Demise of the Planktic Foraminifer Genus Morozovella during the Early Eocene Climatic Optimum: New Records from ODP Site 1258 (Demerara Rise, Western Equatorial Atlantic) and Site 1263 (Walvis Ridge, South Atlantic)
Abstract
1. Introduction
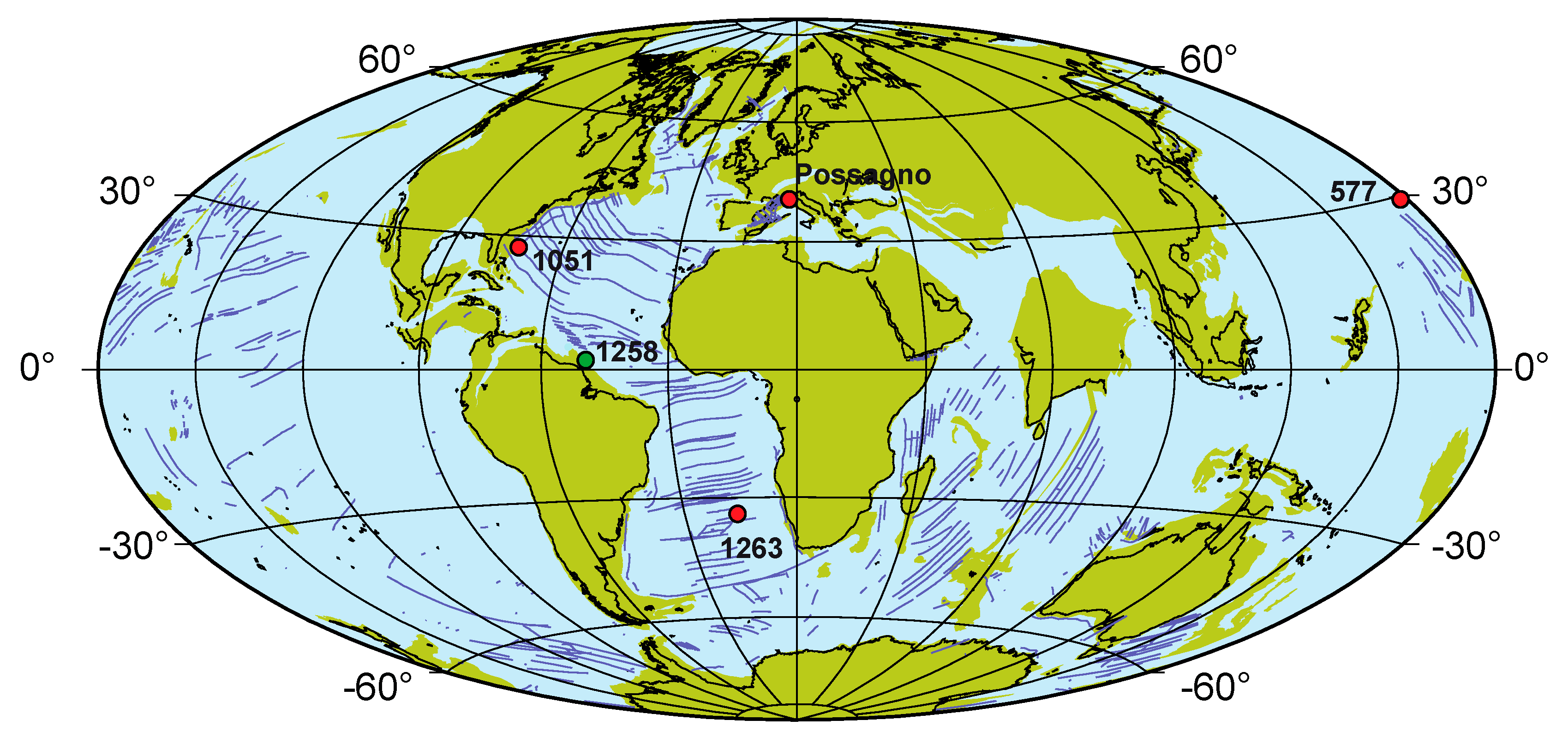
2. ODP Site 1258: Location and Previous Work
3. Materials and Methods
3.1. Early Eocene Sampling Strategy at Site 1258
3.2. Proxy for Carbonate Dissolution: Fragmentation Index
3.3. Planktic Foraminiferal Analysis
4. Results
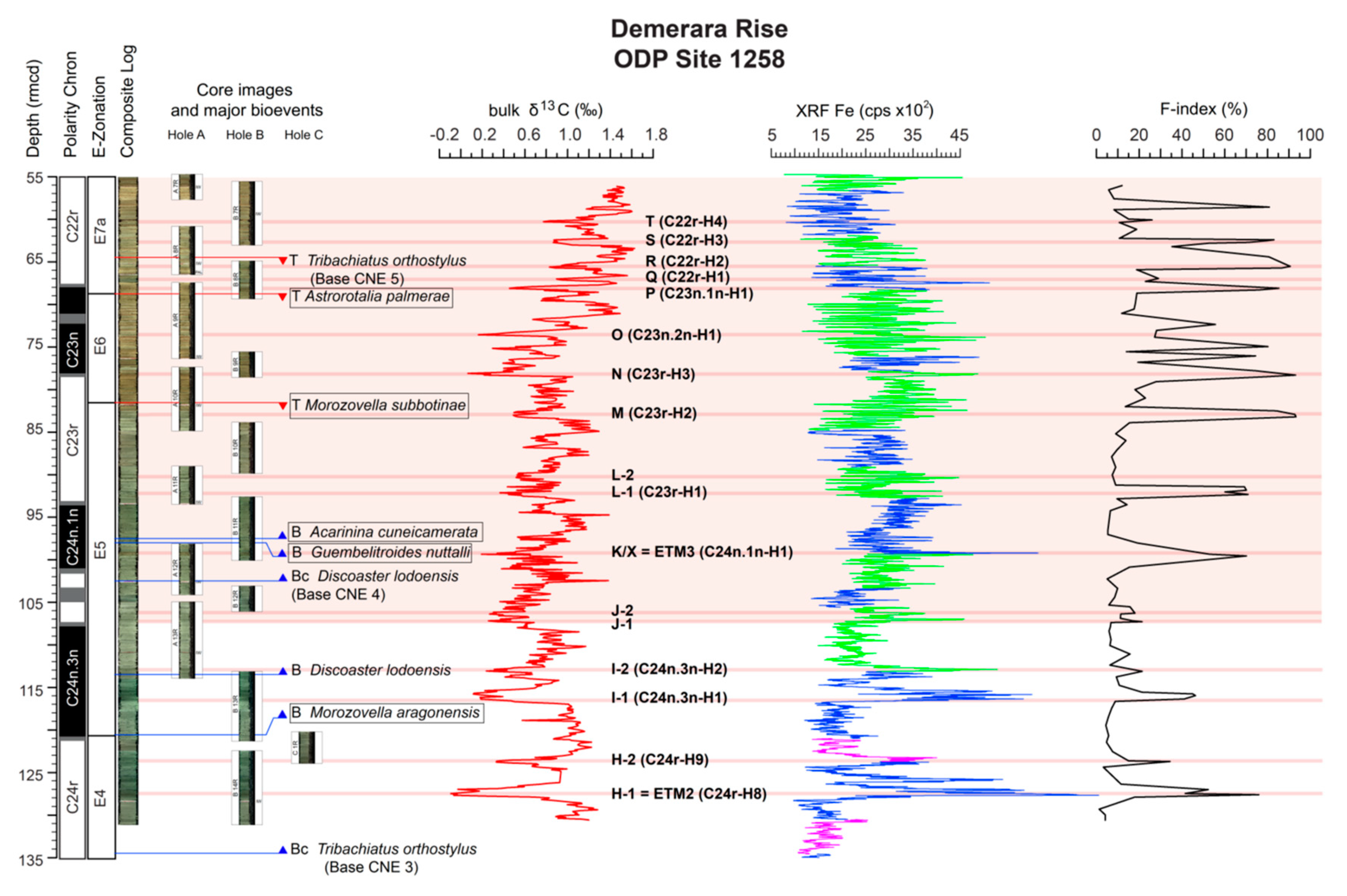
4.1. Variations in Dissolution Proxy
4.2. Biostratigraphy
- the B of M. aragonensis lies 4.85 m below the I-1 CIE that is at 115.8 rmcd;
- the T of M. subbotinae lies 1.49 m above the M CIE that is at 83.0 rmcd;
- the B of Acarinina cuneicamerata, the suggested marker for the E6/E7a boundary [52], is found at 97.53 rmcd, 5.4 m below the L1 that is at 92,13 rmcd CIE and well below the T of M. subbotinae;
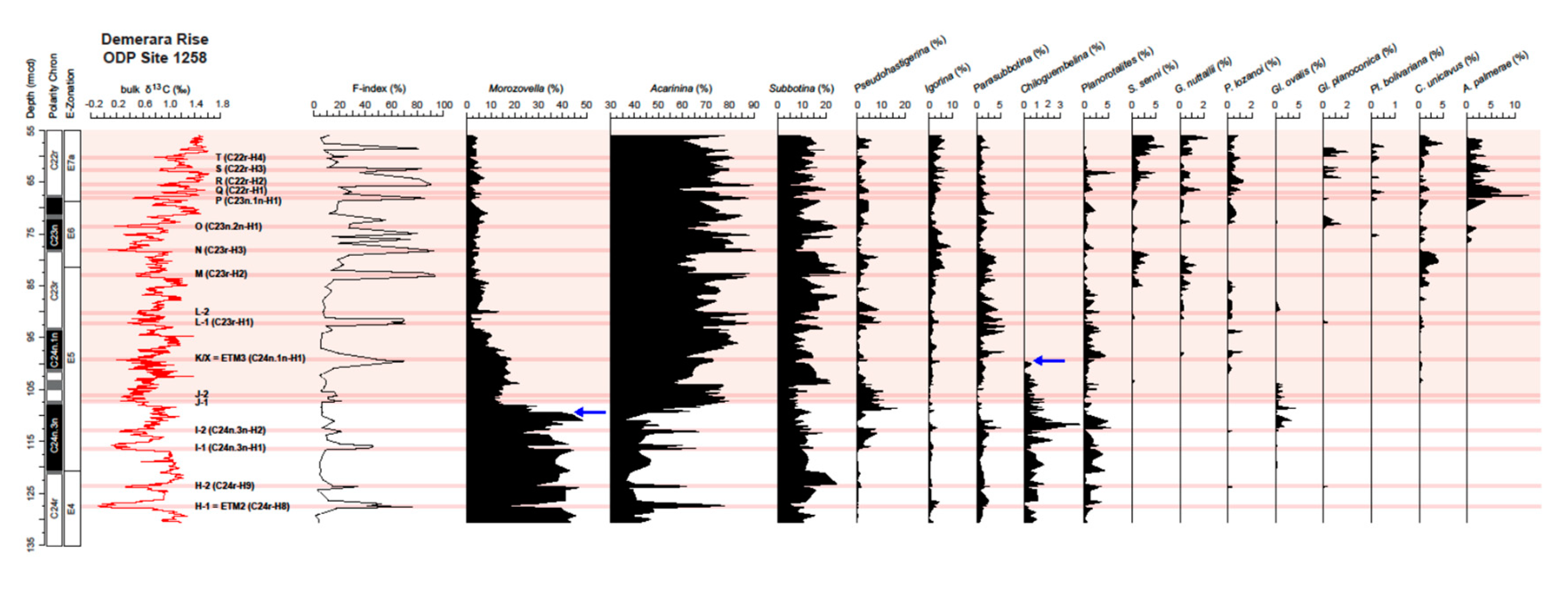
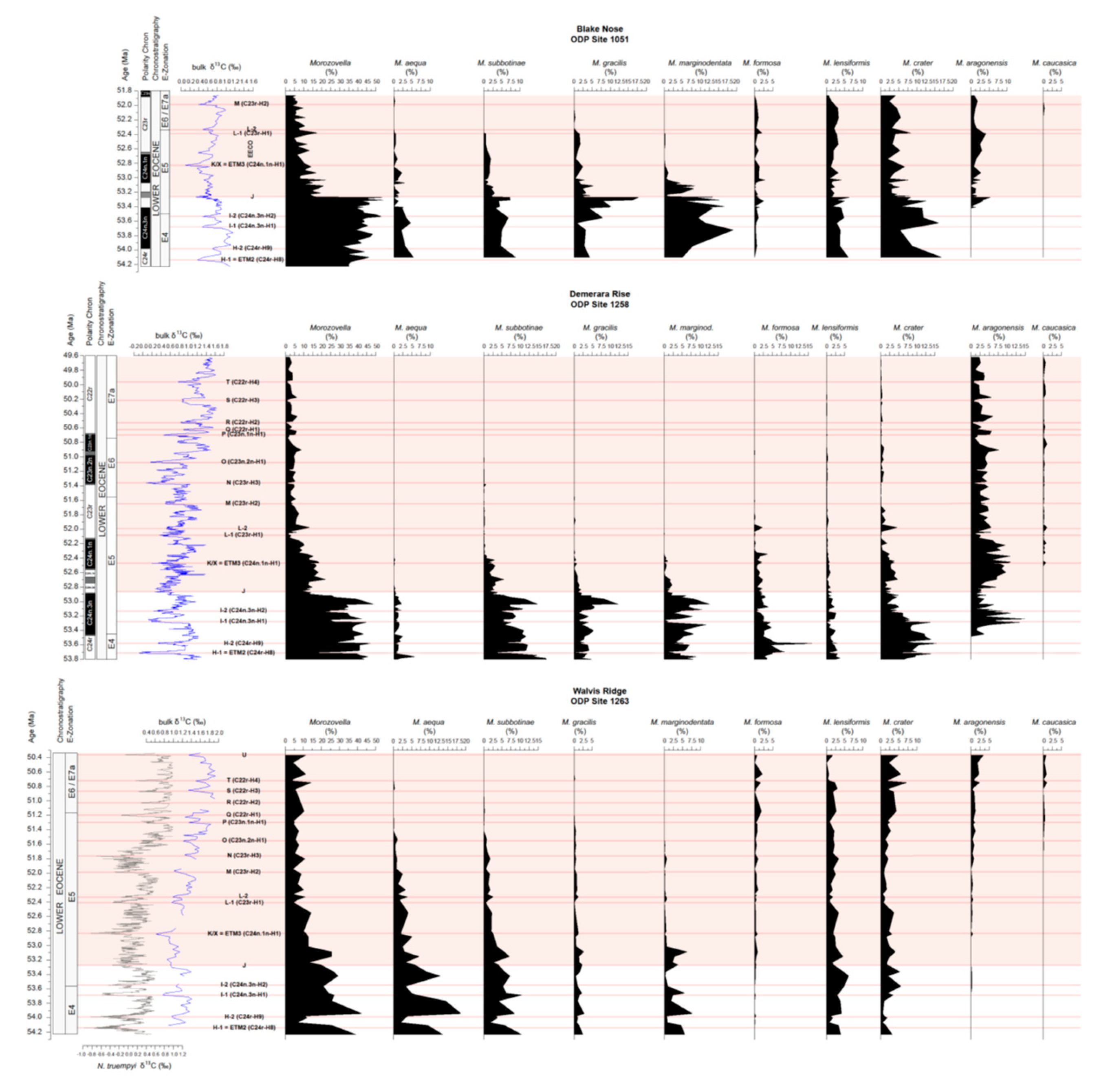
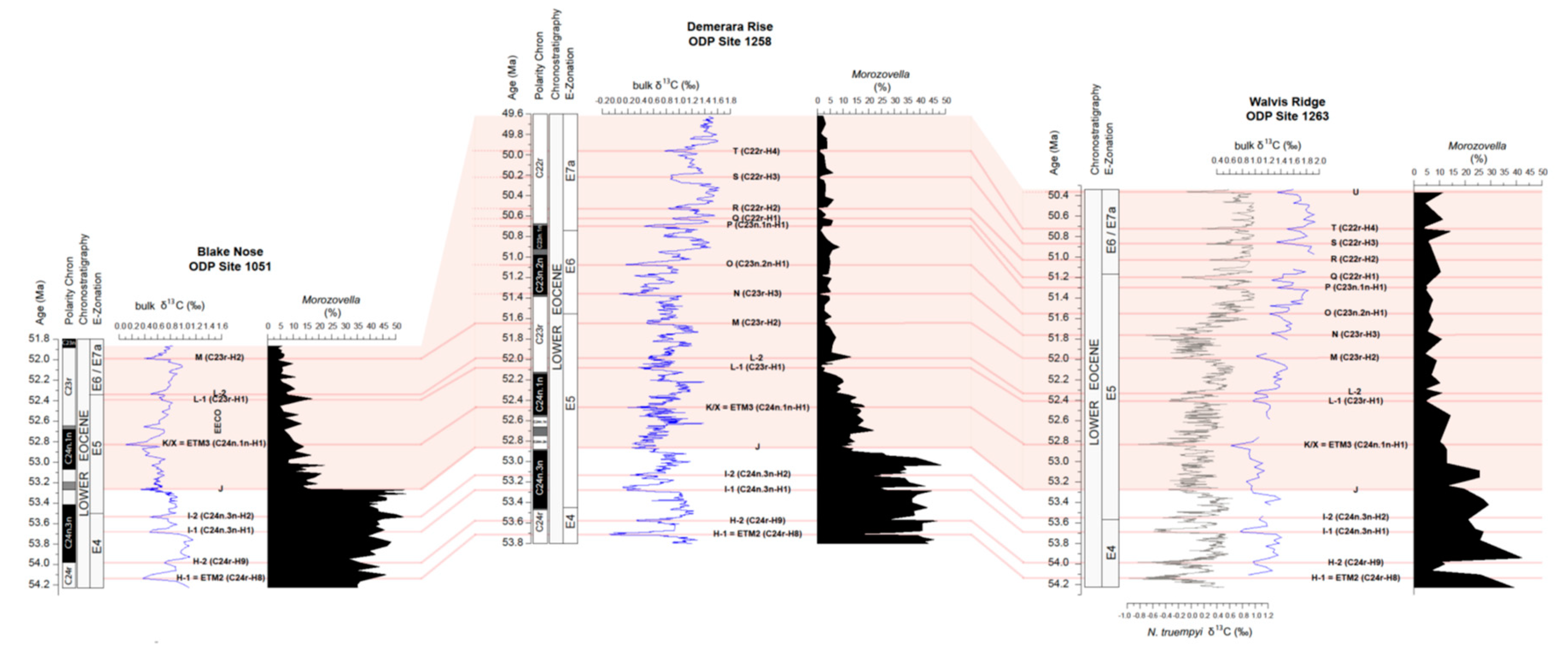
