Parameters in Paddy Fields of Cuddalore District, Tamil Nadu. Int. J. Res. Environ. Sci. Technol. 2011, 1, 7–15. [Google Scholar]
- Singh, P.K.; Kishore, S.; Prakash, S.; Singh, K. Cyanophycean Algae Inhabiting Sodic Soil Exhibit Diverse Morphology: An Adaptation to High Exchangeable Sodium. Ecoprint 2008, 15, 15–21. [Google Scholar] [CrossRef]
- Mataloni, G.; González, G.G.; Bolter, M.; Convey, P.; Fermani, P. What shapes edaphic communities in mineral and ornithogenic soils of Cierva Point, Antarctic Peninusula? Polar Sci. 2010, 4, 405–419. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef] [PubMed]
- Mateo, P.; Leganés, F.; Perona, E.; Loza, V.; Fernández-Piñas, F. Cyanobacteria as bioindicators and bioreporters of environmental analyses in aquatic ecosystems. Biodivers. Conserv. 2015. [Google Scholar] [CrossRef]
- Loza, V.; Perona, E.; Mateo, P. Molecular fingerprinting of cyanobacteria from river biofilms as a water quality monitoring tool. Appl. Environ. Microbiol. 2013, 79, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Saxena, P.N. Preliminary studies on algal succession in raw and stabilized sewage. Hydrobiologia 1969, 34, 503–512. [Google Scholar] [CrossRef]
- Venkateshwarlu, V. Taxonomy and ecology of algae in the river Moosi. Hyderabad (India). Nova Hedwigia. 1976, 27, 661–676. [Google Scholar]
- Rao, C.B. On the distribution of algae in a group of six small ponds. II. Algal periodicity. J. Ecol. 1955, 43, 291–308. [Google Scholar] [CrossRef]
