Verde Macael: A Serpentinite Wrongly Referred to as a Marble
Abstract
:1. Introduction
2. Location and Geological Setting of the Studied Samples
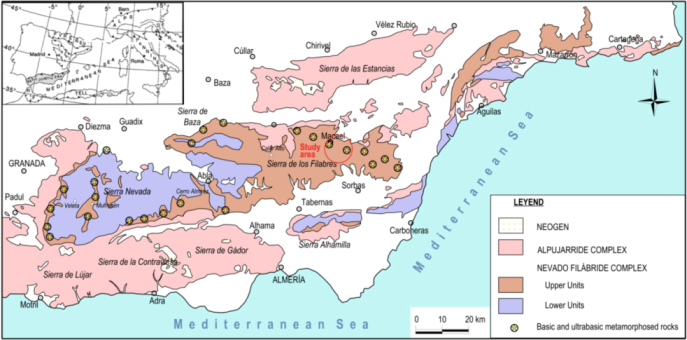
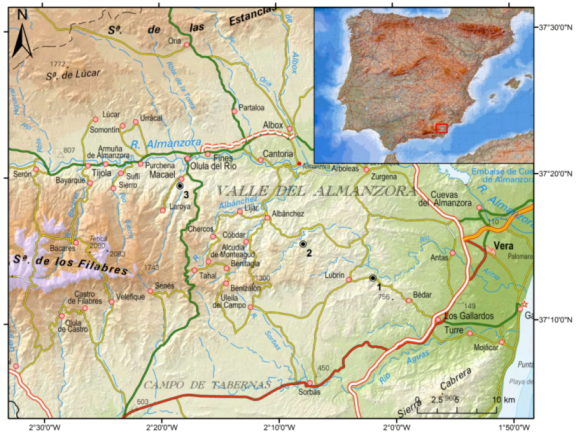
3. Methodology



4. Results
4.1. Textures and Mineralogy
| Sample | Main | Subordinate | Accessories and trace |
|---|---|---|---|
| Verde Macael 1 | |||
| MI-101 | Antigorite (89%) | Magnetite (11%) | |
| MI-201 | Antigorite (88%) | Magnetite (12%) | |
| MI-301 | Antigorite (89%) | Magnetite (11%) | |
| MI-401 | Antigorite (89%) | Magnetite (11%) | Calcite (<1%) |
| MI-501 | Antigorite (89%) | Magnetite (11%) | Calcite (<1%) |
| Verde Macael 2 | |||
| CA-101 | Talc (45%) | Calcite (25%), Dolomite (22%) | |
| CA-201 | Calcite (65%) | Augite (35%) | |
| CA-202 | Chlorite (70%) | Calcite (30%) | Talc (<1%) |
| CA-301 | Antigorite (87%) | Magnetite (13%) | Magnesite (<1%) |
| CA-401 | Antigorite (82%) | Magnetite (15%) | Magnesite (<1%) |
| CA-402 | Antigorite (87%) | Magnetite (8.5%) | Magnesite (4.5%) |
| CA-501 | Antigorite (100%) | Calcite (<1%) | |
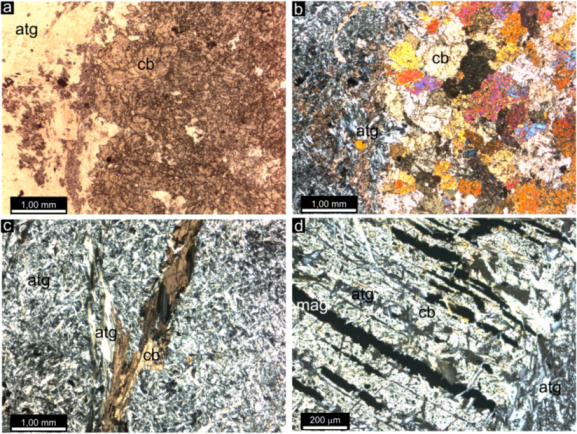

4.2. Physical and Mechanical Parameters
| Sample | Bulk Specific Gravity (g/cm3) | Open Porosity (%) | Absorption (% weight) | Compressive Strength (MPa) |
|---|---|---|---|---|
| Verde Macael 1 | 2.68 | 0.7 | 0.30 | 252 |
| Verde Macael 2 | 2.90 | 1.4 | 0.40 | 279 |
| Blanco Macael | 2.72 | 0.2 | 0.07–0.08 1 | 125 |

4.3. Sound Wave Speed Propagation
| Sample | Vp (X) | Vp (Y) | Vp (Z) | Vp (mean) | ΔM% |
|---|---|---|---|---|---|
| Verde Macael 1 | 5.72 | 5.73 | 5.83 | 5.76 | 0.98 |
| Verde Macael 2 | 4.75 | 5.26 | 5.07 | 5.03 | 7.92 |
| Blanco Macael | 5.89 | 5.76 | 5.06 | 5.57 | 13.10 |
5. Discussion
6. Conclusions
Acknowledgements
References
- Pereira, M.D.; Yenes, M.; Blanco, J.A.; Peinado, M. Characterization of serpentinites to define their appropriate use as dimension stone. In Building Stone Decay: From Diagnosis to Conservation; Prikryl, R., Smith, B.J., Eds.; Geological Society: London, UK, 2007; Geological Society Special Publication No. 271; pp. 55–62. [Google Scholar]
- Ismael, I.; Hassan, M. Characterization of some Egyptian serpentinites used as ornamental stones. Chin. J. Geochem. 2008, 27, 140–149. [Google Scholar] [CrossRef]
- Pereira, D.; Peinado, M.; Blanco, J.A. Misuse of natural stone for construction and the consequences in buildings. Case of study of serpentinites. J. Mater. Civ. Eng. 2012. [Google Scholar] [CrossRef]