4.3. Variations in Abundance of Planktic Foraminiferal Genera
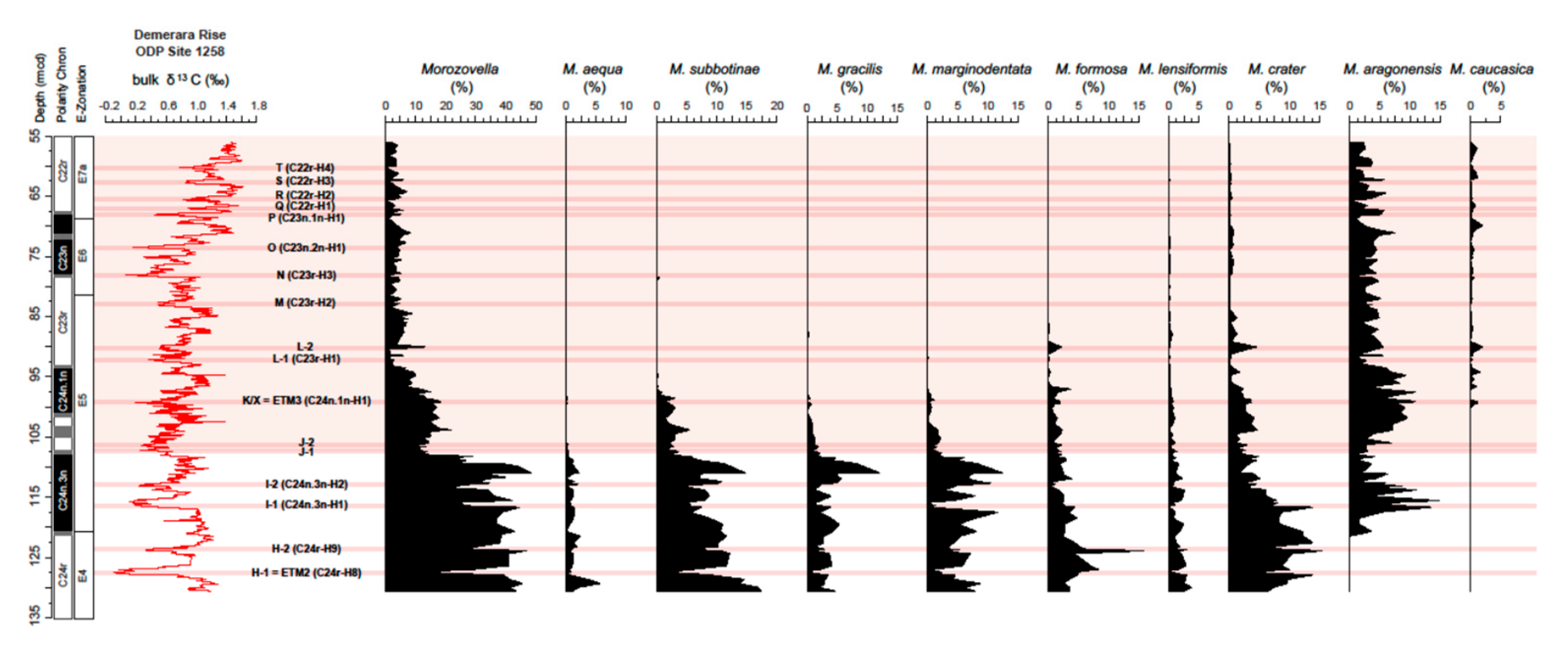
4.4. Variations in Morozovella Species Abundance and Test Size
4.5. Changes in Morozovellid Species Abundance and Test Size from Site 1263
5. Discussion
5.1. The F-Index Record and Impact of Carbonate Dissolution
5.2. Problems in Early Eocene Planktic foraminiferal Biozones
5.3. The Planktic Foraminiferal Record across the EECO at Site 1258
5.3.1. Permanent Morozovella and Acarinina Switch in Abundances
5.3.2. Changes in Morozovellid Species Population and Test Size
5.3.3. Chiloguembelinid Virtual Disappearance at the EECO
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature: List of Species Cited in Text and Figures
| Astrorotalia palmerae (Cushman and Bermudez, 1937) |
| Catapsydrax unicavus Bolli, Loeblich and Tappan, 1957 |
| Globanomalina ovalis Haque, 1956 |
| Globanomalina planoconica (Subbotina, 1953) |
| Guembelitrioides nuttalli (Hamilton, 1953) |
| Morozovella aequa (Cushman and Renz, 1942) |
| Morozovella aragonensis (Nuttall, 1930) |
| Morozovella caucasica (Glaessner, 1937) |