- Venkateshwarlu, V. An ecological study of the algae of the river Moosi. Hyderabad (India) with special reference to water pollution. II. Factors influencing the distribution of algae. Hydrobiologia 1969, 34, 352–362. [Google Scholar] [CrossRef]
- Moisander, P.H.; Steppe, T.F.; Hall, N.S.; Kuparinen, J.; Paerl, H.W. Variability in nitrogen and phosphorus limitation for Baltic Sea phytoplankton during nitrogenfixing cyanobacterial blooms. Mar. Ecol. Prog. Ser. 2003, 262, 81–95. [Google Scholar] [CrossRef]
- Tanaka, T.; Rassoulzadegean, F.; Thingstad, T.F. Orthophosphate uptake by heterotrophic bacteria, cyanobacteria, and autotrophic nanoflagellates in Villefranche Bay, northwestern Mediterranean: Vertical, seasonal, and short-term variations of the competitive relationship for phosphorus. Limnol. Oceanogr. 2004, 49, 1063–1072. [Google Scholar] [CrossRef]
- Rangel, L.M.; Silva, L.H.S.; Rosa, P.; Roland, F.; Huszar, V.L.M. Phytoplankton biomass is mainly controlled by hydrology and phosphorus concentrations in tropical hydroelectric reservoirs. Hydrobiologia 2012, 693, 13–28. [Google Scholar] [CrossRef]
- Santos, V.B.; Araujo, S.F.; Leite, L.F.; Nunes, L.A.; Melo, J.W. Soil microbial biomass and organic matter fractions during transition from conventional to organic farming systems. Geoderma 2012, 170, 227–231. [Google Scholar] [CrossRef]
- Pereg, L.; McMillan, M. Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 2015, 80, 349–358. [Google Scholar] [CrossRef]
- Guerriero, G.; D’Errico, G.; Di Giaimo, R.; Rabbito, D.; Olanrewaju, O.S.; Ciarcia, G. Reactive oxygen species and glutathione antioxidants in the testis of the soil biosentinel Podarcis sicula (Rafinesque 1810). Environ. Sci. Pollut. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]