- Pereira, M.D.; Peinado, M.; Blanco, J.A.; Yenes, M.; Fallick, A.; Upton, B. Serpentinite: A potential natural stone in Spain. In Dimension Stone; Prikryl, R., Ed.; Taylor and Francis: London, UK, 2004; pp. 85–87. [Google Scholar]
- Meierding, T.C. Weathering of serpentine stone buildings in the Philadelphia region: A geograffic approach related to acidic deposition. In Stone Decay in the Architectural Environment; Turkington, A.V., Ed.; Geological Society of America: Boulder, CO, USA, 2005; Special Paper No. 399; pp. 17–25. [Google Scholar]
- Harrell, J.A. Survey of Ornamental Stones in Mosques and Other Islamic Buildings of the Pre-Ottoman Period in Cairo, Egypt. Available online: http://www.eeescience.utoledo.edu/Faculty/Harrell/Egypt/Mosques/Survey_Intro.htm (accessed on 4 November 2011).
- Malesani, P.; Pecchioni, E.; Cantisani, E.; Fratini, F. Geolithology and provenance of materials of some historical buildings and monuments in the centre of Florence (Italy). Episodes 2003, 26, 250–255. [Google Scholar]
- Marino, L.; Corti, M.; Coli, M.; Tanini, C.; Nenci, C. The “Verde di Prato” Stones of Cathedral and Baptistery of Florence (abstract). In Proceedings of 32nd International Geological Congress, Florence, Italy, 20–28 August, 2004; p. 283.
- Sharp, J. Cosmati pavements at Westminster abbey. Nexus Netw. J. 1999, 1, 99–104. [Google Scholar] [CrossRef]
- Egeler, C.G. On the tectonics of the eastern Betic Cordilleras (SE Spain). Geol. Rundsch. 1963, 52, 260–269. [Google Scholar]
- Puga, E.; Diaz de Federico, A.; Nieto, J.M. Tectonostratigraphic subdivision and petrological characterisation of the deepest complexes of the Betic zone: A review. Geodin. Acta 2002, 15, 23–43. [Google Scholar]
- Cordillera Betica y Baleares. [in Spanish]; In Geologia de Espana; Vera, J.A. (Ed.) SGE-IGME: Madrid, Spain, 2004; pp. 345–464.
- Schultz, L.G. Quantitative Interpretation of Mineralogical Composition from X-ray and Chemical Data for Pierre Shale; U.S. Government Printing Office: Washington, DC, USA, 1964; U.S. Geological Survey Professional Paper 391-c; pp. 1–31.
- UNE-EN-ISO-17025: Evaluación de la Conformidad. Requisitos Generales Para la Competencia de los Laboratorios de Ensayo y de Calibración; [in Spanish]; Spanish Association for Standarization and Certification (AENOR): Madrid, Spain, 2005.
