Uses for Incomplete Ammonite Sutures: Lateral Lobe and Second Saddle as Markers of Sutural Complexity
Abstract
:1. Introduction
- The modified method offers a new way to take a full ontogeny of sutures from just one shell, rather than compositing data from many shells.
- For broad-scale analyses, our modification widens the potential sample size by enabling the use of specimens broken or crushed during post-mortem compaction.
2. Materials
3. Methods
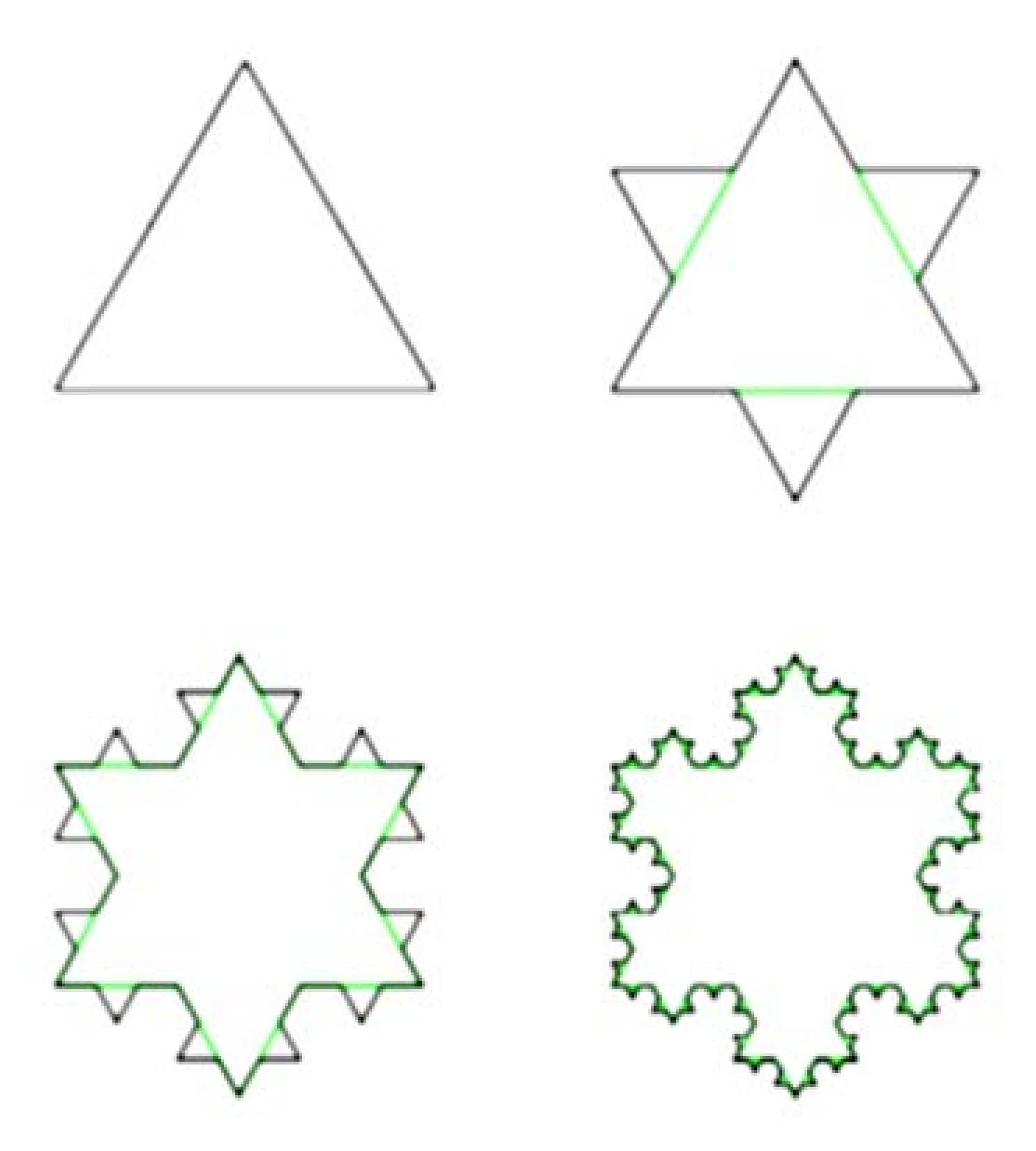
- The Richardson Step Method. The Richardson Step Method, described by [10], is one such approach that has been widely applied to ammonite sutures [9,10,13,15,16]. In this approach, rule size can be expressed in terms of actual mensuration units (e.g., 5, 10, or 20 mm), or alternatively as fractions of the straight-line distance (Lmax) between the ends of the hemisuture (e.g., 1/5, 1/10, or 1/20 of the total straight-line distance from the external to the umbilical lobe) (Figure 2).