| Morozovella crater (Hornibrook, 1958) |
| Morozovella formosa (Bolli, 1957) |
| Morozovella gracilis (Bolli, 1957) |
| Morozovella lensiformis (Subbotina, 1953) |
| Morozovella marginodentata (Subbotina, 1953) |
| Morozovella subbotinae (Morozova, 1939) |
| Praemurica lozanoi (Colom, 1954) |
| Pseudoglobigerinella bolivariana (Petters, 1954) |
| Subbotina senni (Beckmann, 1953) |
References
- Zachos, J.C.; Dickens, G.R.; Zeebe, R.E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 2008, 451, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Westerhold, T.; Röhl, U.; Donner, B.; Zachos, J.C. Global Extent of Early Eocene Hyperthermal Events: A New Pacific Benthic Foraminiferal Isotope Record from Shatsky Rise (ODP Site 1209). Paleoceanogr. Paleoclimatol. 2018, 33. [Google Scholar] [CrossRef]
- Hollis, C.J.; Dunkley Jones, T.; Anagnostou, E.; Bijl, P.K.; Cramwinckel, M.J.; Cui, Y.; Dickens, G.R.; Edgar, K.M.; Eley, Y.; Evans, D.; et al. The DeepMIP contribution to PMIP4: Methodologies for selection, compilation and analysis of latest Paleocene and early Eocene climate proxy data, incorporating version 0.1 of the DeepMIP database. Geosci. Model Dev. 2019, 12, 3149–3206. [Google Scholar] [CrossRef]