- UNE-EN-1936: Métodos de Ensayo Para Piedra Natural. Determinación de la Densidad Real y Aparente y de la Porosidad Abierta y Total; [in Spanish]; Spanish Association for Standarization and Certification (AENOR): Madrid, Spain, 2007.
- UNE-EN-13755: Métodos de Ensayo Para Piedra Natural. Determinación de la Absorción de Agua a Presión Atmosférica; [in Spanish]; Spanish Association for Standarization and Certification (AENOR): Madrid, Spain, 2008.
- UNE-EN-1926: Métodos de Ensayo Para la Piedra Natural. Determinación de la Resistencia a la Compresión Uniaxial; [in Spanish]; Spanish Association for Standarization and Certification (AENOR): Madrid, Spain, 2007.
- UNE-EN-1457: Métodos de Ensayo Para Piedra Natural. Determinación de la Velocidad de Propagación del Sonido; [in Spanish]; Spanish Association for Standarization and Certification (AENOR): Madrid, Spain, 2005.
- Guyader, J.; Denis, A. Propagation des ondes les roches anisotropes sous contrainte evaluation de la qualite des schiste ardoisiers. [in French]. Bull. Eng. Geol. 1986, 33, 49–55. [Google Scholar] [CrossRef]
- Wicks, F.J.; Whittaker, E.J.W. Serpentinte textures and serpentinization. Can. Mineral. 1977, 15, 459–488. [Google Scholar]
- Lasa, I. Marmoles. [in Spanish]; In Roc-Maquina. La Piedra Natural de España. Directorio, 21st ed; Reed Business Information: Bilbao, Spain, 2010; pp. 344–409. [Google Scholar]
- Lopez-Arce, P.; Doehne, E.; Martin, W.; Pinchin, S. Magnesium sulfate salts and historic building materials: Experimental simulation of limestone flaking by relative humidity cycling and crystallization of salts. Mater. Constr. 2008, 58, 125–142. [Google Scholar]
- Vilhelm, J.; Rudajev, V.; Zivor, R.; Lokajicek, T.; Pros, Z. Comparison of field and laboratory seismic velocity anisotropy measurement (scaling factor). Acta Geodyn. Geomater. 2008, 5, 161–169. [Google Scholar]
- Christensen, N.I. Ophiolites, seismic velocities and oceanic crustal structure. Tectonophysics 1978, 47, 131–157. [Google Scholar] [CrossRef]
- Bezacier, L.; Reynard, B.; Bass, J.D.; Sanchez-Valle, C.; van de Moortele, B. Elasticity of antigorite, seismic detection of serpentinites, and anisotropy in subduction zones. Earth Planet. Sci. Lett. 2010, 289, 198–208. [Google Scholar] [CrossRef]
- Luque, A.; Ruiz-Agudo, E.; Cultrone, G.; Sebastian-Pardo, E.; Siegesmund, S. Direct observation of microcrack development in marble caused by thermal weathering. Environ. Earth Sci. 2011, 62, 1375–1386. [Google Scholar] [CrossRef]
- Christensen, N.I. Serpentinites, peridotites, and seismology. Int. Geol. Rev. 2004, 46, 795–816. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Navarro, R.; Pereira, D.; Gimeno, A.; Barrio, S.D. Verde Macael: A Serpentinite Wrongly Referred to as a Marble. Geosciences 2013, 3, 102-113. https://doi.org/10.3390/geosciences3010102
Navarro R, Pereira D, Gimeno A, Barrio SD. Verde Macael: A Serpentinite Wrongly Referred to as a Marble. Geosciences. 2013; 3(1):102-113. https://doi.org/10.3390/geosciences3010102
Chicago/Turabian StyleNavarro, Rafael, Dolores Pereira, Ana Gimeno, and Santiago Del Barrio. 2013. "Verde Macael: A Serpentinite Wrongly Referred to as a Marble" Geosciences 3, no. 1: 102-113. https://doi.org/10.3390/geosciences3010102
APA StyleNavarro, R., Pereira, D., Gimeno, A., & Barrio, S. D. (2013). Verde Macael: A Serpentinite Wrongly Referred to as a Marble. Geosciences, 3(1), 102-113. https://doi.org/10.3390/geosciences3010102