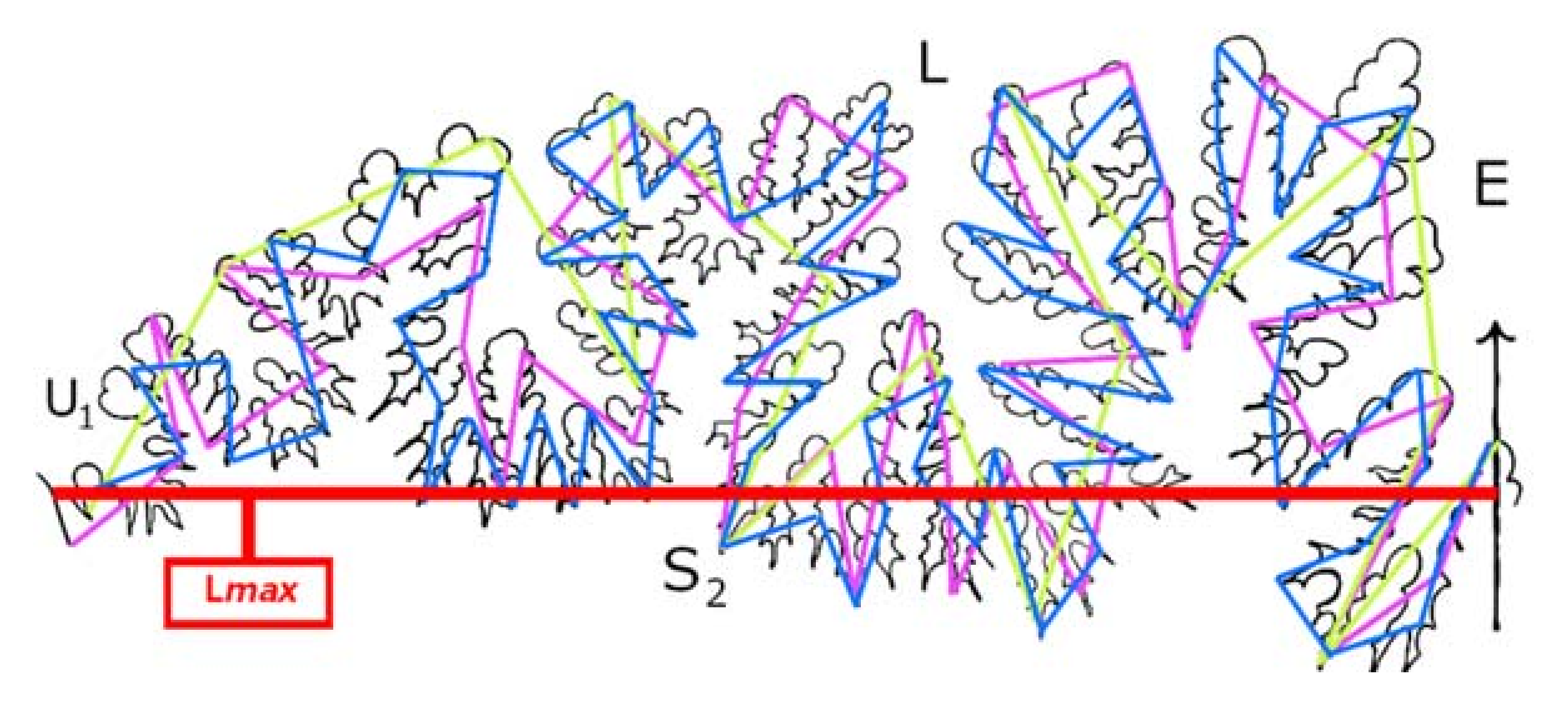
- b.
- The LLS Step Method. In contrast to the Richardson Method, our LLS Method measures only the lateral lobe and the second saddle (S2) (Figure 3 and Figure 4). In the LLS Step Method, rule size is equal to a given fraction of Lmax, which for LLS is the length between the top of the lateral lobe (L) and the base of S2 (e.g., 1/5, 1/10, 1/20 of this distance). We do not use actual mensuration units (e.g., 5 mm, 10 mm, 20 mm). In Figure 3, the magenta line is the full length of the maximum length from which rule sizes can be drawn, Lmax, and the green line shows a rule size of 1/10 the length of the lateral lobe–saddle pair. One-tenth hemisuture lengths were used by [10] in their study of ammonite sutures.