- Bijl, P.K.; Schouten, S.; Sluijs, A.; Reichart, G.-J.; Zachos, J.C.; Brinkhuis, H. Early Paleogene temperature evolution of the southwest Pacific Ocean. Nature 2009, 461, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Caballero, R. The early Eocene equable climate problem revisited. Clim. Past 2011, 7, 603–633. [Google Scholar] [CrossRef]
- Hollis, C.J.; Taylor, K.W.R.; Handley, L.; Pancost, R.D.; Huber, M.; Creech, J.B.; Hines, B.R.; Crouch, E.M.; Morgans, H.E.G.; Crampton, J.S.; et al. Early Paleogene temperature history of the Southwest Pacific Ocean: Reconciling proxies and models. Earth Planet. Sci. Lett. 2012, 349, 53–66. [Google Scholar] [CrossRef]
- Pross, J.; Contreras, L.; Bijl, P.K.; Greenwood, D.R.; Bohaty, S.M.; Schouten, S.; Bendle, J.A.; Röhl, U.; Tauxe, L.; Raine, J.I.; et al. Persistent near-tropical warmth on the Antarctic continent during the early Eocene Epoch. Nature 2012, 488, 73–77. [Google Scholar] [CrossRef]
- Inglis, G.N.; Farnsworth, A.; Lunt, D.; Foster, G.L.; Hollis, C.J.; Pagani, M.; Jardine, P.E.; Pearson, P.N.; Markwick, P.; Galsworthy, A.M.J.; et al. Descent toward the icehouse: Eocene sea surface cooling inferred from GDGT distributions. Paleoceanography 2015, 30, 100–1020. [Google Scholar] [CrossRef]
- Kennett, J.P.; Stott, L.D. Abrupt deep-sea warming; palaeoceanographic changes and benthic extinctions at the end of the Palaeocene. Nature 1991, 353, 225–229. [Google Scholar] [CrossRef]
- Thomas, E.; Zachos, J.C.; Bralower, T.J. Deep-sea environments on a warm Earth: Latest Paleocene–early Eocene. In Warm Climates in Earth History; Huber, B., MacLeod, K., Wing, S., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 132–160. [Google Scholar]
- Lourens, L.J.; Sluijs, A.; Kroon, D.; Zachos, J.C.; Thomas, E.; Röhl, U.; Bowles, J.; Raffi, I. Astronomical pacing of late Palaeocene to early Eocene global warming events. Nature 2005, 435, 1083–1087. [Google Scholar] [CrossRef]
- Nicolo, M.J.; Dickens, G.R.; Hollis, C.J.; Zachos, J.C. Multiple early Eocene hyperthermals: Their sedimentary expression on the New Zealand continental margin and in the deep sea. Geology 2007, 35, 99–702. [Google Scholar] [CrossRef]
