Landscape Mapping, Ichnological and Benthic Foraminifera Trends in a Deep-Water Gateway, Discovery Gap, NE Atlantic
Abstract
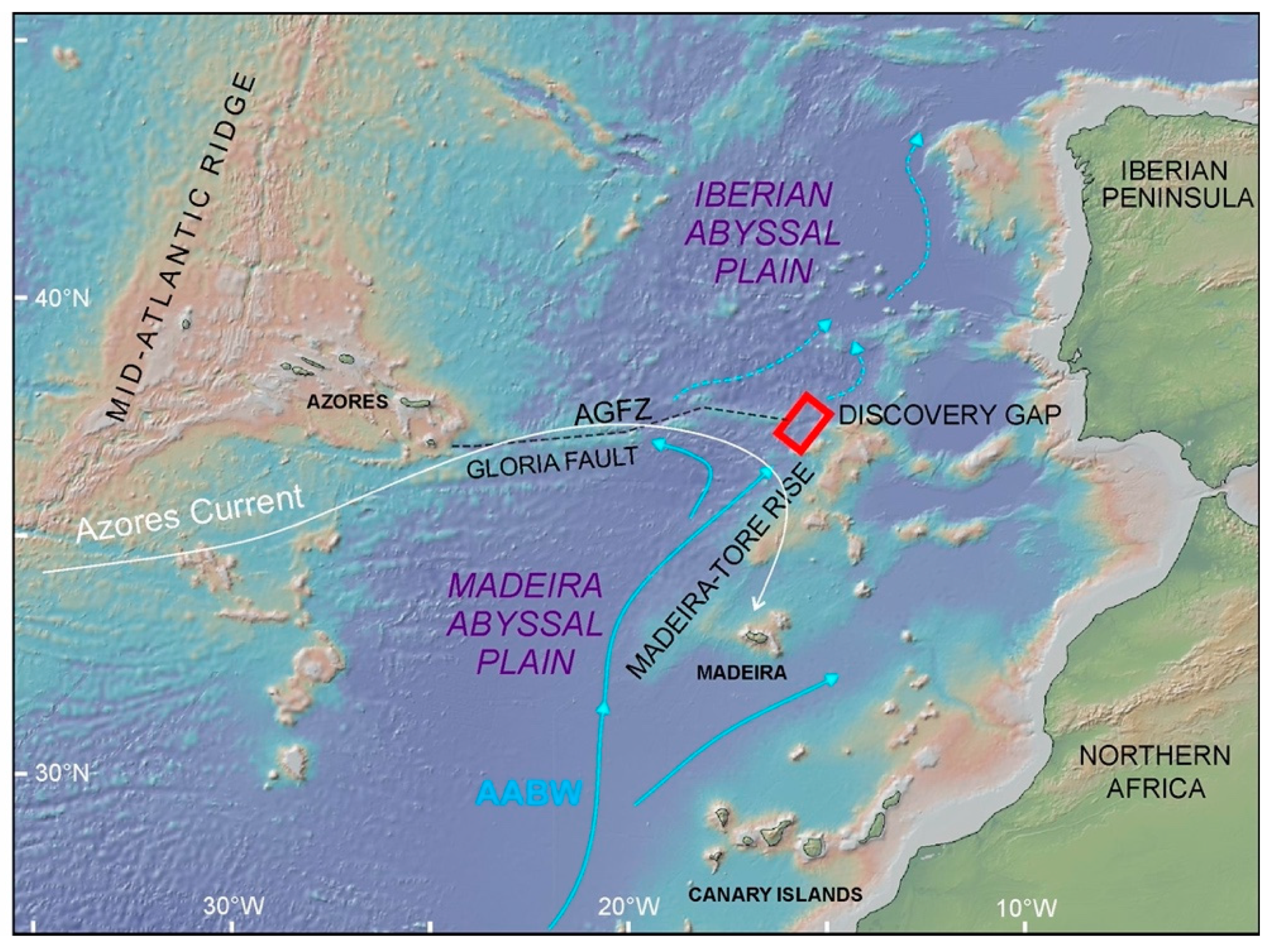
:1. Introduction
2. Materials and Methods
2.1. Identification of Seabed Landscapes
2.1.1. Bathymetric Position Index
- Crests—areas on the elevations with B-BPIstd ≥ 1;
- Depressions—areas in the lowering of the relief, B-BPIstd ≤ −1;
- Horizontal surfaces (Flats)—areas of the terrain with −1 < B-BPIstd < 1 and slope lower or equal to 3°;
- Slopes—areas of the inclined bathymetric surface with −1 < B-BPIstd < 1 and slope higher than the threshold value for slope.
2.1.2. Bottom Substrates
2.1.3. Water Temperature, Oxygen, and Suspended Particulate Matter Concentration
2.1.4. Landscape Mapping
- Mesoscale relief defined with the BPI (crests, depressions, flats, slopes);
- Bottom substrate types (softground, firmground and hardground);
- Near-bottom water potential temperature with 2 °C as threshold value representing the upper boundary of the AABW.
2.2. Sediment Cores
2.2.1. CT-Scanning
2.2.2. Ichnology
2.2.3. Benthic Foraminifera
- 5.
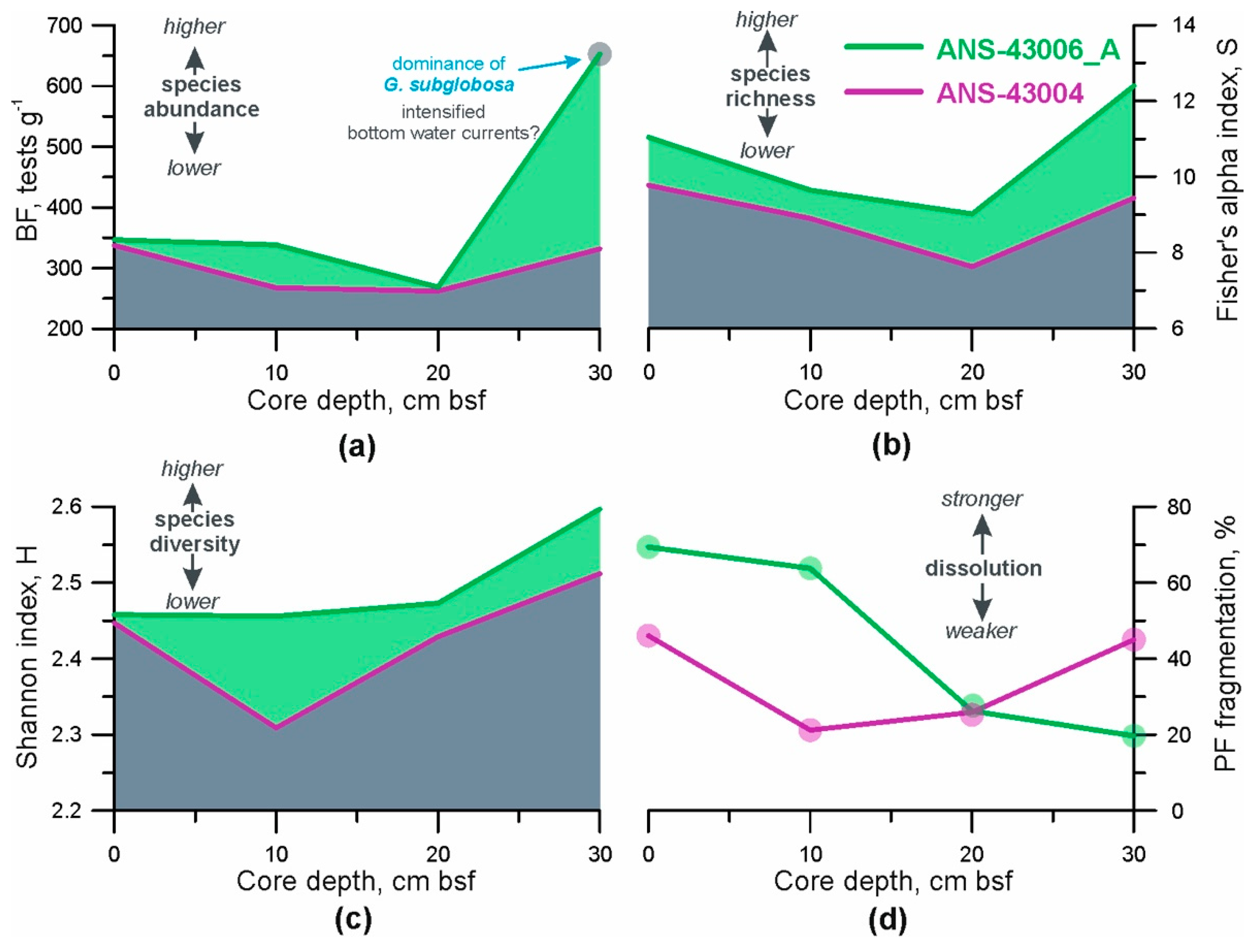
- Total BF abundance. This parameter was calculated as the number of benthic foraminifera per 1 g of dry bulk sediment [55]. A combination of sufficient food supply to the seafloor and relatively high oxygen content in the pore and bottom water creates favourable conditions for mass development of benthic organisms (e.g., [18,21]). Consequently, under such conditions, the concentration of BF tests in the sediments should be higher;
- 6.
