Abstract
Mesoamerican nephropathy (MeN) is a non-traditional chronic kidney disease in some areas of Mesoamerica. The health risk from nephrotoxic metals, such as arsenic (As), lead (Pb), mercury (Hg), vanadium (V), cadmium (Cd), rubidium (Rb), chromium (Cr), and nickel (Ni), was assessed in drinking water and soils. These metals, even at low concentrations, have the capacity to induce epigenetic damage and a nephrotoxic effect. The quantification of metals in soils was made through X-ray fluorescence spectrometry (XRF) and inductively coupled plasma optical emission spectrophotometry (ICP-OES), while the quantification of metals in water was carried out through inductively coupled plasma mass spectrometry (ICPMS) and atomic absorption (AA) spectrometry. The levels of As, Hg, Cd, and V in water were within the permissible limits, whereas Pb was found to be double and triple the value recommended by the World Health Organization. The non-carcinogenic risk from As in soil was evaluated using the Hazard Index (HI), and the route of ingestion was found to be the most important route. The results indicate that consuming water or ingesting soil particles with Pb and As poses a health risk to humans. Therefore, these findings identify the presence of toxicants in an exposure scenario and justify further research into these metals in people and the analysis of exposure routes.
1. Introduction
Chronic kidney disease (CKD) is a public health problem that affects millions of people worldwide, and according to global statistics, it is estimated that 850 million people live with this disease [1]; an average of one in ten adults suffers from kidney disease [2]. In Panama, a country located at the southern end of Mesoamerica, it has been observed that the prevalence of CKD in recent years has increased, becoming one of the main causes of mortality [3,4]. The main risk factors for CKD are hypertension, diabetes, and being over 65 years of age. However, during recent decades, there has been a significant number of young renal patients between the ages of 20 and 30 years who are not associated with these risk factors, which has given rise to another comparable pathology to non-traditional chronic kidney disease called Mesoamerican nephropathy (MeN) [5]. Similar cases have occurred in all Mesoamerican countries, mainly near the Pacific coast and low-altitude areas [6]. The etiology of this type of CKD is a pending task for the international scientific community; however, there is a list of possible causal factors or hypotheses, including metabolic, occupational, infectious, toxicants, and even genetic aspects [7,8]. Among the toxics, some heavy metals and nephrotoxic metalloids of environmental origin have been pointed out [9,10], such as Hg, As, Cd, Pb, and Zn, as capable of harming the kidneys [8,11] and contributing to chronic kidney disease of non-traditional causes [12]. Studies show a relationship between exposure to heavy metals and metalloids in areas shared with human populations and the occurrence of kidney function damage [12,13]. The mechanism of the toxicological action of these metals is complex. It occurs mainly through the generation of free radicals (e.g., reactive oxygen species, ROS), which affects the correct function of the nephron due to the damage of molecules such as mitochondrial and nuclear DNA, lipids, and proteins [14]. Heavy metals are also known as genotoxic because they can inhibit DNA repair [15]; the severity of kidney damage depends mainly on the type of exposure and the concentration of the toxic metal. Exposure to metals at low but sustained doses over prolonged periods leads to bioaccumulative processes that produce clinical manifestations that may take many years to show symptoms [16]. Cadmium, Pb, and Hg are considered to cause damage to the kidneys at the tubulointerstitial level, similar to the damage identified in research with patients who have MeN [12,17]. The geogenic nature of the site may determine the concentration of these metals in soils and water. These properties in the soil and water may have risks that go unnoticed, such as the hazards to the population of ash, volcanic rocks, and soil formation with toxic metal contents [18]. The ability of these toxicants to cause MeN is based on an exposure scenario that includes the convergence of toxicants, environmental factors, and the susceptible or at-risk population. This comprehensive approach allows us to understand how the interaction of these metals with local environmental–geographical conditions and individual susceptibilities can trigger renal damage. Our research group recognizes the multifactorial etiology of this disease, which includes genetic, occupational, and environmental factors that have been widely documented in recent publications from our group [8]. The objective of this study was to evaluate the risk of kidney damage by nephrotoxic metals such as As, Pb, Hg, V, Cd, Rb, Cr, and Ni found in drinking water and soils of communities with a high prevalence of Mesoamerican nephropathy (MeN) in the province of Coclé, Panama.
2. Materials and Methods
The survey area was located in the province of Coclé, Panama, in the center of the country on the Pacific coast (Figure 1). Coclé is located between the El Valle Volcano and the volcanic complex of La Yeguada, which classifies it as a volcanic zone. Coclé soils are rich in heavy metals and other minerals due to their geological origin, historically attracting the extraction of gold, copper, and other metals [19]. Based on data from the Ministry of Health, this province has the highest prevalence of renal morbidity in Panama [20]. The area is characterized by environmental, geographic, and socioeconomic conditions similar to other regions of Mesoamerica, which display a high prevalence of Mesoamerican nephropathy5. Soil and water samples were collected in communities with a high prevalence of chronic kidney disease in the province of Coclé, Panama, to determine the concentrations of nephrotoxic metals and perform a risk analysis for ingestion that will help to identify whether there is a risk to the renal health for the population in the area.