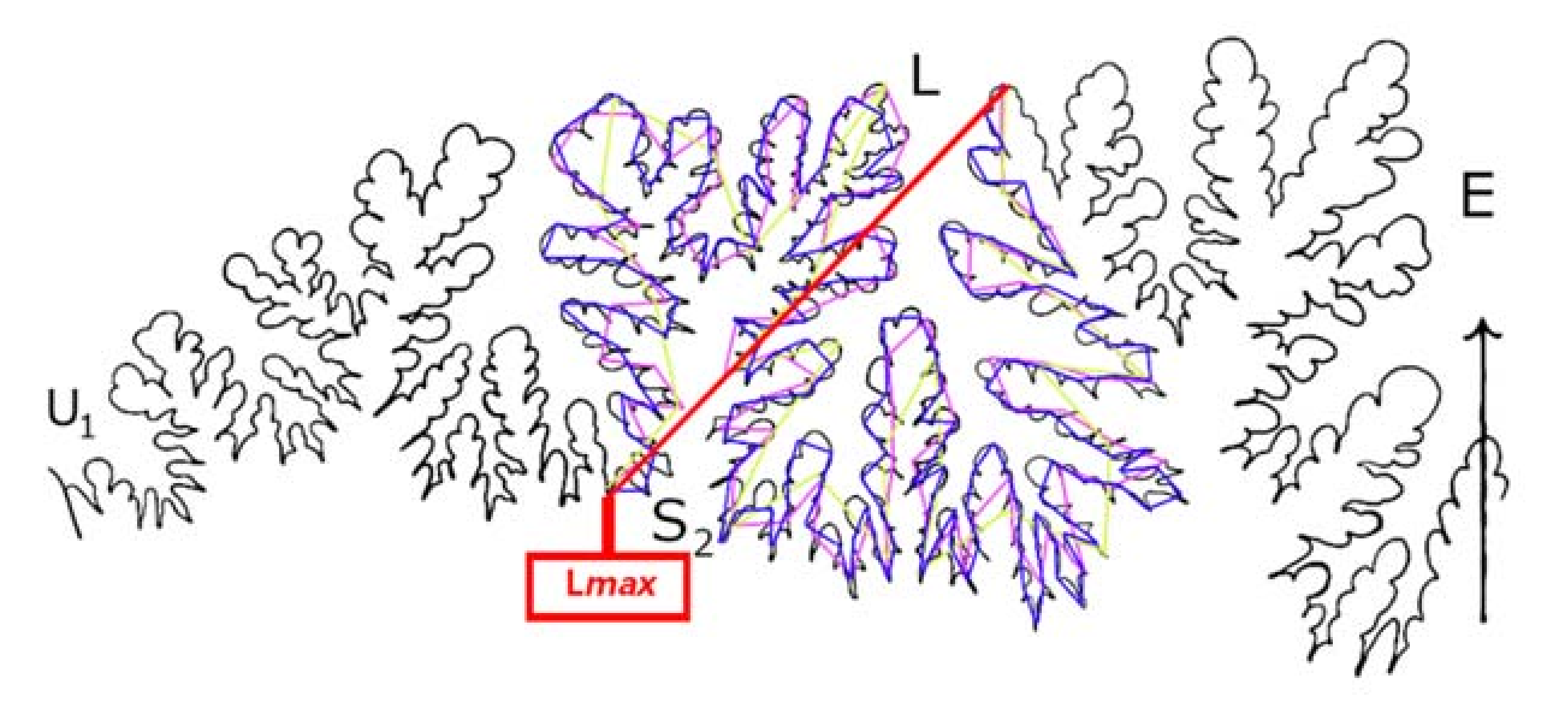
- c.