- BF ecological indices. The Palaeontological Statistics (PAST) data analysis package was applied to the foraminiferal data (the number of each BF species at given depths in the cores) to obtain the distribution patterns of two ecological indices [56]. The Shannon index (H) accounts for both the number of taxa and the individuals in a community, so here we use it as a parameter of heterogeneity (diversity) of each sample [57]. Fisher’s alpha diversity index (S) describes the relationship between the taxa and the number of individuals of those taxa [58] and consequently, reflects the species richness ([57,59]);
- 7.
- Fragmentation index of planktic foraminifera (PF). It is well-known that changes in foraminiferal abundances are commonly affected by dissolution in the deep open ocean (e.g., [27,60]). Therefore, it is necessary to consider how strong the past dissolution signal might have been and whether it predominated over the export productivity and lateral food advection. The PF fragmentation index was used as a dissolution proxy and calculated according to the equations formulated by [61]. We counted planktic foraminifera in the >150 μm sediment fraction which is considered to be the most sensitive to changes in hydrological conditions (e.g., [62]). A PF fragment was defined as a test portion less than 60% of its original size [62]. If a relationship between the curves of total BF abundance and PF fragmentation index can be identified then it may be possible to suggest that dissolution plays a major role in BF distribution. A higher value of PF fragmentation, together with increased content of pitted benthic foraminifera shells in the same sample, indicates stronger dissolution.
2.3. Integration of the Data
3. Results
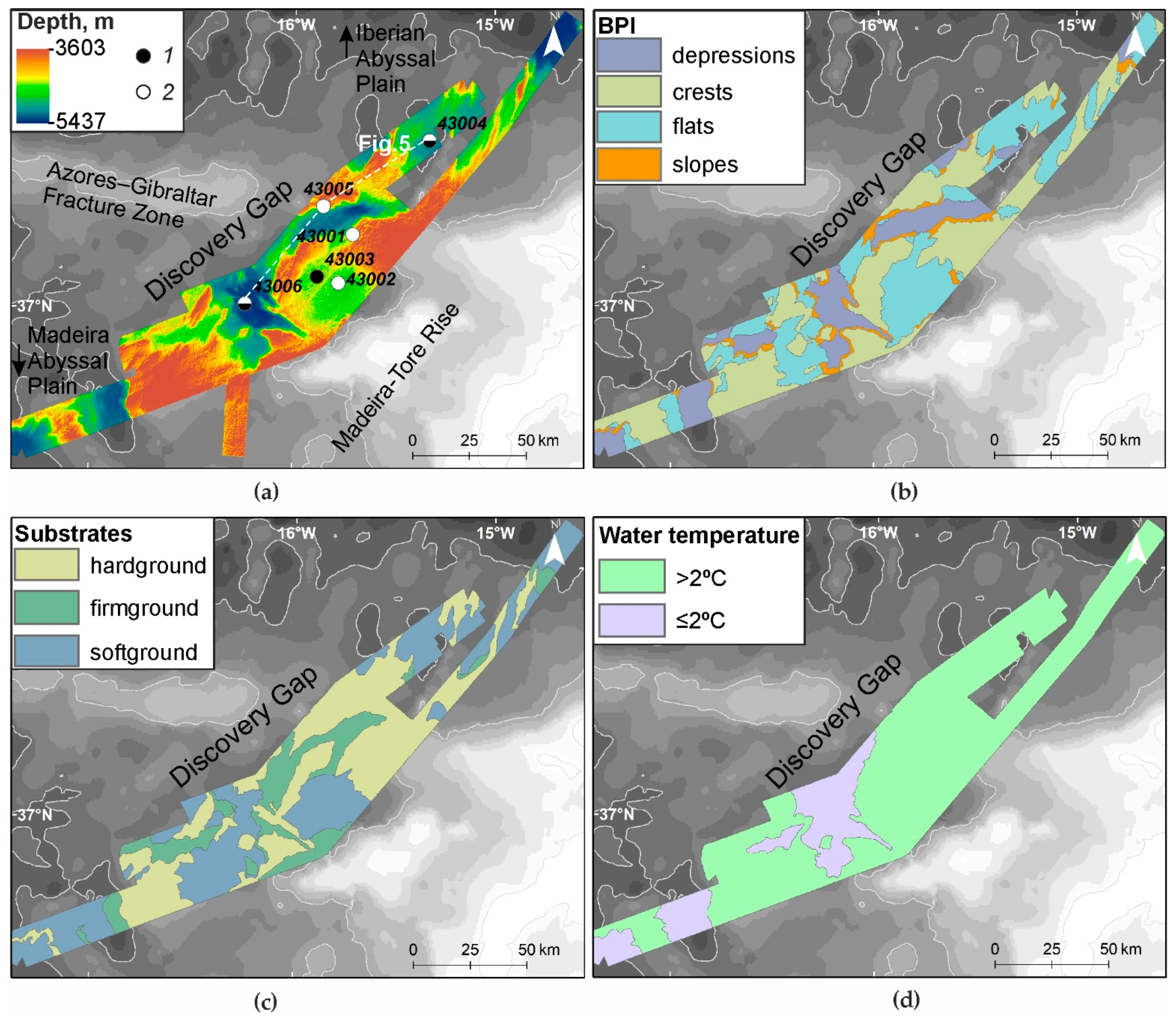
3.1. Relief and Substrate Types
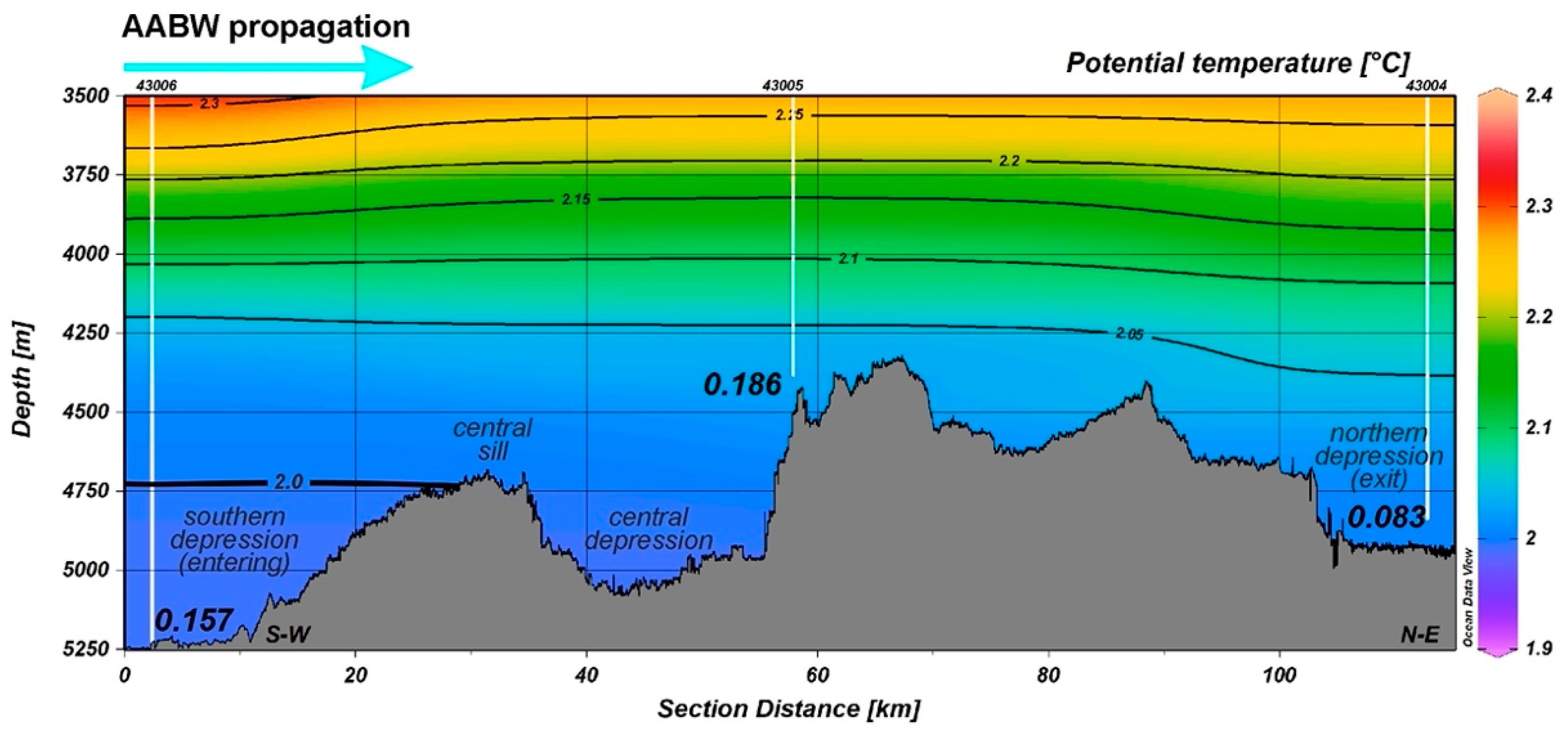
3.2. Temperature, Oxygen and SPM Concentration in the Near-Bottom Waters
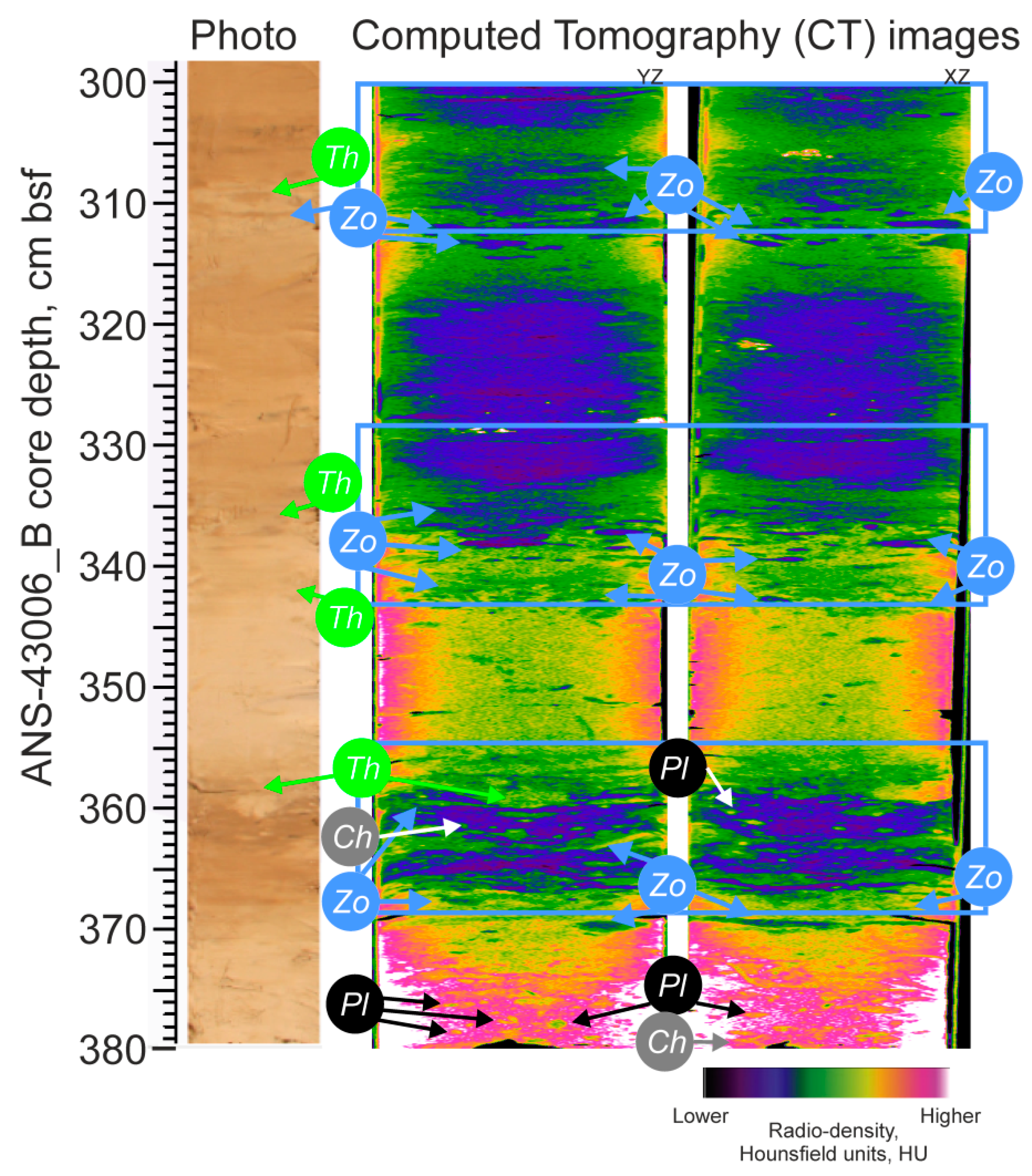
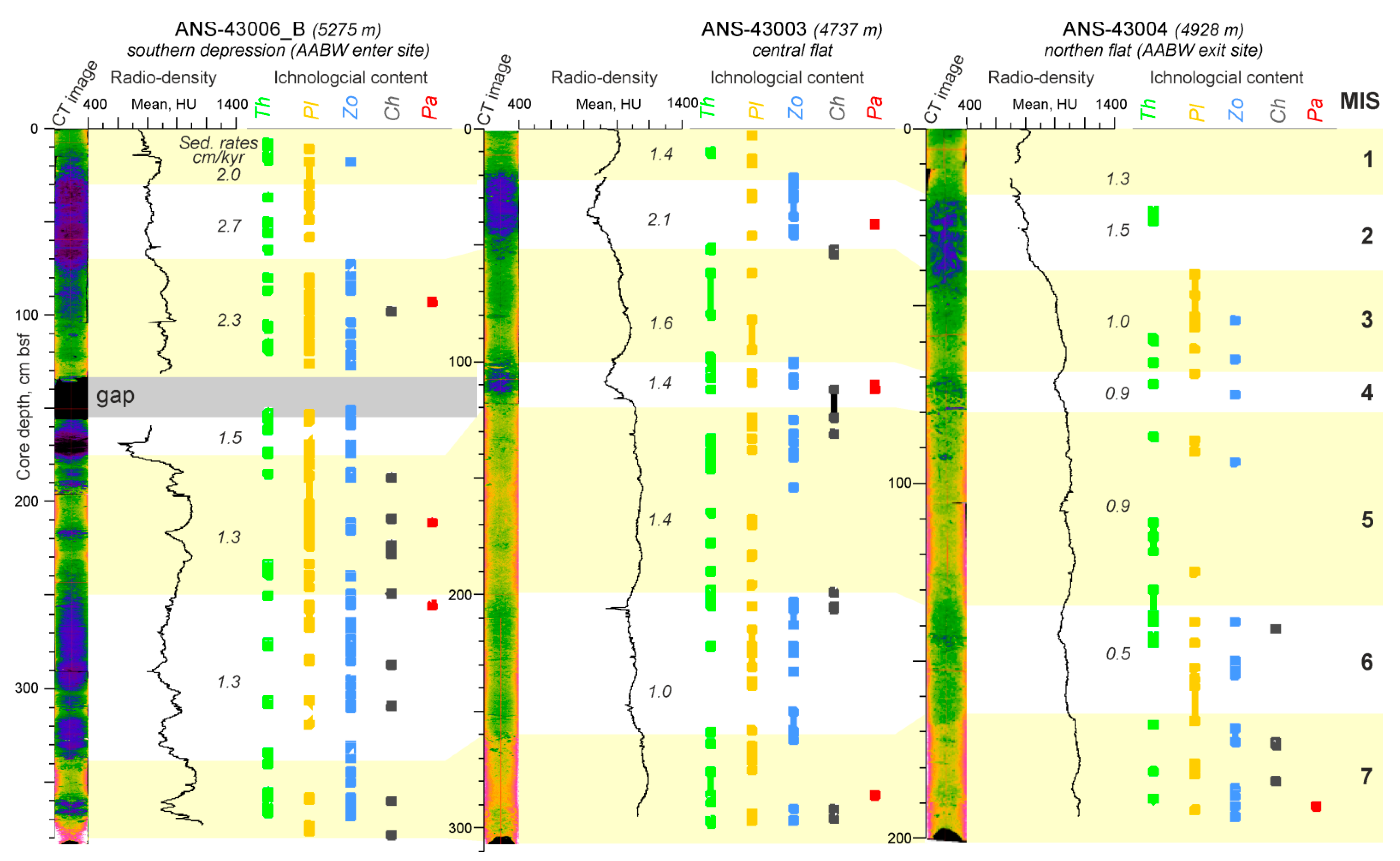
3.3. Ichnology
3.4. CT-Scanning
3.5. Benthic Foraminifera
3.6. Seabed Landscapes
4. Discussion
4.1. Landscapes Variability in the Deep-Water Gateway
4.2. Environmental Drivers of Ichnofossils and Benthic Foraminifera Spatial Variation in the Deep-Water Gateway
4.3. Temporal Variation of the Environmental Factors in Discovery Gap
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harris, P.T.; Baker, E.K. GeoHab Atlas of seafloor geomorphic features and benthic habitats—Synthesis and lessons learned. Seafloor Geomorphol. Benthic Habitat 2020, 969–990. [Google Scholar] [CrossRef]
- Harris, P.T.; Macmillan-Lawler, M.; Rupp, J.; Baker, E.K. Geomorphology of the oceans. Mar. Geol. 2014, 352, 4–24. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Brandt, A.; Danovaro, R.; De Mol, B.; Escobar, E.; German, C.R.; Levin, L.A.; Martinez Arbizu, P.; Menot, L.; Buhl-Mortensen, P.; et al. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences 2010, 7, 2851–2899. [Google Scholar] [CrossRef] [Green Version]
- Roff, J.C.; Taylor, M.E. National Frameworks for Marine Conservation—A Hierarchical Geophysical Approach. Aquat. Conserv. Mar. Freshw. Ecosyst. 2000, 10, 209–223. [Google Scholar] [CrossRef]