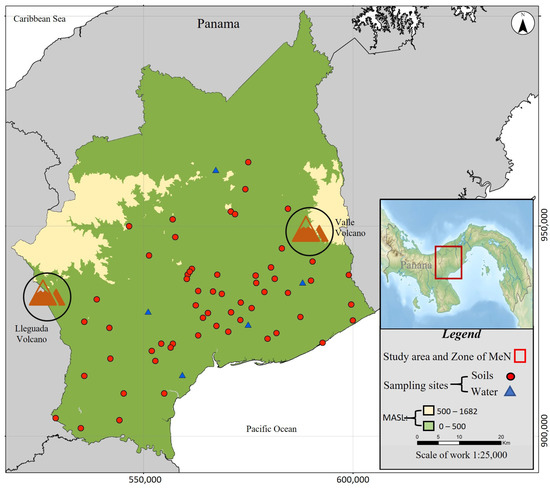
Figure 1.
Map of the study area soil (red circle, ●) and water (blue triangle ▲) sample collection sites in Coclé, Panama: MASL, meters above sea level.
2.1. Sample Collection
Samples of natural (untreated) water intended for human consumption were collected from the catchment sources of five rural aqueducts in Coclé, Panama. Five sampling sites were established, and four sampling events were conducted, two in the dry season and two in the rainy season in 2020 and 2021, for 20 samples. The samples were collected in one-liter polyethylene containers, previously washed with an acid solution of HNO3 (1 + 1) reagent grade. The samples were immediately fixed at pH < 2 with analytical grade HNO3 and refrigerated at a temperature below 4 °C until their analysis (APHA et al., 2017) [21]. Each sample was coded and georeferenced using a navigator Global Positioning System (GPS) Brand Garmin Etrex 10 model. Seventy soil samples were collected at an altitude below or equal to 500 m above sea level (Figure 1), according to the non-probabilistic sampling method by judgment [22] at a depth of 10 cm using a 40 cm long stainless steel soil sampler (Type T) and a sample ejector; approximately 500 g of soil was collected and placed in a polyethylene plastic bag and transferred at room temperature to the laboratory for treatment.
2.2. Water Analysis
The analyses were conducted at the Environmental Contamination Research Center of the University of Costa Rica and at the Research Center and Chemical and Microbiological Services of Costa Rica Institute of Technology. Both laboratories have ISO IEC 17025:2017 accreditation. The metals As, Hg, Cd, Pb, V, and Si were quantified using atomic adsorption (AA) spectrometry following the standardized techniques of the (APHA et al., 2017) [21]. Samples with turbidity higher than one nephelometric turbidity unit (NTU) were subjected to digestion using 5% of concentrated HNO3, and subsequently, the corresponding measurements were made; in the case of As, the digestion consisted of adding HNO3 at 1% and H2O2 at 2%. The calibration curve was measured for each element, and the quality control and the blank were checked.
For quality control for the analyses, a calibration curve was prepared for the working range of a Sigma-Aldrich periodic table mix 1 multi-elemental metals dilution. The results of the linear regression for the working range had to meet the following criterion: R > 0.999. Reagent blank analysis included results lower than confidence limits for each analyte (mercury: 0.01 μg/L, and for the rest of the metals, 0.1 μg/L). Reference Material (RM) analysis included recovery percentages between 85% and 115%. The sequence was defined as follows: calibration curve—blank-MR-10 samples—fortified sample—10 samples—MR-10 samples—fortified sample. Analysis of fortified samples included recovery percentages between 80% and 120%, which were intercalated into the measurement sequence every 10 samples alternately. The sequence was defined as follows: calibration curve—blank-MR-10 samples—fortified sample—10 samples—MR-10 samples—fortified sample.
2.3. Soil Analysis
Samples were processed within 24 h after collection, starting with air drying at room temperature (25 °C–30 °C) for 48 h; then, samples were manually sieved through a stainless steel sieve with a 2.0 mm. mesh opening. After that, they were stored in polyethylene bags at room temperature until analysis. Samples were analyzed at the Environmental Soil Chemistry Laboratory at Texas Tech University, USA. Rb and Ni were quantified by the X-ray fluorescence spectrometry technique (XRF), and inductively coupled plasma optical emission spectrophotometry (ICP-OES) was used to quantify As, Cr, Pb, and V.
2.3.1. Soil Analysis by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
Samples were processed in triplicate at all stages, including weighing, acidification, digestion, dilution, and reading in the ICP-OES. Briefly, 0.5 g of soil was weighed on an analytical balance; triplicate quality control (QC) was included after every 11 samples (Loam B, High Purity Standards, North Charleston, SC, USA). Samples were placed in Teflon tubes with an ultrapure trace-level acid solution of 9 mL of HNO3 and 3 mL of HCl. Afterward, the samples were digested by assisted acid digestion and microwave with CEM Mars 6 equipment, Charlotte, NC, USA, using the EPA-3051A method [23]; once the digestion was completed, the sample was filtered through a syringe filter with a 0.20-micron mesh opening and was diluted in a 2% trace-level ultrapure HNO3 solution.
The spectrometer used was an ICP-OES Thermo Scientific iCAP 7600 series. Yttrium (Y) was used as an internal standard. For the calibration curve, two standards with different concentrations were used: (a) multielement standard with concentrations of 0.01 ppm, 0.10 ppm, 1.0 ppm, and 10 ppm, and (b) standard solution of Si and Rb at concentrations of 0.10 ppm, 1.0 ppm, 10.0 ppm, and 50.0 ppm. The following wavelengths were used for reading the samples, according to the metal of interest: As, 189 nm and 197 nm; Cd, 228 nm; Pb, 220 nm; and V, 289 nm and 311 nm.