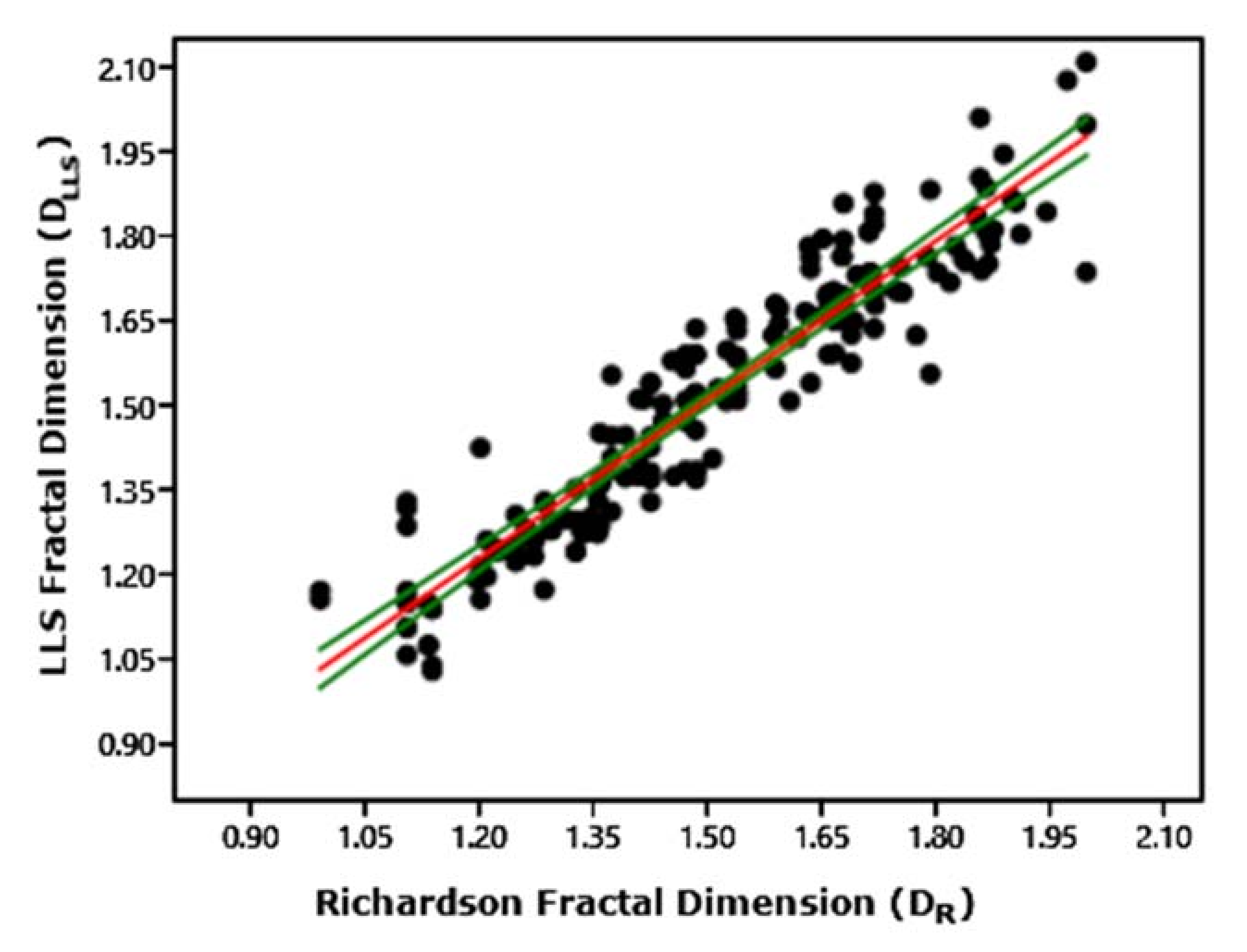
- Conversion of LLS Data to Richardson Data.
4. Results and Discussion
- Taxonomic Implications of the Richardson/LLS Conversion Value:
- b.
- Shell Surface Flatness and the LLS Method. The issue of distortion from whorl curvature when tracing LLS suture elements from ammonites photographed in profile was ruled out as insignificant by a series of Kruskal–Wallis analyses. We tested genera with various degrees of lateral compression based on Raup’s [46] coiling parameters and Raup and Chamberlain’s [47] whorl expansion rates. This test was based on a process in which lateral lobes and saddles were counted in the LLS Method from sutures present on actual shell surfaces, and then repeating the process using profile photographs of these same shells (see File S2 in the Supplementary Marterials for this manuscript, for data).
- c.
- Ontogeny
- d.