- Hernández-Molina, F.J.; Serra, N.; Stow, D.A.V.; Llave, E.; Ercilla, G.; van Rooij, D. Along-slope oceanographic processes and sedimentary products around the Iberian margin. Geo Mar. Lett. 2011, 31, 315–341. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Molina, F.; Maldonado, A.; Stow, D. Chapter 18 Abyssal Plain Contourites. Cycl. Dev. Sedimentol. Basins 2008, 60, 345–378. [Google Scholar] [CrossRef]
- Heezen, B.C.; Tharp, M.; Ewing, M. The Floors of the Oceans: I. The North Atlantic; Geological Society of America: New York, NY, USA, 1959; pp. 1–126. [Google Scholar]
- Whitehead, J.A. Topographic control of oceanic flows in deep passages and straits. Rev. Geophys. 1998, 36, 423–440. [Google Scholar] [CrossRef] [Green Version]
- Morozov, E.G.; Demidov, A.N.; Tarakanov, R.Y.; Zenk, W. Abyssal Channels in the Atlantic Ocean; Springer: Dordrecht, The Netherlands, 2010; Volume 53, ISBN 978-90-481-9357-8. [Google Scholar]
- Johnson, D.A. The Vema Channel: Physiography, structure, and sediment—Current interactions. Mar. Geol. 1984, 58, 1–34. [Google Scholar] [CrossRef]
- Riehl, T.; Wölfl, A.-C.; Augustin, N.; Devey, C.W.; Brandt, A. Discovery of widely available abyssal rock patches reveals overlooked habitat type and prompts rethinking deep-sea biodiversity. Proc. Natl. Acad. Sci. USA 2020, 117, 15450–15459. [Google Scholar] [CrossRef]
- Devey, C.W.; Augustin, N.; Brandt, A.; Brenke, N.; Köhler, J.; Lins, L.; Schmidt, C.; Yeo, I. Habitat characterization of the Vema Fracture Zone and Puerto Rico Trench. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2018, 148, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Morozov, E.G.; Tarakanov, R.Y. Discovery gap: The terminal point of Antarctic bottom water spreading. Dokl. Earth Sci. 2012, 446, 1190–1192. [Google Scholar] [CrossRef]
- Glazkova, T.; Hernández-Molina, F.J.; Dorokhova, E.V.; Mena, A.; Roque, C.; Rodríguez-Tovar, F.J.; Krechik, V.; Llave, E.; Kuleshova, L.A. Sedimentary Processes in the Discovery Gap (Central-NE Atlantic): An Example of a Deep Marine Gateway. Deep Sea Res. Part I Oceanogr. Res. Pap. 2021, in press. [Google Scholar]
- Gooday, A.J.; Levin, L.A.; Linke, P.; Heeger, T. The Role of Benthic Foraminifera in Deep-Sea Food Webs and Carbon Cycling. In Deep-Sea Food Chains and the Global Carbon Cycle; Rowe, G.T., Pariente, V., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 63–91. [Google Scholar]
- Wetzel, A. Deep-Sea Ichnology: Observations in Modern Sediments to Interpret Fossil Counterparts. Acta Geol. Pol. 2010, 60, 125–138. [Google Scholar]
- Dorador, J.; Rodríguez-Tovar, F.J.; Mena, A.; Francés, G. Lateral variability of ichnological content in muddy contourites: Weak bottom currents affecting organisms’ behavior. Sci. Rep. 2019, 9, 17713. [Google Scholar] [CrossRef]
- Gooday, A.J. Benthic foraminifera (protista) as tools in deep-water palaeoceanography: Environmental influences on faunal characteristics. Adv. Mar. Biol. 2003, 46, 1–90. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Murray, J.W. Ecology and Paleoecology of Benthic Foraminifera; Routledge: Harlow, UK, 1991. [Google Scholar]
- Van der Zwaan, G.; Duijnstee, I.; den Dulk, M.; Ernst, S.; Jannink, N.; Kouwenhoven, T. Benthic foraminifers: Proxies or problems?: A review of paleocological concepts. Earth Sci. Rev. 1999, 46, 213–236. [Google Scholar] [CrossRef]