The reference material used for verification (i.e., quality assurance/quality control, QA/QC) for the digestion of soil samples was soil reference material purchased from High Purity Standards, North Charleston, SC, USA. The “Loam B” reference soil was analyzed in triplicate during each digestion set, and up to 30 individual replicates were digested per batch. For the ICP-OES itself, aqueous standards were purchased from SCP Science (now known as Analytichem). The standards used were a multielement standard (SCP28AES) along with single element standards to create calibration curves for analysis of elements of interest in the digested soils, including the digested Loam B. Percent recovery of select elements from the Loam B via microwave digestion (EPA 3051A) followed by ICP-OES were arsenic, 99.3%; vanadium, 87.43%; cadmium, 92.0%; lead, 94.0; chromium, 90.08%. These values were averages from 30 digestion replicates. All samples, reference soil material, and blanks were analyzed under the same conditions. In addition, an internal standard of yttrium was used during the ICP-OES analysis to verify consistent results between samples and blanks.
2.3.2. Analysis of Soil Samples via X-ray Spectrometry
The analysis was performed using a primary X-ray spectrometer (Vanta Family—portable X-ray Fluorescence Analyzer, New York, NY, USA). The sample data were calibrated using a pure silica standard and two soil standards, SRM 2710A (NIST, 2018a) and SRM 2711A (NIST, 2018b) [24,25]. The standards were read every ten samples, and the recovery percentage was calculated based on the results. The equipment read the soil sample directly using the Geochem method (2 Beams) for 240 s. The software algorithm generated the concentration results of the different metals in units of mg kg−1.
2.4. Calculation of the Non-Carcinogenic Risk Assessment
The evaluation of non-carcinogenic risk was calculated using the methodology proposed by the U.S. Environmental Protection Agency [26], which consists of the calculation of the Hazard Index (HI) equation (Equation (1)). This index describes the Hazard Quotient (HQ) by considering three routes of exposure: dermal, inhalation, and ingestion. The individual non-cancer risk (HQ) equation (Equation (2)) is calculated by dividing the Chronic Daily Dose (CDD) per route of exposure (dermal, inhalation, and ingestion) (CDD mg/kg/day) by the reference dose for each toxicant. The Chronic Daily Dose (CDD) formulas (Equations (3)–(5)) are calculated by considering the routes of dermal exposure, inhalation, ingestion, and the concentration of the toxicant of interest. If the HI risk index exceeds 1, it is considered that there is a risk of adverse effects on human health; if the value is less than one, it is considered no risk. Formulas:
HI = ∑ HQ
HI: Hazard Index (non-carcinogenic risk sum index);
HQ: Hazard Quotient (non-carcinogenic risk).
CDD: Chronic Daily Dose;
DRef: Reference dose according to USEPA.
CDDder: Chronic Daily Dose dermal;
C: Content of metal in soil mg kg−1;
SA: Skin Surface Area: 4350 cm2 for adults, 1600 cm2 for children;
SAF: Skin Adhesion Factor: 0.2 mg (cm2)−1;
DAF: Dermal Absorption Factor: 0.001;
ED: Exposure Duration: 6 years for children and 30 years for adults;
EF: Exposure Frequency: in this study: 365 days year−1;
BW: Body Weight average: 20 kg for children and 70 kg for adults;
AT: Average Time for non-carcinogens: EDX 365 days;
CF: Conversion Factor: 1 × 10−6 kg mg−1.
CDDinh: Chronic Daily Dose by inhalation;
IRinh: Inhalation Rate: 5 m3 day−1 for children and 15 m3 day−1 per day for adults;
PEF: Particle Emission Factor: 1.36 × 109 m3 kg−1.
CDDing Chronic Daily Dose by ingestion;
IRing: Ingestion Rate: 200 mg day−1 for children and 100 mg day−1 for adults.
2.5. Total Risk Assessment
The assessment of total risk for accidental geophagy in adults and children was conducted using the methodology proposed by the EPA and the General Direction of Public Health of Madrid, Spain [27], which consists of determining the delivered dose (Ds), the Hazard Quotient (HQ), and the combined Hazard Index (HIc) that describes the sum of the hazard quotients (HQc) of each metal, according to the following formulas:
C: Concentration of the metal in soil mg kg−1;
T: Contact rate per unit of time. For adults = 50 mg day−1. For children = 125 mg day−1 (average value of the actual range 50–200 mg day−1);
F: Frequency of exposure, F = days of exposure per year/(365 days × years of exposure);
D: Duration of exposure: 6 years for children and 30 years for adults;
M: Body mass. For adults = 70 kg, for children = 35 kg;
P: Period 70 years.
Ds: Calculated delivered dose;
DdR: Dose of constant reference dose per metal according to USEPA.
HI = HQ1 + HQ2 + HQ3 + HQn
HQ: Calculated hazard quotient per metal.
2.6. Mapping
The maps were generated with ArcGIS software version 10.8.2 from modifying a raster layer of the province of Coclé extracted from the Panamanian Spatial Data Infrastructure portal [28]. The concentrations for each metal were located according to their georeferenced location in the raster layer, and the concentrations of each metal were interpolated using the Inverse Distance Weighted (IDW) method to estimate the concentration over the entire province of Coclé.