- Preservation
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buckland, W. Geology and Mineralogy Considered with Reference to Natural Theology; Willian Pickering: London, UK, 1836. [Google Scholar]
- Westermann, G.E.G. Form, structure and function of shell and siphuncule in coiled Mesozoic ammonoids. R. Ont. Mus. Life Sci. Contrib. 1971, 78, 1–39. [Google Scholar]
- Mandelbrot, B.B. The Fractal Geometry of Nature; W.H. Freeman: New York, NY, USA, 1982. [Google Scholar]
- Ward, P.D. Comparative shell shape distributions in Jurassic-Cretaceous ammonites and Jurassic-Tertiary Nautilids. Paleobiology 1980, 6, 32–43. [Google Scholar] [CrossRef]
- Vicencio, R. Models for the Morphology and Morphogenesis of the Ammonoid Shell. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, 1973. Unpublished. [Google Scholar]
- Guex, J. Associations virtuelles et discontinuités dans la distribution des espèces fossils: Un exemple intéressant. Bull. Soc. Vaud. Sci. Nat. 1981, 75, 179–197. [Google Scholar] [CrossRef]
- Bayer, U. Pattern Recognition Problems in Geology and Paleontology; Springer: Berlin, Germany, 1985. [Google Scholar]
- Damiani, G. Significato Funzionalle Dell’evoluzione dei setti e delle Line di Sutura dei Nautiloidi e Degli Ammonoidi. In Atti I Convengo Internationale: Fossili, Evoluzione, Ambiente, Pergola 1984; Pallini, G., Ed.; Tecnoscienza: Roma, Italy, 1986. [Google Scholar]
- García-Ruiz, J.M.; Checa, A. A model for the morphogenesis of ammonoid septal sutures. Geobios 1993, 26, 157–162. [Google Scholar] [CrossRef]
- Lutz, T.; Boyajian, G. Fractal Geometry of Ammonoid Sutures. Paleobiology 1995, 21, 329–342. [Google Scholar] [CrossRef]
- Oloriz, F.; Palmqvist, P.; Perez-Claros, J.A. Shell features, main colonized environments, and fractal analysis of sutures in Late Jurassic ammonites. Lethaia 2007, 30, 191–204. [Google Scholar] [CrossRef]
- Klug, C.; Hoffmann, R. Ammonoid septa and sutures. In Ammonoid Paleobiology, Volume I: From Anatomy to Ecology; Klug, C., De Baets, K., Kruta, I., Mapes, R., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 45–90. [Google Scholar]
- Pérez-Claros, J.; Palmqvist, P.; Oloriz, F. First and second orders of suture complexity in ammonites: A new methodological approach using fractal analysis. Math. Geol. 2002, 34, 323–332. [Google Scholar] [CrossRef]
- Pérez-Claros, J.; Olóriz, F.; Palmqvist, P. Sutural complexity in Late Jurassic ammonites and its relationship with phragmocone size and shape: A multidimensional approach using fractal analysis. Lethaia 2007, 40, 253–272. [Google Scholar] [CrossRef]
- Canfield, D.J.; Anstey, R.L. Harmonic analysis of cephalopod suture patterns. Math. Geol. 1981, 13, 23–35. [Google Scholar] [CrossRef]
- Oloriz, F.; Palmqvist, P. Sutural complexity and bathymetry in ammonites: Fact or artifact? Lethaia 1995, 28, 167–170. [Google Scholar] [CrossRef]
- Olóriz, F.; Palmqvist, P.; Pérez-Claros, J.A. Recent Advances in Morphometric Approaches to Covariation of Shell Features and the Complexity of Suture Lines in Late Jurassic Ammonites, with Reference to the Major Environments Colonized. In Advancing Research on Living and Fossil Cephalopods; Olóriz, F., Rodríguez-Tovar, F.J., Eds.; Springer: Boston, MA, USA, 1999. [Google Scholar] [CrossRef]
- García-Ruiz, J.; Checa, A.; Rivas, P. On the origin of ammonite sutures. Paleobiology 1990, 16, 349–354. [Google Scholar] [CrossRef]
- Hariri, K.E.; Bachnou, A. Describing Ammonite shape using Fourier analysis. J. Afr. Earth Sci. 2004, 39, 347–352. [Google Scholar] [CrossRef]
- Jiang, J.; Plotnick, R.E. Fractal Analysis of the Complexity of United States Coastlines. Math. Geol. 1998, 30, 535–546. [Google Scholar] [CrossRef]
- Reeside, J.B. A comparison of the genera Metaplacenticeras Spath and Placenticeras Meek. Shorter Contrib. Gen. Geol. 1926. [Google Scholar] [CrossRef]
- Cobban, W.A. New Species of Baculites from the Upper Cretaceous of Montana and South Dakota. J. Paleontol. 1951, 25, 817–821. Available online: http://www.jstor.org/stable/1299823 (accessed on 21 September 2021).