- Milker, Y.; Schmiedl, G. A taxonomic guide to modern benthic shelf foraminifera of the western Mediterranean Sea. Palaeontol. Electron. 2012, 15, 1–134. [Google Scholar] [CrossRef]
- Smart, C.W.; Thomas, E.; Bracher, C.M. Holocene variations in North Atlantic export productivity as reflected in bathyal benthic foraminifera. Mar. Micropaleontol. 2019, 149, 1–18. [Google Scholar] [CrossRef]
- Altenbach, A.V.; Pflaumann, U.; Schiebel, R.; Thies, A.; Timm, S.; Trauth, M. Scaling Percentages and Distributional Patterns of Benthic Foraminifera with Flux Rates of Organic Carbon. J. Foraminifer. Res. 1999, 29, 173–185. [Google Scholar]
- Loubere, P.; Fariduddin, M. Quantitative estimation of global patterns of surface ocean biological productivity and its seasonal variation on timescales from centuries to millennia. Glob. Biogeochem. Cycles 1999, 13, 115–133. [Google Scholar] [CrossRef]
- Sun, X.; Corliss, B.H.; Brown, C.W.; Showers, W.J. The effect of primary productivity and seasonality on the distribution of deep-sea benthic foraminifera in the North Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2006, 53, 28–47. [Google Scholar] [CrossRef]
- Jorissen, F.J.; Fontanier, C.; Thomas, E. Chapter Seven Paleoceanographical Proxies Based on Deep-Sea Benthic Foraminiferal Assemblage Characteristics. Dev. Mar. Geol. 2007, 1, 263–325. [Google Scholar] [CrossRef]
- Fontanier, C.; Jorissen, F.; Licari, L.; Alexandre, A.; Anschutz, P.; Carbonel, P. Live benthic foraminiferal faunas from the Bay of Biscay: Faunal density, composition, and microhabitats. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 751–785. [Google Scholar] [CrossRef]
- Stefanoudis, P.V.; Bett, B.J.; Gooday, A.J. Abyssal hills: Influence of topography on benthic foraminiferal assemblages. Prog. Oceanogr. 2016, 148, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Kaskela, A.; Kotilainen, A.; Al-Hamdani, Z.; Leth, J.; Reker, J. Seabed geomorphic features in a glaciated shelf of the Baltic Sea. Estuar. Coast. Shelf Sci. 2012, 100, 150–161. [Google Scholar] [CrossRef]
- Lundblad, E.R.; Wright, D.; Miller, J.; Larkin, E.M.; Rinehart, R.; Naar, D.F.; Donahue, B.T.; Anderson, S.M.; Battista, T. A Benthic Terrain Classification Scheme for American Samoa. Mar. Geod. 2006, 29, 89–111. [Google Scholar] [CrossRef] [Green Version]
- Rinehart, R.W.; Wright, D.J.; Lundblad, E.R.; Larkin, E.M.; Murphy, J.; Cary-Kothera, L. ArcGIS 8.x Benthic Terrain Modeler: Analysis in American Samoa. In Proceedings of the 24th Annual ESRI User Conference, San Diego, CA, USA, 9 August 2004. [Google Scholar]
- Dudkov, I.; Dorokhova, E. Multibeam bathymetry data of Discovery Gap in the eastern North Atlantic. Data Brief. 2020, 31, 105679. [Google Scholar] [CrossRef]
- Mikuláš, R.; Dronov, A. Palaeoichnology—Introduction to the Study of Trace Fossils; Institute of Geology, Academy of Sciences of Czech Republic: Prague, Czech, 2006; ISBN 9788090351158. [Google Scholar]
- Catuneanu, O. Principles of Sequence Stratigraphy; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780444515681. [Google Scholar]
- Ekdale, A.A.; Bromley, R.G.; Pemberton, S.G. Ichnology: The Use of Trace Fossils in Sedimentology and Stratigraphy; SEPM (Society for Sedimentary Geology): Broken Arrow, OK, USA, 1984; ISBN 978-1-56576-244-2. [Google Scholar]