3. Results and Discussion
3.1. Metals in Water
The levels of As, Hg, Cd, and V in water (Table 1) were found to be within the maximum permissible limits for water intended for human consumption and domestic use, according to the Drinking Water Guidelines of the World Health Organization (WHO) [29]. For As, maximum values of 6.00 µg L−1 were reported, while the maximum value allowed in drinking water is 10 µg L−1. Cd was reported with a maximum value of 2.10 µg L−1, while the permitted maximum value according to the same standard is 3.00 µg L−1 (Table 1). On the other hand, a concentration of Pb of 33.0 µg L−1 was found in the community of Coclé, while a Pb concentration of 21.17 µg L−1 was reported in the community of Santa Rita, both of which exceed the maximum permissible value in water intended for human consumption of 10 µg L−1 according to WHO [29].

Table 1.
Concentration of metals in water for human consumption in communities of Coclé.
Extended human consumption of water in the communities of Coclé and Santa Rita with concentrations of Pb that exceed the maximum permitted value may represent a risk to public health due to the capacity of this metal to cause kidney damage. Pb, Cd, and Hg are metals with high toxicity levels, indicating that even at very low concentrations, they represent a hazard to human populations [30]. Likewise, the presence of heavy metals in water for human consumption, even at concentrations within the limit permitted, does not guarantee the innocuousness for special populations, such as the immunocompromised, children, pregnant women, and elderly adults [31]. In addition, heavy metals are not biodegradable and tend to bio-accumulate, which increases their hazardousness to health, even in low concentrations [32].
Despite concentrations within the acceptable limits or values for the metals As, Cd, Hg, and Si, these are ingested as a mixture in the water, and their components are added together, which can have a blending effect in the organism (As+ Hg + Cd+ Pb + V + Si). This occurs when independent toxicants that impact a common organ are added together, altering their kinetics and toxicity and generating synergistic effects that may have toxic capacities greater than the fractional sum of each component [33]. In particular, lead levels in water should be monitored due to its toxic and bioaccumulative capacity in the body [34]. Lead is capable of causing adverse effects on human health, particularly its effects on renal health, which are varied and have been associated with a reduction in glomerular filtration rate, tubular nephropathy, tubular necrosis, and glomerular sclerosis [35].
3.2. Metals in Soil
In the province of Coclé, the variation in the concentration of the different metals shows heterogeneity in the geographic distribution of these metals (Table 2).

Table 2.
Descriptive statistics of metal concentrations.
No consensus has been reached regarding the hazardous levels of heavy metals in soils due to direct contact with people and their effect on human health; however, there is a uniform protocol on the hazardousness of these metals and their toxicological effects [41]. The most hazardous trace elements in the soil include Pb, Cd, Hg, Cu, Sn, V, Cr, Mo, Co, and Ni, and three metalloids (Sb, As, and Se) [42]. Heavy metal toxicity can damage vital organs, such as kidneys, liver, blood, lungs, and brain [30], and generate serious diseases such as Parkinson’s, muscular dystrophy, and MeN [43].
The results were compared with the 95th percentile reference value [36], which refers to the concentration of metals in continental soils of the United States. For As, the average value of all samples analyzed is greater than the 95th percentile reference value of the concentrations obtained for this metal in soils of the United States, as reported by [36] (Figure 2). Meanwhile, the average concentrations of Pb, Cr, V, Si, and Rb were lower than the 95th percentile value. Although the average value of all the measurements of V and Rb is below the 95th percentile, there were 3 sites with maximum values exceeding the reference for rubidium and 18 exceeding the reference value for vanadium (Figure 2). Additionally, all As values found in Coclé soils are above the health risk reference value defined by [37] and the limit for soils in residential areas [40].
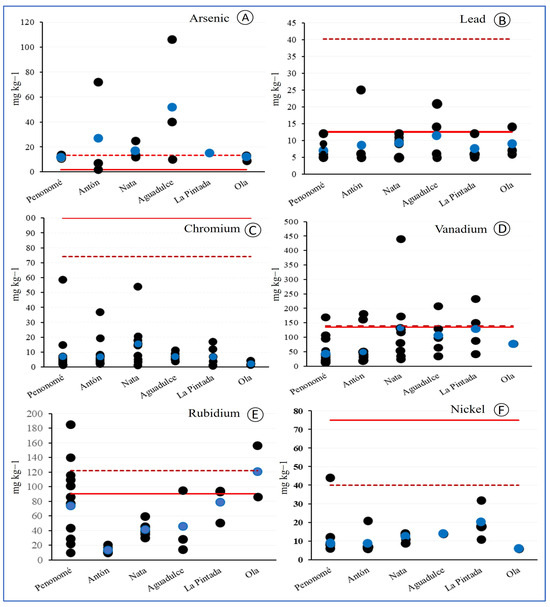
Figure 2.
Nephrotoxic metals by districts in Coclé. (A) concentration of As; (B) concentration of Pb; (C) concentration of Cr; (D) concentration of V; (E) concentration of Rb; (F) concentration of Ni. Blue circles represent the average value for the series of data. The red dashed line indicates the 95th percentile of metal reported in soils (Smith et al., 2019) [36]. The solid red line indicates the guide value of the chemical elements in the continental crust (Taylor, 1964) [38].
3.3. Geographical Distribution of Metals in Soil
The study area is bounded by two volcanoes, La Yeguada and El Valle, and the geographical distribution of the analyzed metals was heterogeneous (Figure 1). As increases toward the southern ends (east and west) with a tendency toward the coastal zone (Figure 3A), in contrast to Rb, which presents increased concentrations toward the northeast (NE) side of Coclé (Figure 3E). The concentration of Pb, V, Zn, and Cr is higher toward the southern end (Figure 3). Overall, all the metals analyzed are found in higher concentrations in low-lying areas and near the coast.