- Cobban, W.A. Two New Species of Baculites from the Western Interior Region. J. Paleontol. 1958, 32, 660–665. Available online: http://www.jstor.org/stable/1300784 (accessed on 21 September 2021).
- Cobban, W.A. The Late Cretaceous Ammonites Scaphites Leei Reeside and Scaphites Hippocrepis DeKay in the Western Interior of the United States; United States Government Printing Office: Washington, DC, USA, 1969; 29p. [Google Scholar] [CrossRef]
- Cobban, W.A. Baculites from the Lower Part of the Pierre Shale and Equivalent Rocks in the Western Interior. J. Paleontol. 1962, 36, 704–718. Available online: http://www.jstor.org/stable/1301354 (accessed on 21 September 2021).
- Schindewolf, O. On development, evolution, and terminology of ammonoid suture line. Bull. Mus. Comp. Zool. Harv. Coll. 1953, 112, 217–237. [Google Scholar]
- Schindewolf, O. Studien zur Stammesgeschichte der Ammoniten. Lief 1. Ash. Akad. Wiss. Lit. Mainz Math.-Nat Kl 1960, 10, 639–743. [Google Scholar]
- Schindewolf, O. Studien zur Stammesgeschichte der Ammoniten. Lief 3. Ash. Akad. Wiss. Lit. Mainz Math.-Nat Kl, 1965; 13, 139–238. [Google Scholar]
- Cobban, W.A.; Jeletzky, J.A. A New Scaphite from the Campanian Rocks of the Western Interior of North America. J. Paleontol. 1965, 39, 794–801. [Google Scholar]
- Kullmann, J.; Wiedmann, J. Significance of sutures in phylogeny of Ammonoidea. In The University of Kansas Paleontological Contributions; The University of Kansas: Lawrence, KS, USA, 1970; pp. 1–25. [Google Scholar]
- Schlegelmilch, R. Die Ammoniten des Suddeutschen Lias; Gustav Fischer Verlag: Jena, Germany, 1976. [Google Scholar]
- Schlegelmilch, R. Die Ammoniten des Suddeutschen Malms; Gustav Fischer Verlag: Jena, Germany, 1994. [Google Scholar]
- Stevens, G. A revision of the Lytoceratinae (Subclass Ammonoidea) including Lytoceras taharoaense n. sp., Upper Jurassic, New Zealand. N. Z. J. Geol. Geophys. 1985, 28, 153–185. [Google Scholar] [CrossRef]
- Lominazde, T.; Sharikazde, M.; Kvantaliani, I. Phylogeny and systematics of Perisphinctids as interpreted from suture ontogenies. Geobios 1993, 26 (Suppl. 1), 275–286. [Google Scholar]
- Kennedy, W.J.; Cobban. Campanian Ammonites from the Annona Chalk near Yancy, Arkansas. J. Paleontol. 1993, 67, 183–197. [Google Scholar] [CrossRef]
- Kennedy, W.J.; Cobban, W.A.; Landman, N.H. Two species of Placenticeras (Ammonitina) from the Upper Cretaceous (Campanian) of the Western Interior of the United States. In American Museum Novitates; The American Museum of Natural History: New York, NY, USA, 1996. [Google Scholar]
- Shevyrev, A.A. The development of the suture line in Mesozoic ammonoids and the terminology of its elements. Int. Geol. Rev. 1963, 5, 1659–1669. [Google Scholar] [CrossRef]
- Joly, B. Aptian and Albian Phylloceratids (Ammonoidea) from the Vocontian Basin (SE France). Carnets Geol. 2008, CG2008 (M04), 1–60. [Google Scholar]
- Hoffmann, R. New insights on the phylogeny of the Lytoceratoidea (ammonitina) from the septal lobe and its functional interpretation. Rev. Paleobiol. 2010, 29, 1–159. [Google Scholar]
- Ifrim, C.; Stinnesbeck, W.; Garza, R.R.; Ventura, J.F. Hemipelagic cephalopods from the Maastrichtian (late Cretaceous) Parras Basin at La Parra, Coahuila, Mexico, and their implications for the correlation of the lower Difunta Group. J. S. Am. Earth Sci. 2010, 29, 597–618. [Google Scholar] [CrossRef]