- Damuth, J.E. Use of high-frequency (3.5–12 kHz) echograms in the study of near-bottom sedimentation processes in the deep-sea: A review. Mar. Geol. 1980, 38, 51–75. [Google Scholar] [CrossRef]
- Dorokhova, E.V.; Krechik, V.A.; Ponomarenko, E.P.; Dudkov, I.Y.; Shakhovskoy, I.B.; Napreenko-Dorokhova, T.N.; Ezhov, V.E.; Malafeev, G.V.; Kuleshova, L.A.; Glazkova, T.A. Integrated Oceanographic Research of Discovery Gap (Eastern North Atlantic) during the Cruise 43 of the R/V Akademik Nikolaj Strakhov. Oceanology 2021, 61, 144–146. [Google Scholar] [CrossRef]
- Wüst, G. Schichtung und Zirkulation des Atlantischen Ozeans. In Wissenschaftliche Ergebnisse, Deutsche Atlantische Expedition auf dem Forschungs-und Vermessungsschiff “Meteor” 1925–1927; Defant, A., Ed.; Walter de Gruyter & Co: Berlin, Germany, 1936; p. 411. [Google Scholar]
- ISO 5813:1983, I. Water Quality—Determination of Dissolved Oxygen: Iodometric Method; International Organization for Standardization: Geneva, Switzerland, 1983. [Google Scholar]
- Neukermans, G.; Ruddick, K.; Loisel, H.; Roose, P. Optimization and quality control of suspended particulate matter concentration measurement using turbidity measurements. Limnol. Oceanogr. Methods 2012, 10, 1011–1023. [Google Scholar] [CrossRef] [Green Version]
- McCave, I. Properties of suspended sediment over the HEBBLE area on the Nova Scotian Rise. Mar. Geol. 1985, 66, 169–188. [Google Scholar] [CrossRef]
- Mena, A.; Frances, G.; Perez-Arlucea, M.; Aguiar, P.; Barreiro-Vázquez, J.-D.; Iglesias, A.; Barreiro-Lois, A. A novel sedimentological method based on CT-scanning: Use for tomographic characterization of the Galicia Interior Basin. Sediment. Geol. 2015, 321, 123–138. [Google Scholar] [CrossRef]
- Rorden, C.; Brett, M. Stereotaxic Display of Brain Lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Dorador, J.; Rodríguez-Tovar, F.J. Digital image treatment applied to ichnological analysis of marine core sediments. Facies 2014, 60, 39–44. [Google Scholar] [CrossRef]
- Dorador, J.; Rodríguez-Tovar, F.J. High-resolution image treatment in ichnological core analysis: Initial steps, advances and prospects. Earth Sci. Rev. 2018, 177, 226–237. [Google Scholar] [CrossRef]
- Dorador, J.; Rodríguez-Tovar, F.J.; Hernández-Molina, F.J.; Stow, D.A.V.; Alvarez-Zarikian, C.; Acton, G.; Bahr, A.; Balestra, B.; Ducassou, E.; Flood, R.; et al. Quantitative estimation of bioturbation based on digital image analysis. Mar. Geol. 2014, 349, 55–60. [Google Scholar] [CrossRef]
- Rodríguez-Tovar, F.J.; Dorador, J. Ichnofabric characterization in cores: A method of digital image treatment. Ann. Soc. Geol. Pol. 2015, 85. [Google Scholar] [CrossRef] [Green Version]
- Knaust, D. Atlas of Trace Fossils in Well Core; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Rodríguez-Tovar, F.J.; Dorador, J. Ichnological analysis of Pleistocene sediments from the IODP Site U1385 “Shackleton Site” on the Iberian margin: Approaching paleoenvironmental conditions. Palaeogeogr. Palaeoclim. Palaeoecol. 2014, 409, 24–32. [Google Scholar] [CrossRef]