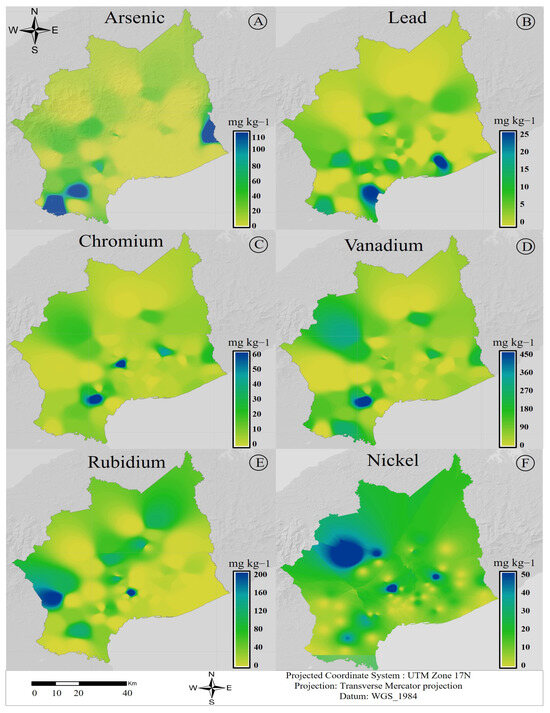
Figure 3.
Geographical distribution of nephrotoxic metals in soils in Coclé. Represents the spatial distribution of metals as interpolated through ArcGIS: (A) distribution of As; (B) distribution of Pb; (C) distribution of Cr; (D) distribution of V; (E) distribution of Rb; (F) distribution of Ni. All results are expressed in mg kg−1.
Figure 4 shows the geographical distribution and concentration of the nephrotoxic metals analyzed, As, Cr, Pb, Rb, Ni, and V, in relation to populations diagnosed with CKD, according to information from the Ministry of Health [20]. The convergence of both factors in the same geographical area should draw attention to the risk to which these populations are subjected. Epidemiological studies have shown that chronic exposure to these metals can cause kidney damage [44,45]. Throughout Central America, the areas affected by MeN are characterized by high wind traffic, similar land use and land cover, and low altitudes where the affected population is located. These conditions facilitate the access of soil particles to people, either by oral or inhalation, where the smallest particles, smaller than 20 µm, can enter directly into the bloodstream through the respiratory tract.
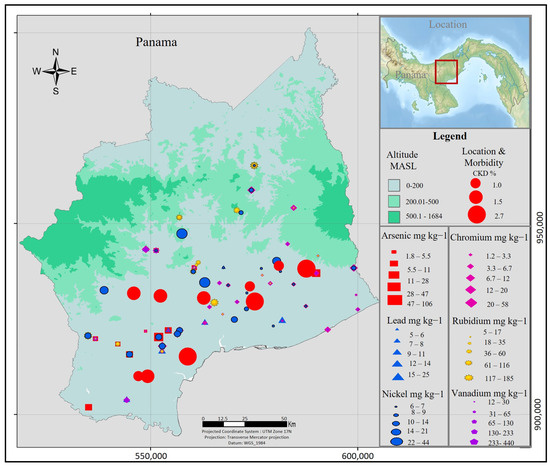
Figure 4.
Geographical distribution morbidity to CKD and metals concentrations in Coclé, Panama. Data Source: Prevalence of CKD: Ministry of Panama Health [20], metals concentration: Environmental Soil Chemistry Lab Texas Tech University, USA. Map created by ArGis Software (version 10.8.2).
3.4. Evaluation of Non-Carcinogenic Toxicological Risk
Toxic metals enter the body primarily by ingestion and inhalation routes, with the possibility of causing non-cancerous effects, such as Mesoamerican nephropathy, these being a low-dose pathology [8,46]. The results regarding the risk of occurrence in children and adults of the non-carcinogenic effects of the nephrotoxic metals are shown in Table 3. For all three routes of exposure evaluated, the ingestion route is the most important in children as well as in adults. Likewise, for all metals and concentration levels of the toxicant reported (average and maximum), the risk was consistently higher in children than in adults, which indicates that children are at a higher risk than adults of developing health problems as a consequence of being exposed to these metals found in the soils in Coclé.

Table 3.
Evaluation of non-carcinogenic risk.
The calculated values were mostly below 1, which indicates the absence of non-carcinogenic risk. However, the exception was for the maximum arsenic concentration and the ingestion route of exposure, which exceeded the reference HI = 1. This is indicative of the metal’s ability to cause adverse effects on human health other than cancer, which may include kidney damage.
3.5. Blend Effect Evaluation, Total Risk, and Genotoxic Evaluation
As shown in Table 4, the risk analysis calculations are presented. This risk is calculated based on apparent damage to health; however, it is relevant to evaluate the genotoxic hazards, which are heritable, and the occurrence of genotoxic hazards is defined as “the identification of the type and nature of the adverse effects that an agent has as its inherent capacity to cause in an organism, system or (sub)population”, derived from the same genetic information [47].

Table 4.
Evaluation of the combined effect of nephrotoxic metals.