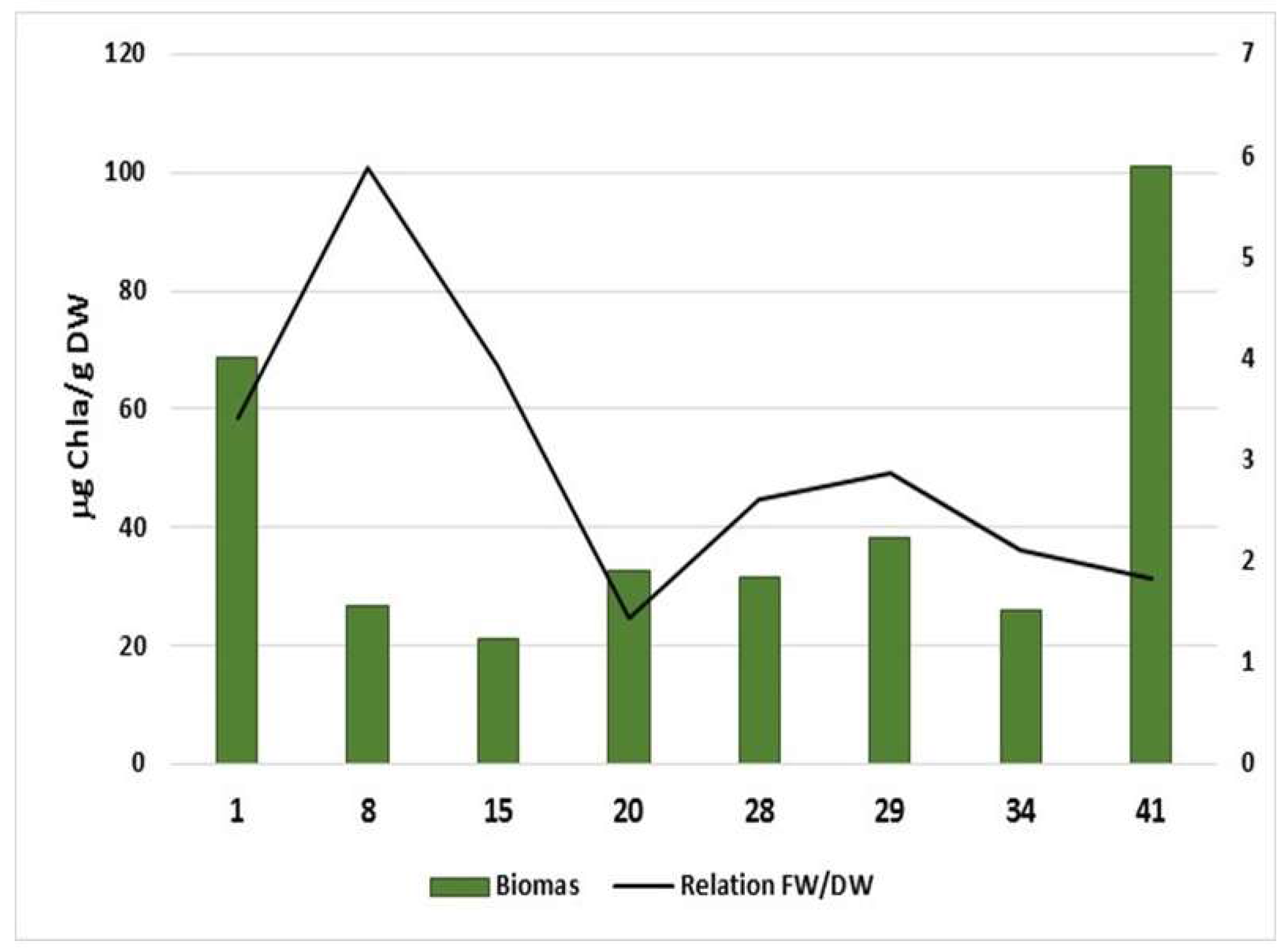
| Sample Nº | Name | Coordenates | |
|---|---|---|---|
| X | Y | ||
| 1 | Gigüela | 0446572 | 4338602 |
| 8 | E. Casablanca | 0438910 | 4333770 |
| 15 | Entradilla | 0439461 | 4332245 |
| 20 | Molemocho | 0440304 | 4331318 |
| 28 | V. de los Toros | 0436149 | 4331316 |
| 29 | S. Almochinares | 0435363 | 4330524 |
| 34 | Zacatena | 0434206 | 4329673 |
| 41 | L. Navaseca | 0446536 | 4328067 |
| Sample Nº | Name | Colour | DO | Eh | pH | OM | N | C/N | P2O5 | P | EC | CaCO3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry | Wet | (%) | (mV) | (%) | (%) | (mg/kg) | (g/kg) | (dS/m) | (%) | ||||
| 1 | Gigüela | 5Y 5/1 | 5 Y 2,5/1 | 90.1 | −171.9 | 8.0 | 11.8 | 0.239 | 28.8 | 22.1 | 1.06 | 1.27 | 37.9 |
| 8 | E. Casablanca | 2.5 Y 5/2 | 2.5 Y 3/1 | 74.2 | −73.8 | 7.5 | 23.3 | 0.440 | 30.6 | 40.7 | 1.21 | 3.61 | 33.8 |
| 15 | Entradilla | 2.5 Y 6/3 | 2.5 Y 4/2 | 67.8 | −23.5 | 8.1 | 19.3 | 0.532 | 21.1 | 15.4 | 0.26 | 1.05 | 72.4 |
| 20 | Molemocho | 2.5 Y 5/3 | 2.5 Y 3/3 | 112.1 | 39.8 | 7.8 | 3.0 | 0.075 | 23.0 | 42.6 | 0.89 | 0.48 | 7.60 |
| 28 | V. de los Toros | 5 Y 3/1 | 5 Y 2.5/2 | 15.4 | −169.4 | 7.9 | 21.3 | 0.604 | 20.5 | 54.3 | 0.94 | 1.61 | 28.0 |
| 29 | S. Almochinares | 5 Y 4/2 | 5 Y 3/2 | 49.3 | −168.3 | 7.9 | 37.9 | 0.261 | 84.3 | 50.7 | 0.26 | 1.38 | 37.4 |
| 34 | Zacatena | 5 Y 5/1 | 5 Y 2.5/2 | 36.8 | −163.4 | 7.7 | 10.6 | 0.247 | 25.0 | 36.5 | 0.71 | 1.70 | 37.9 |