- Dorador, J.; Rodríguez-Tovar, F.J. Stratigraphic variation in ichnofabrics at the “Shackleton Site” (IODP Site U1385) on the Iberian Margin: Paleoenvironmental implications. Mar. Geol. 2016, 377, 118–126. [Google Scholar] [CrossRef]
- Licari, L.; Mackensen, A. Benthic foraminifera off West Africa (1° N to 32° S): Do live assemblages from the topmost sediment reliably record environmental variability? Mar. Micropaleontol. 2005, 55, 205–233. [Google Scholar] [CrossRef]
- Poli, M.S.; Meyers, P.A.; Thunell, R.C.; Capodivacca, M. Glacial-interglacial variations in sediment organic carbon accumulation and benthic foraminiferal assemblages on the Bermuda Rise (ODP Site 1063) during MIS 13 to 10. Paleoceanography 2012, 27. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, T.L.; Thomsen, E. Ecology of deep-sea benthic foraminifera in the North Atlantic during the last glaciation: Food or temperature control. Palaeogeogr. Palaeoclim. Palaeoecol. 2017, 472, 15–32. [Google Scholar] [CrossRef]
- Ovsepyan, E.A.; Ivanova, E.V. Glacial–interglacial interplay of southern- and northern-origin deep waters in the São Paulo Plateau—Vema Channel area of the western South Atlantic. Palaeogeogr. Palaeoclim. Palaeoecol. 2018, 514, 349–360. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Softwar Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Wagner, P.J.; Harper, D.A.T. Numerical Palaeobiology: Computer-Based Modelling and Analysis of Fossils and Their Distributions; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar] [CrossRef]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The Relation between the Number of Species and the Number of Individuals in a Random Sample of an Animal Population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Schwing, P.T.; O’Malley, B.J.; Romero, I.C.; Martínez-Colón, M.; Hastings, D.W.; Glabach, M.A.; Hladky, E.M.; Greco, A.; Hollander, D.J. Characterizing the variability of benthic foraminifera in the northeastern Gulf of Mexico following the Deepwater Horizon event (2010–2012). Environ. Sci. Pollut. Res. 2016, 24, 2754–2769. [Google Scholar] [CrossRef] [PubMed]
- Corliss, B.H.; Honjo, S. Dissolution of Deep-Sea Benthonic Foraminifera. Micropaleontology 1981, 27, 356–378. [Google Scholar] [CrossRef]
- Ohkushi, K.; Thomas, E.; Kawahata, H. Abyssal benthic foraminifera from the northwestern Pacific (Shatsky Rise) during the last 298 kyr. Mar. Micropaleontol. 1999, 38, 119–147. [Google Scholar] [CrossRef]
- Berger, W.H.; Bonneau, M.-C.; Parker, F.L. Foraminifera on the Deep-Sea Floor: Lysocline and Dissolution Rate. Oceanol. Acta 1982, 5, 249–258. [Google Scholar]
- Harris, P.T. Surrogacy; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128149607. [Google Scholar]
- Saunders, P.M. Flow through Discovery Gap. J. Phys. Oceanogr. 1987, 17, 631–643. [Google Scholar] [CrossRef] [Green Version]
- Tarakanov, R.Y.; Morozov, E.; Gritsenko, A.M.; Demidova, T.A.; Makarenko, N.I. Transport of Antarctic Bottom Water through passages in the East Azores Ridge (37° N) in the East Atlantic. Oceanology 2013, 53, 432–441. [Google Scholar] [CrossRef]
- Boyer, T.P.; Baranova, O.K.; Coleman, C.; Garcia, H.E.; Grodsky, A.; Locarnini, R.A.; Mishonov, A.V.; Paver, C.R.; Reagan, J.R.; Seidov, D.; et al. World Ocean Database 2018; Mishonov, A.V., Ed.; National Centers for Environmental Information: Asheville, NC, USA, 2018.