Since the late 1960s, the assessment of the genotoxic effects of chemical products has received more and more attention, and these effects have become an essential part of the hazard identification step. The growing interest in identifying genotoxic chemicals has generated many in vivo, in vitro methods for detecting or predicting a broad range of genetic toxicity endpoints (Menz et al., 2023) [47]. For a thorough overview of current approaches concerning the identification of genotoxic properties of chemical substances, reference is made to detailed guidance documents and the scientific opinions of international risk assessment bodies [48,49,50,51].
4. Conclusions
The results of this study provide useful information that considers heavy metals as agents that have the potential to contribute to the etiology of MeN following an environmental approach. In addition, it provides relevant data on risk analyses that could be extrapolated to other regions in Mesoamerica that are identified as areas with a high prevalence of MeN and similar environmental and geographic characteristics to the region of study. The results open new lines of research into the possible connection between nephrotoxic metals and chronic kidney disease (CKD). Pb concentrations in water above the maximum permitted value and As, V, and Rb in soil above the 95th percentile reflect the potential risk of these metals in environmental matrices to cause health problems. These values suggest the need for further research into the effect of these metals on people and, subsequently, to relate to renal function tests to confirm these findings and develop effective mitigation strategies to reduce exposure to these metals and protect the kidney health of people living in these areas.
These results have allowed us to establish that the “ingestion” route is the most important exposure route. While it was determined, except for arsenic, that the non-carcinogenic risks and the total intake risks of the metals analyzed were within the parameters recommended by EPA, in comparison to adults, children are at a higher risk of being affected by these types of substances. This is mainly because their tolerance to these toxicants is lower than that of adults, they have a smaller body surface area, and their organs and immune system are still developing. Considering the hazardous nature of these heavy metals, the greatest risk is for vulnerable people, such as children, the elderly, and pregnant women living in the area. The exposure of pregnant women to these toxicants in the early stages of gestation is of special concern due to the alteration that may occur in the endocrine system of the fetus. These toxicants can cause hereditary and selective effects on the organs responsible for the detoxification of heavy metals, especially due to the combined effect of these substances. It is suggested in future research to consider soil parameters, such as pH, total organic matter and particle size, wind strength, and direction at different times of the year since these factors may influence the availability and mobility of toxicants. This future work is needed due to the high prevalence of chronic kidney disease in the area.
Author Contributions
B.V.-R.: Conceptualization and design of the study, data collection and analysis, writing and supervision. V.M.-C.: Investigation, writing—review and editing. M.G.S.: Fundraising, investigation, writing—review and editing, A.J.Z.: Data collection and analysis. M.V.-A.: Review and data visualization. S.P.U.C.: Data visualization and validation. D.R.: Review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was made possible thanks to the support of the Sistema Nacional de Investigación (SNI) of Secretaría Nacional de Ciencia, Tecnología e Innovación (Panama). Financial contract for covering project expenses. SNI 56-2021.
Data Availability Statement
The data used in this research are available on request from the corresponding author.
Acknowledgments
The National Research System (SNI) of the National Secretariat of Science and Technology of Panama (SENACYT) for funding this project. The Water Laboratory and Physico-Chemical Services of the Autonomous University of Chiriquí for the important collaboration to perform field activities, and at the Environmental Soil Chemistry Laboratory of Texas Tech University.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to publish the results.
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Owolabi, M.O. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Li, P.K.-T.; Garcia-Garcia, G.; Lui, S.-F.; Andreoli, S.; Fung, W.W.-S.; Hradsky, A.; Kumaraswami, L.; Liakopoulos, V.; Rakhimova, Z.; Saadi, G.; et al. Kidney Health for Everyone Everywhere—From Prevention to Detection and Equitable Access to Care. Braz. J. Med. Biol. Res. 2020, 53, e9614. [Google Scholar] [CrossRef]
- Hernández, A.; Carrasquilla, C.; Castillo, I.; Barrios, K.; Bernal, D. Factores que influyen en el deterioro de la calidad de vida del paciente con nefropatía diabética en hemodiálisis. Enfoque 2024, 34, 57–77. [Google Scholar] [CrossRef]
- Ministerio de Salud de Panamá. Análisis de Situación de Salud con Énfasis en Macro Indicadores en la República de Panamá. 2018. Available online: https://www.minsa.gob.pa/informacion-salud/analisis-de-situacion-de-salud-asis (accessed on 8 August 2024).
- Correa-Rotter, R.; García-Trabanino, R. Mesoamerican Nephropathy. Semin. Nephrol. 2019, 39, 263–271. [Google Scholar] [CrossRef]
- Hoy, W.E.; Ordunez, P. Epidemia de Enfermedad Renal Crónica en Comunidades Agrícolas de Centroamérica. Definición de Casos, Base Metodológica y Enfoques Para la Vigilancia de Salud Pública; OPS: Washington, DC, USA, 2017; Available online: https://iris.paho.org/handle/10665.2/34157 (accessed on 8 September 2023).
- Rotter, R.C.; Trabanino, R.G. Nefropatía Mesoamericana: Una Nueva Enfermedad Renal Crónica de Alta Relevancia Regional. 2018. Available online: https://www.medigraphic.com/cgi-bin/new/resumen.cgi?IDARTICULO=82350 (accessed on 8 August 2024).
- Valdés-Rodríguez, B.; Montero-Campos, V.; Siebecker, M.G. Causes of Chronic Kidney Disease of Non-Traditional Origin in Central America: An Approach Based on Medical Geology. Geosciences 2023, 13, 360. [Google Scholar] [CrossRef]
- Baker, A. Chronic Kidney Disease May Be “Black Lung of Climate Change”. TIME. 2023. Available online: https://time.com/6303020/chronic-kidney-disease-climate-change/ (accessed on 8 August 2024).