| 41 | L. Navaseca | 5 Y 5/2 | 5 Y 3/2 | 143.3 | 25.4 | 8.3 | 8.8 | 0.090 | 56.8 | 12.1 | 5.39 | 1.32 | 38.7 |
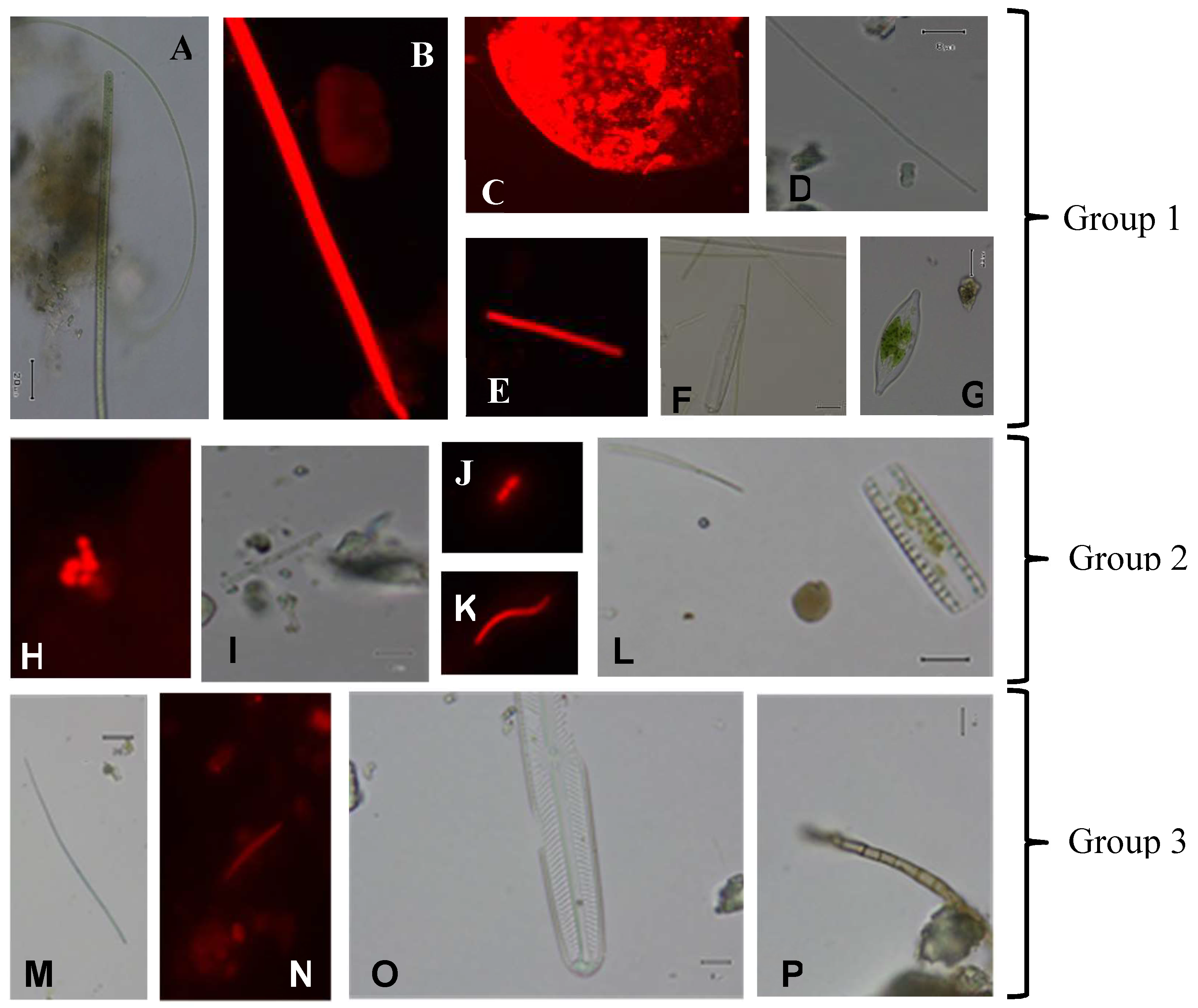
| Sample Nº | Cyanobacteria | Diattoms | Green Algae | Fungi |
|---|---|---|---|---|
| 1 | ++ | ++(+) | - | + |
| 8 | +++ | ++ | - | - |
| 15 | ++ | + | - | - |
| 20 | ++ | + | - | - |
| 28 | + | + | - | - |
| 29 | + | + | + | - |
| 34 | + | + | - | + |
| 41 | ++ | ++ | + | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Navarro, F.J.; Perona, E.; Cubero, S.; Allende, F.; Bravo, S.; Jiménez-Ballesta, R. Primary Producers and Anthropic Signs Related to the Flood Plain Soils of the Tablas de Daimiel Wetland. Geosciences 2018, 8, 106. https://doi.org/10.3390/geosciences8040106
Garcia-Navarro FJ, Perona E, Cubero S, Allende F, Bravo S, Jiménez-Ballesta R. Primary Producers and Anthropic Signs Related to the Flood Plain Soils of the Tablas de Daimiel Wetland. Geosciences. 2018; 8(4):106. https://doi.org/10.3390/geosciences8040106
Chicago/Turabian StyleGarcia-Navarro, Francisco J., Elvira Perona, Sara Cubero, Fernando Allende, Sandra Bravo, and Raimundo Jiménez-Ballesta. 2018. "Primary Producers and Anthropic Signs Related to the Flood Plain Soils of the Tablas de Daimiel Wetland" Geosciences 8, no. 4: 106. https://doi.org/10.3390/geosciences8040106
APA StyleGarcia-Navarro, F. J., Perona, E., Cubero, S., Allende, F., Bravo, S., & Jiménez-Ballesta, R. (2018). Primary Producers and Anthropic Signs Related to the Flood Plain Soils of the Tablas de Daimiel Wetland. Geosciences, 8(4), 106. https://doi.org/10.3390/geosciences8040106