- Yasuda, H. Late Miocene–Holocene paleoceanography of the western equatorial Atlantic: Evidence from deep-sea benthic foraminifers. In Proceedings of the Ocean Drilling Program, Scientific Results; Shackleton, N.J., Curry, W.B., Richter, C., Bralower, T.J., Eds.; Ocean Drilling Program: College Station, TX, USA; pp. 395–431. [CrossRef]
- Levin, L.A.; Etter, R.J.; Rex, M.A.; Gooday, A.J.; Smith, C.R.; Pineda, J.; Stuart, C.T.; Hessler, R.R.; Pawson, D. Environmental Influences on Regional Deep-Sea Species Diversity. Annu. Rev. Ecol. Syst. 2001, 32, 51–93. [Google Scholar] [CrossRef] [Green Version]
- Volk, T.; Hoffert, M.I. Ocean Carbon Pumps: Analysis of Relative Strengths and Efficiencies in Ocean-Driven Atmospheric CO2 Changes. Submar. Landslides 2013, 32, 99–110. [Google Scholar] [CrossRef]
- Longhurst, A.; Sathyendranath, S.; Platt, T.; Caverhill, C. An estimate of global primary production in the ocean from satellite radiometer data. J. Plankton Res. 1995, 17, 1245–1271. [Google Scholar] [CrossRef] [Green Version]
- Hensen, C.; Duarte, J.C.; Vannucchi, P.; Mazzini, A.; Lever, M.A.; Terrinha, P.; Géli, L.; Henry, P.; Villinger, H.; Morgan, J.; et al. Marine Transform Faults and Fracture Zones: A Joint Perspective Integrating Seismicity, Fluid Flow and Life. Front. Earth Sci. 2019, 7, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Van Andel, T.H.; Komar, P.D. Ponded Sediments of the Mid-Atlantic Ridge between 22° and 23° North Latitude. GSA Bull. 1969, 80, 1163–1190. [Google Scholar] [CrossRef]
- MacEachern, J.A.; Pembeton Gingras, M.K.; Bann, S.G. The ichnofacies concept: A fifty-year retrospective. In Trace Fossils, Concepts, Problems, Prospects; Miller, W., III, Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 50–75. [Google Scholar]
- MacEachern, J.A.; Bann, K.L.; Gingras, M.K.; Zonneveld, J.-P.; Dashtgard, S.E.; Pemberton, S.G. The Ichnofacies Paradigm. In Cyclic Development of Sedimentary Basins; Elsevier BV: Amsterdam, The Netherlands, 2012; Volume 64, pp. 103–138. [Google Scholar]
- Buatois, L.; Mangano, G. Ichnology. Organism-Substrate Interraction in Space and TIME; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780521855556. [Google Scholar]
- Rodríguez-Tovar, F.J.; Hernández-Molina, F.J. Ichnological analysis of contourites: Past, present and future. Earth Sci. Rev. 2018, 182, 28–41. [Google Scholar] [CrossRef]
- Durden, J.M.; Bett, B.J.; Ruhl, H.A. Subtle variation in abyssal terrain induces significant change in benthic megafaunal abundance, diversity, and community structure. Prog. Oceanogr. 2020, 186, 102395. [Google Scholar] [CrossRef]
- Priede, I.G.; Bergstad, O.A.; Miller, P.; Vecchione, M.; Gebruk, A.; Falkenhaug, T.; Billett, D.S.M.; Craig, J.; Dale, A.; Shields, M.A.; et al. Does Presence of a Mid-Ocean Ridge Enhance Biomass and Biodiversity? PLoS ONE 2013, 8, e61550. [Google Scholar] [CrossRef] [Green Version]
- Niedzielski, T.; Høines, Å.; Shields, M.A.; Linley, T.D.; Priede, I. A multi-scale investigation into seafloor topography of the northern Mid-Atlantic Ridge based on geographic information system analysis. Deep Sea Res. Part II Top. Stud. Oceanogr. 2013, 98, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Frey, D.I. Bottom Gravity Currents in the Deep-Water Atlantic Channels; Shirshov Institute of Oceanology, Russian Academy of Sciences: Moscow, Russia, 2018. [Google Scholar]
- Sivkov, V.; Kravchishina, M.; Klyuvitkin, A. Suspended matter in the channel and adjacet slopesof the Rio Grande Rise. In Abyssal Channels in the Atlantic Ocean; Morozov, E.G., Demidov, A.N., Tarakanov, R.Y., Zenk, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 159–165. [Google Scholar]
- Thomsen, L.; Vanweering, T.; Gust, G. Processes in the benthic boundary layer at the Iberian continental margin and their implication for carbon mineralization. Prog. Oceanogr. 2002, 52, 315–329. [Google Scholar] [CrossRef]
- Wetzel, A.; Werner, F.; Stow, D. Chapter 11 Bioturbation and Biogenic Sedimentary Structures in Contourites. In Developments in Sedimentology; Elsevier BV: Amsterdam, The Netherlands, 2008; Volume 60, pp. 183–202. ISBN 9780444529985. [Google Scholar]
- Nemirovskaya, I.A.; Kravchishina, M.D.; Nemirovskaya, I. Variability of suspended particulate matter concentrations and organic compounds in frontal zones of the Atlantic and Southern oceans. Oceanology 2016, 56, 55–64. [Google Scholar] [CrossRef]
- Alvarez-Salgado, X.A. Contribution of upwelling filaments to offshore carbon export in the subtropical Northeast Atlantic Ocean. Limnol. Oceanogr. 2007, 52, 1287–1292. [Google Scholar] [CrossRef] [Green Version]
- Roden, G.I. Effect of Seamounts and Semounts Chains on Oceano Circulation and Thermohaline Strucutres. Geophys. Monogr. Ser. 1987, 43, 335–354. [Google Scholar]
- Vandorpe, T.; Collart, T.; Cnudde, V.; Lebreiro, S.M.; Hernández-Molina, F.J.; Alonso, B.; Mena, A.; Antón, L.; Van Rooij, D. Quantitative characterisation of contourite deposits using medical CT. Mar. Geol. 2019, 417, 106003. [Google Scholar] [CrossRef]
- Duplessy, J.C.; Shackleton, N.J.; Fairbanks, R.G.; Labeyrie, L.; Oppo, D.; Kallel, N. Deepwater source variations during the last climatic cycle and their impact on the global deepwater circulation. Paleoceanography 1988, 3, 343–360. [Google Scholar] [CrossRef]
- Kaiho, K. Benthic foraminiferal dissolved-oxygen index and dissolved-oxygen levels in the modern ocean. Geology 1994, 22, 719. [Google Scholar] [CrossRef]
- Thunell, R.C. Carbonate dissolution and abyssal hydrography in the Atlantic Ocean. Mar. Geol. 1982, 47, 165–180. [Google Scholar] [CrossRef]
- Raymo, M.; Ruddiman, W.; Shackleton, N.; Oppo, D. Evolution of Atlantic-Pacific δ13C gradients over the last 2.5 m.Y. Earth Planet. Sci. Lett. 1990, 97, 353–368. [Google Scholar] [CrossRef]