- Chapman, C.; Hess, H.; Lucas, R.; Glaser, J.; Saran, R.; Bragg-Gresham, J.; Wegman, D.; Hansson, E.; Minson, C.; Schlader, Z.J. Occupational Heat Exposure and the Risk of Chronic Kidney Disease of Nontraditional Origin in the United States. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R141–R151. [Google Scholar] [CrossRef]
- Lunyera, J.; Smith, S.R. Heavy Metal Nephropathy: Considerations for Exposure Analysis. Kidney Int. 2017, 92, 548–550. [Google Scholar] [CrossRef]
- Díaz García, J.D.; Arceo, E. Daño Renal Asociado a Metales Pesados: Trabajo de Revisión. Rev. Colomb. Nefrol. 2017, 5, 43–45. [Google Scholar] [CrossRef][Green Version]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between Exposure to Heavy Metals and the Risk of Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef]
- Azeh Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? Karcioglu, O., Arslan, B., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Kocadal, K.; Alkas, F.; Battal, D.; Saygi, S. Cellular Pathologies and Genotoxic Effects Arising Secondary to Heavy Metal Exposure: A Review. Hum. Exp. Toxicol. 2020, 39, 3–13. [Google Scholar] [CrossRef]
- Ortega-Moctezuma, O.; Zárate-Pérez, J.; Alba-Alba, C.M.; Jiménez-Hernández, M.; Ramírez-Girón, N. Enfermedad renal crónica asociada a la exposición a metales pesados y productos agroquímicos en Latinoamérica. Enferm. Nefrol. 2023, 26, 120–131. [Google Scholar] [CrossRef]
- Vervaet, B.A.; Nast, C.C.; Jayasumana, C.; Schreurs, G.; Roels, F.; Herath, C.; Kojc, N.; Samaee, V.; Rodrigo, S.; Gowrishankar, S.; et al. Chronic Interstitial Nephritis in Agricultural Communities is a Toxin-Induced Proximal Tubular Nephropathy. Kidney Int. 2020, 97, 350–369. [Google Scholar] [CrossRef]
- Soltani-Gerdefaramarzi, S.; Ghasemi, M.; Ghanbarian, B. Geogenic and Anthropogenic Sources Identification and Ecological Risk Assessment of Heavy Metals in the Urban Soil of Yazd, Central Iran. PLoS ONE 2021, 16, e0260418. [Google Scholar] [CrossRef]
- Redwood, S.D. The History of Mining and Mineral Exploration in Panama: From Pre-Columbian Gold Mining to Modern Copper Mining. BSGM 2020, 72, A180720. [Google Scholar] [CrossRef]
- Ministry of Health of Panama. Panama, Pan. Personal Communication Request for Official Data: National Statistics of Renal Disease. 2020. Available online: https://www.minsa.gob.pa/informacion-salud/estadisticas-de-salud (accessed on 8 August 2024).
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA-AWWA-WEF: Washington, DC, USA, 2017. [Google Scholar]
- Oribhabor, C.B.; Anyanwu, C.A. Research Sampling and Sample Size Determination: A Practical Application. J. Educ. Res. 2019, 2, 47–56. Available online: https://www.researchgate.net/publication/336723498_Research_Sampling_and_Sample_Size_Determination_A_practical_Application (accessed on 8 August 2024).
- USEPA. United States Environmental Protection Agency, Method 3051A Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. 2007. Available online: https://www.epa.gov/sites/production/files/2015-12/documents/3051a.pdf (accessed on 8 August 2024).
- Zimmerman, A.J.; Gutierrez, D.G.; Campos, V.M.; Weindorf, D.C.; Deb, S.K.; Chacón, S.U.; Landrot, G.; Flores, N.G.G.; Siebecker, M.G. Arsenic Speciation in Titanium Dioxide (TiO2) Waste Produced via Drinking Water Filtration: Potential Environmental Implications for Soils, Sediments, and Human Health. Environ. Adv. 2021, 3, 100036. [Google Scholar] [CrossRef]
- Zimmerman, A.J.; Garcia Gutierrez, D.; Shaghaghi, N.; Sharma, A.; Deonarine, A.; Landrot, G.; Weindorf, D.C.; Siebecker, M.G. Mobility and Bioaccessibility of Arsenic (As) Bound to Titanium Dioxide (TiO2) Water Treatment Residuals (WTRs). Environ. Pollut. 2023, 326, 121468. [Google Scholar] [CrossRef]
- USEPA. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2002. Available online: https://semspub.epa.gov/work/HQ/175878.pdf (accessed on 8 August 2024).
- Martín Olmedo, P.; Ma José Carroquiño, S.; Ordoñez, I.; Moya, J. La Evaluación de riesgos en salud. Guía Metodológica. Aplicaciones Prácticas de la Metodología de Evaluación de Riesgos en Salud por Exposición a Químicos. 2016. Available online: https://www.diba.cat/documents/467843/96195101/Evaluacion_riesgos_salud_Guia_metodologica.pdf/37481f80-8641-4a42-a647-eb7f24808d33 (accessed on 8 August 2024).
- IPDE. Geoservicios. Infraestructura Panameña de Datos Espaciales (IPDE). Available online: https://www.ipde.gob.pa/geoservicios/ (accessed on 6 February 2024).