- Galácz, A.; Kassai, P. New species and stratigraphic data on Lower Bajocian (Middle Jurassic) lytoceratids (Ammonoidea) from Lókút, Bakony Mts, Hungary. Paläontolog. Z. 2012, 86, 281–295. [Google Scholar] [CrossRef]
- Dias-Canas, J.S.; Patarroyo, P. Vista de Pachydiscus del Campaniano Superior—Maastrichtiano Inferior de la formación Penderisco, cordillera occidental (Antioquia—Colombia) (unal.edu.co). Geol. Colomb. 2014, 39, 15–22. [Google Scholar]
- Hoffmann, R.; Maisch, M. Systematics and phylogenetic position of the lytoceratid ammonite genus Holcolytoceras Spath, 1924 (Cephalopoda, Ammonoidea) from the Lower Pliensbachian of Europe. Neues Jahrb. Geol. Paläontolog. 2018, 288, 173–182. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Slattery, J.; Harries, P.J.; Sandness, A.L. A review of Late Cretaceous (Campanian and Maastrichtian heteromorphic ammonite paleobiology, paleoecology, and diversity in the Western Interior of North America. In Invertebrates: Spineless Wonders, 18th Annual Tate Conference; Cavigelli, J.P., Ed.; Tate Geological Museum, Casper College: Casper, WY, USA, 2012; pp. 76–93. [Google Scholar]
- Raup, D.M. Geometric analysis of shell coiling: Coiling in ammonoids. J. Paleontol. 1967, 41, 43–65. [Google Scholar]
- Raup, D.M.; Chamberlain, J.A. Equations for volume and center of gravity in ammonoid shells. J. Paleontol. 1967, 41, 566–574. [Google Scholar]
- Boyajian, G.; Lutz, T. Evolution of biological complexity and its relation to taxonomic longevity in the Ammonoidea. Geology 1992, 20, 983–986. [Google Scholar] [CrossRef]
- Weaver, J.S.; Chamberlain, J.A., Jr. Equations of motion for post-mortem sinking of cephalopod shells and the sinking of Nautilus. Paleobiology 1976, 2, 8–18. [Google Scholar] [CrossRef]
- Chamberlain, J.A., Jr.; Weaver, J.S. Equations of motion for post-mortem sinking of cephalopod shells. Math. Geol. 1978, 10, 675–691. [Google Scholar] [CrossRef]
- Chamberlain, J.A., Jr.; Ward, P.D.; Weaver, J.S. Post-mortem ascent of Nautilus shells: Implications for cephalopod paleobiogeography. Paleobiology 1981, 7, 494–509. [Google Scholar] [CrossRef]
- Proceedings of the California Academy of Sciences Ser.3, Geology; California Academy of Sciences: San Francisco, CA, USA, 1897; Volume 1.
- Natural History Museum: Suture Patterns within Subclass Ammonoidea | Natural History Museum. Available online: https://natmus.humboldt.edu/exhibits/fossil-focus-exhibits/suture-patterns-within-subclass-ammonoidea (accessed on 21 September 2021).
- Mikhailov, A.Y. Boreal Jurassic Ammonites (Dorsoplanitinae) and Zonal Subdivision of the Volgian Stage; Academy of Sciences of the USSR, Geological Institute: Moscow, Russia, 1963. [Google Scholar]
- Maisch, M.; Hoffmann, R. Lytoceratids (Cephalopoda, Ammonoidea) from the Lower Posidonienschiefer Formation (Tenuicostatum Zone, Early Jurassic) of BadenWürttemberg (south-western Germany). N. Jb. Geol. Paläont. Abh. 2017. [Google Scholar] [CrossRef]
- Muséum National d’Histoire Naturelle (MNHN). Consultation des Collections. Available online: https://science.mnhn.fr/institution/mnhn/list?collectionCode=f&genus=Lytoceras (accessed on 9 November 2021).
- Cobban, W.A.; Kennedy, W.J. Maastrichtian Ammonites Chiefly from the Prairie Bluff Chalk in Alabama and Mississippi. Memoir (Paleontol. Soc.) 1995, 44, 1–40. [Google Scholar] [CrossRef]
- Paleontological Research Institution Digital Atlas of Ancient Life. Cadoceras sublaeve. Available online: https://www.digitalatlasofancientlife.org/vc/mollusca/cephalopoda/ammonoidea/ (accessed on 21 September 2021).