- Fariduddin, M.; Loubere, P. The surface ocean productivity response of deeper water benthic foraminifera in the Atlantic Ocean. Mar. Micropaleontol. 1997, 32, 289–310. [Google Scholar] [CrossRef]
- Kuleshova, L.A.; Ovsepyan, E.A. Paleoceanographic Reconstructions for the Southwestern Atlantic during the Middle-Late Pleistocene Based on Benthic Foraminiferal Assemblages. Vestn. Mosk. Univ. Seriya 5 Geogr. 2019, 3, 72–82. [Google Scholar]
- Dorador, J.; Wetzel, A.; Rodríguez-Tovar, F.J. Zoophycos in deep-sea sediments indicates high and seasonal primary productivity: Ichnology as a proxy in palaeoceanography during glacial-interglacial variations. Terra Nova 2016, 28, 323–328. [Google Scholar] [CrossRef]
- Dorador, J.; Rodríguez-Tovar, F.J.; Mena, A.; Francés, G. Deep-sea bottom currents influencing tracemaker community: An ichnological study from the NW Iberian margin. Mar. Geol. 2021, 437, 106503. [Google Scholar] [CrossRef]
- Sarnthein, M.; Stattegger, K.; Dreger, D.; Erlenkeuser, H.; Grootes, P.; Haupt, B.J.; Jung, S.; Kiefer, T.; Kuhnt, W.; Pflaumann, U.; et al. Fundamental Modes and Abrupt Changes in North Atlantic Circulation and Climate over the last 60 ky—Concepts, Reconstruction and Numerical Modeling. N. N. Atl. A Chang. Environ. 2001, 365–410. [Google Scholar] [CrossRef]
- Ternois, Y.; Sicre, M.-A.; Paterne, M. Climatic changes along the northwestern African Continental Margin over the last 30 kyrs. Geophys. Res. Lett. 2000, 27, 133–136. [Google Scholar] [CrossRef]
- Weaver, P.P.E.; Rothwell, R.G. Sedimentation on the Madeira Abyssal Plain over the last 300 000 years. Geol. Soc. Spec. Publ. 1987, 31, 71–86. [Google Scholar] [CrossRef]
- Lebreiro, S.; Weaver, P.; Howe, R. Sedimentation on the Madeira Abyssal Plain: Eocene-Pleistocene history of turbidite infill. Proc. Ocean Drill. Progr. Sci. Results 1998, 157, 523–531. [Google Scholar] [CrossRef]
- Huguet, C.; de Lange, G.J.; Gustafsson, Ö.; Middelburg, J.J.; Sinninghe Damsté, J.S.; Schouten, S. Selective preservation of soil organic matter in oxidized marine sediments (Madeira Abyssal Plain). Geochim. Cosmochim. Acta 2008, 72, 6061–6068. [Google Scholar] [CrossRef]
- Weaver, P.P.E.; Kuijpers, A. Climatic control of turbidite deposition on the Madeira Abyssal Plain. Nat. Cell Biol. 1983, 306, 360–363. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Digital Elevation Model resolution, m | 100 |
| Neighborhood form | ring |
| Outer radius, number of sells | 200 |
| Inner radius, number of sells | 20 |
| Scale coefficient | 20,000 |
| Minimum incline value for the slope, degree | 3 |
| Station Number | Latitude | Longitude | Type of Data | Core Length, cm |
|---|---|---|---|---|
| ANS-43001 | 37°18.30′ N | 15°42.51′ W | Hydrologycal | |
| ANS-43002 | 37°06.73′ N | 15°46.70′ W | Hydrologycal | |
| ANS-43003 | 37°08.23′ N | 15°53.07′ W | Sediment core | 302 |
| ANS-43004 | 37°40.75′ N | 15°19.69′ W | Hydrologycal/sediment core | 195 |
| ANS-43005 | 37°24.97′ N | 15°51.27′ W | Hydrologycal | |
| ANS-43006 | 37°01.56′ N | 16°14.45′ W | Hydrologycal/two sediment cores (A, B) | 395 (A), 391 (B) |
| Number | Landscape | Area, km2 | Area, % |
|---|---|---|---|
| 1 | Crests, hardground, water temperature >2 °C | 2704.7 | 28.8 |
| 2 | Crests, softground, water temperature >2 °C | 767.3 | 8.2 |
| 3 | Crests, firmground, water temperature >2 °C | 334.4 | 3.6 |
| 4 | Crests, hardground, water temperature ≤2 °C | 58.9 | 0.6 |
| 5 | Crests, softground, water temperature ≤2 °C | 20.8 | 0.2 |
| 6 | Crests, firmground, water temperature ≤2 °C | 7.4 | 0.1 |
| 7 | Depressions, hardground, water temperature >2 °C | 235.4 | 2.5 |
| 8 | Depressions, softground, water temperature >2 °C | 161.9 | 1.7 |
| 9 | Depressions, firmground, water temperature >2 °C | 333.2 | 3.5 |
| 10 | Depressions, hardground, water temperature ≤2 °C | 110.8 | 1.2 |
| 11 | Depressions, softground, water temperature ≤2 °C | 512.6 | 5.5 |
| 12 | Depressions, firmground, water temperature ≤2 °C | 350.5 | 3.7 |
| 13 | Flats, hardground, water temperature >2 °C | 518.0 | 5.5 |
| 14 | Flats, softground, water temperature >2 °C | 1902.8 | 20.2 |
| 15 | Flats, firmground, water temperature >2 °C | 273.4 | 2.9 |
| 16 | Flats, hardground, water temperature ≤2 °C | 52.0 | 0.6 |
| 17 | Flats, softground, water temperature ≤2 °C | 299.9 | 3.2 |
| 18 | Flats, firmground, water temperature ≤2 °C | 205.1 | 2.2 |
| 19 | Slopes, hardground, water temperature >2 °C | 282.4 | 3.0 |
| 20 | Slopes, firmground, water temperature >2 °C | 71.7 | 0.8 |
| 21 | Slopes, hardground, water temperature ≤2 °C | 117.3 | 1.2 |
| 22 | Slopes, softground, water temperature ≤2 °C | 21.7 | 0.2 |
| 23 | Slopes, firmground, water temperature ≤2 °C | 62.3 | 0.6 |
| Station | Depth, m | SPM, mg/L | Oxygen, mL/L |
|---|---|---|---|
| ANS-43001 | 4533 | 0.034 | 5.30 |
| ANS-43002 | 4673 | 0.145 | 5.69 |
| ANS-43004 | 4840 | 0.083 | 5.32 |
| ANS-43005 | 4384 | 0.186 | 5.61 |
| ANS-43006 | 5250 | 0.157 | 5.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorokhova, E.V.; Rodríguez-Tovar, F.J.; Dorokhov, D.V.; Kuleshova, L.A.; Mena, A.; Glazkova, T.; Krechik, V.A. Landscape Mapping, Ichnological and Benthic Foraminifera Trends in a Deep-Water Gateway, Discovery Gap, NE Atlantic. Geosciences 2021, 11, 474. https://doi.org/10.3390/geosciences11110474
Dorokhova EV, Rodríguez-Tovar FJ, Dorokhov DV, Kuleshova LA, Mena A, Glazkova T, Krechik VA. Landscape Mapping, Ichnological and Benthic Foraminifera Trends in a Deep-Water Gateway, Discovery Gap, NE Atlantic. Geosciences. 2021; 11(11):474. https://doi.org/10.3390/geosciences11110474
Chicago/Turabian StyleDorokhova, Evgenia V., Francisco J. Rodríguez-Tovar, Dmitry V. Dorokhov, Liubov A. Kuleshova, Anxo Mena, Tatiana Glazkova, and Viktor A. Krechik. 2021. "Landscape Mapping, Ichnological and Benthic Foraminifera Trends in a Deep-Water Gateway, Discovery Gap, NE Atlantic" Geosciences 11, no. 11: 474. https://doi.org/10.3390/geosciences11110474