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating First Addendum, 4th ed + 1st add; World Health Organization: Geneva, Switzerland, 2017; Available online: https://iris.who.int/handle/10665/254637 (accessed on 8 August 2024).
- Zaynab, M.; Al-Yahyai, R.; Ameen, A.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and Environmental Effects of Heavy Metals. J. King Saud Univ.—Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Mendoza, L.A.; Razo, L.M.D.; Barbier, O.; Saldaña, M.C.M.; González, F.J.A.; Juárez, F.J.; Sánchez, J.L.R. Potable Water Pollution with Heavy Metals, Arsenic, and Fluorides and Chronic Kidney Disease in Infant Population of Aguascalientes. In Water Resources in Mexico; Oswald Spring, Ú., Ed.; Hexagon Series on Human and Environmental Security and Peace; Springer: Berlin/Heidelberg, Germany, 2012; Volume 7, pp. 231–238. [Google Scholar] [CrossRef]
- Ferrey, M.L.; Coreen Hamilton, M.; Backe, W.J.; Anderson, K.E. Pharmaceuticals and Other Anthropogenic Chemicals in Atmospheric Particulates and Precipitation. Sci. Total Environ. 2018, 612, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Pabón Guerrero, S.E.; Benítez Benítez, R.; Sarria Villa, R.A.; Gallo Corredor, J.A. Contaminación del agua por metales pesados, métodos de análisis y tecnologías de remoción. Una revisión. Entre Cienc. E Ing. 2020, 14, 9–18. [Google Scholar] [CrossRef]
- Heredia, D. Metales Pesados y Salud. 2021. Available online: https://revcocmed.sld.cu/index.php/cocmed/article/view/3702/2024 (accessed on 18 June 2024).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Lead; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2020. [CrossRef]
- Smith, D.; Solano, F.; Woodruff, L.G.; Cannon, W.F.; Ellefsen, K.J. Geochemical and Mineralogical Maps, with Interpretation, for Soils of the Conterminous United States: U.S. Geological Survey Scientific Investigations Report 2017-5118; Scientific Investigations Report; Scientific Investigations Report. 2019. Available online: https://pubs.usgs.gov/sir/2017/5118/sir20175118_geo.php (accessed on 8 August 2024).
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in Agricultural Soils of the European Union with Implications for Food Safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R. Abundance of Chemical Elements in the Continental Crust: A New Table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Kabata Pendias, A. Trace Elements from Soil to Human; Kabata-Pendias, A., Mukherjee, A.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Available online: https://www.sidalc.net/search/Record/KOHA-OAI-AGRO:15985/Description (accessed on 8 August 2024).
- US-EPA, Regional Screening Levels (RSLs) Generic Tables. United States Environmental Protection Agency (USEPA). Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 8 August 2024).
- Vodyanitskii, Y.N. Standards for the Contents of Heavy Metals in Soils of Some States. Ann. Agrar. Sci. 2016, 14, 257–263. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, Bioaccumulation, Health Risks and Remediation of Potentially Toxic Metal(Loid)s (As, Cd, Cr, Pb and Hg): An Epitomised Review. Environ. Monit. Assess. 2020, 192, 108. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental Biotechnology: For Sustainable Future; Sobti, R.C., Arora, N.K., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 103–125. [Google Scholar] [CrossRef]
- Isinkaralar, O.; Isinkaralar, K.; Ambade, B. Assessment of Societal Health Risks: Spatial Distribution and Potential Hazards of Toxic Metals in Street Dust Across Diverse Communities. Water Air Soil Pollut. 2024, 235, 302. [Google Scholar] [CrossRef]
- Orr, S.; Bridges, C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, C.; Glaser, J.; Rodríguez-Guzmán, J.; Weiss, I.; Lucas, R.; Peraza, S.; Da Silva, A.S.; Hansson, E.; Johnson, R.J.; Hogstedt, C.; et al. Chronic Kidney Disease of Non-Traditional Origin in Mesoamerica: A Disease Primarily Driven by Occupational Heat Stress. Rev. Panam. Salud Pública 2020, 44, e15. [Google Scholar] [CrossRef]
- Menz, J.; Götz, M.E.; Gündel, U.; Gürtler, R.; Herrmann, K.; Hessel-Pras, S.; Kneuer, C.; Kolrep, F.; Nitzsche, D.; Pabel, U.; et al. Genotoxicity Assessment: Opportunities, Challenges and Perspectives for Quantitative Evaluations of Dose–Response Data. Arch. Toxicol. 2023, 97, 2303–2328. [Google Scholar] [CrossRef]
- European Chemicals Agency. Guidance on Information Requirements and Chemical Safety Assessment: Chapter R.7a: Endpoint Specific Guidance; Publications Office: Luxembourg, 2017. [Google Scholar]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; et al. Clarification of Some Aspects Related to Genotoxicity Assessment. EFS2 2017, 15, e05113. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Overview of the Set of OECD Genetic Toxicology Test Guidelines and Updates Performed in 2014–2015; OECD Publishing: Paris, France, 2016. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization of the United Nations. Chapter 4: Hazard Identification and Characterization: Toxicological and Human Studies. Section 4.5 Genotoxicity. Environmental Health Criteria 240: Principles and Methods for the Risk Assessment of Chemicals in Food. World Health Organization. 2020. Available online: https://www.who.int/docs/default-source/food-safety/publications/section4-5-genotoxicity.pdf (accessed on 8 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).