| SUTURE A Lytoceras sp. | SUTURE B Perisphinctes sp. | SUTURE C Harpoceras sp. | ||||
|---|---|---|---|---|---|---|
| Richardson | LLS | Richardson | LLS | Richardson | LLS | |
| N | 98 | 144 | 47 | 91 | 40 | 54 |
| Df | 1.921 | 2.088 | 1.602 | 1.916 | 1.532 | 1.682 |
| V | 1.469 | 1.936 | 1.350 | |||
| Genus | Species | V | Ave V | Std Dev |
|---|---|---|---|---|
| Lytoceras | L. subsequens | 1.95 | 1.75 | ±0.147 |
| M. submetrerum | 1.925 | |||
| E. phestum | 1.458 | |||
| L. fraasi | 1.864 | |||
| L. trilobeti | 1.840 | |||
| L. exoticum | 1.770 | |||
| L. serorugatum | 2.0 | |||
| L. julietti | 1.517 | |||
| L. alamadense | 1.421 |
| GENUS | V |
|---|---|
| Lytoceras | 1.75 |
| Gaudryceras | 2 |
| Tetragonites | 2 |
| Holcolytoceras | 1.2 |
| ° Psiloceras | 1.55; 1.9 |
| Dorsoplanites | 1.4 |
| Perisphinctes | 2 |
| Stephanoceras | 1.2 |
| Hildoceras | 1.4 |
| Harpoceras | 1.44 |
| Phylloceras | 1.3 |
| ° Pachydiscus | 1.2; 2 |
| Placenticeras | 2 |
| Scaphites | 1.65 |
| * Baculites | 1; 1.5; 2 |
| Kruskal–Wallis Test for Distortion by Curvature | ||||||||
|---|---|---|---|---|---|---|---|---|
| S-Value Section | Coil Profile | Genus | Μean Df Flat | Mean Df Curved | Chi2 | p | Significance (Median D) | |
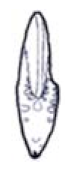 | S ≈ 0.5 | 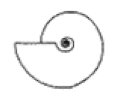 W = 2.0, D = 0.2 | Placenticeras | 1.265 | 1.271 | 2.784 | 0.09852 | No significant distortion. |
 | S ≈ 0.75 | 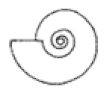 W = 2.0, D = 0.4 | Pachydiscus | 1.648 | 1.754 | 0.5948 | 0.4406 | No significant distortion. |
 | S ≈ 1 | 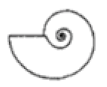 W = 3.0, D = 0.3 | * Gaudryceras | 1.673 | 1.553 | 2.518 | 0.1126 | No significant distortion. |
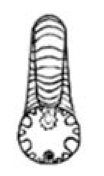 | S ≈ 1 | 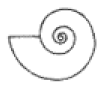 W = 2.5, D = 0.4 | † Lytoceras | 1.651 | 1.707 | 0.9223 | 0.3369 | No significant distortion. |
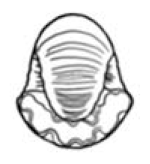 | S ≈ 2 | 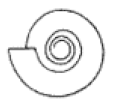 W = 1.5, D = 0.5 | Cadoceras | 1.261 | 1.226 | 0.4963 | 0.4811 | No significant distortion. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marriott, K.; Chamberlain, J.A., Jr. Uses for Incomplete Ammonite Sutures: Lateral Lobe and Second Saddle as Markers of Sutural Complexity. Geosciences 2021, 11, 476. https://doi.org/10.3390/geosciences11110476
Marriott K, Chamberlain JA Jr. Uses for Incomplete Ammonite Sutures: Lateral Lobe and Second Saddle as Markers of Sutural Complexity. Geosciences. 2021; 11(11):476. https://doi.org/10.3390/geosciences11110476
Chicago/Turabian StyleMarriott, Katherine, and John A. Chamberlain, Jr. 2021. "Uses for Incomplete Ammonite Sutures: Lateral Lobe and Second Saddle as Markers of Sutural Complexity" Geosciences 11, no. 11: 476. https://doi.org/10.3390/geosciences11110476
APA StyleMarriott, K., & Chamberlain, J. A., Jr. (2021). Uses for Incomplete Ammonite Sutures: Lateral Lobe and Second Saddle as Markers of Sutural Complexity. Geosciences, 11(11), 476. https://doi.org/10.3390/geosciences11110476