- Zachos, J.C.; McCarren, H.K.; Murphy, B.; Röhl, U.; Westerhold, T. Tempo and scale of late Paleocene and early Eocene carbon isotope cycles: Implications for the origin of hyperthermals. Earth Planet. Sci. Lett. 2010, 299, 242–249. [Google Scholar] [CrossRef]
- Coccioni, R.; Bancalà, G.; Catanzariti, R.; Fornaciari, E.; Frontalini, F.; Giusberti, L.; Jovane, L.; Luciani, V.; Savian, J.; Sprovieri, M. An integrated stratigraphic record of the Palaeocene-lower Eocene at Gubbio (Italy), new insights into the early Palaeogene hyperthermals and carbon isotope excursions. Terra Nova 2012, 24, 380–386. [Google Scholar] [CrossRef]
- Kirtland-Turner, S.; Sexton, P.F.; Charled, C.D.; Norris, R.D. Persistence of carbon release events through the peak of early Eocene global warmth. Nat. Geosci. 2014, 7, 748–751. [Google Scholar] [CrossRef]
- Littler, K.; Röhl, U.; Westerhold, T.; Zachos, J.C. A high-resolution benthic stable isotope record for the South Atlantic: Implications for orbital-scale changes in late Paleocene–early Eocene climate and carbon cycling. Earth Planet. Sci. Lett. 2014, 401, 18–30. [Google Scholar] [CrossRef]
- Slotnick, B.S.; Dickens, G.R.; Hollis, C.J.; Crampton, J.S.; Strong, P.S.; Phillips, A. The onset of the Early Eocene climatic optimum at branch stream, Clarence River Valley. N. Z. J. Geol. Geophys. 2015, 58. [Google Scholar] [CrossRef]
- Lauretano, V.; Hilgen, F.J.; Zachos, J.C.; Lourens, L.J. Astronomically tuned age model for the early Eocene carbon isotope events: A new high-resolution δ13C benthic record of ODP Site 1263 between ~49 and ~54 Ma. Newsl. Stratigr. 2016, 49, 383–400. [Google Scholar] [CrossRef]
- Luciani, V.; Dickens, G.R.; Backman, J.; Fornaciari, E.; Giusberti, L.; Agnini, C.; D’Onofrio, R. Major perturbations in the global carbon cycle and photosymbiont-bearing planktic foraminifera during the early Eocene. Clim. Past 2016, 12, 981–1007. [Google Scholar] [CrossRef]
- Dickens, G.R.; Castillo, M.; Walker, J.C.G. A blast of gas in the latest Paleocene: Simulating first-order effects of massive dissociation of oceanic methane hydrate. Geology 1997, 25, 259–262. [Google Scholar] [CrossRef]
- Pagani, M.; Caldeira, K.; Archer, D.; Zachos, J.C. An ancient carbon mystery. Science 2006, 314, 1556–1557. [Google Scholar] [CrossRef]
- McInerney, F.A.; Wing, S.L. The Paleocene-Eocene Thermal Maximum: A perturbation of carbon cycle, climate, and biosphere with implications for the future. Annu. Rev. Earth Planet. Sci. 2011, 39, 489–516. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Zachos, J.C. Long-term legacy of massive carbon input to the Earth system: Anthropocene versus Eocene. Philos. Trans. R. Soc. A 2013, 371. [Google Scholar] [CrossRef] [PubMed]
- Wing, S.L.; Bown, T.M.; Obradovich, J.D. Early Eocene biotic and climatic change in interior western North America. Geology 1991, 19, 1189–1192. [Google Scholar] [CrossRef]
- Zonneveld, J.P.; Gunnell, G.F.; Bartels, W.S. Early Eocene fossil vertebrates from the southwestern Green River Basin, Lincoln and Uinta Counties, Wyoming. J. Vertebr. Paleontol. 2000, 20, 369–386. [Google Scholar] [CrossRef]
- Wilf, P.; Cúneo, R.N.; Johnson, K.R.; Hicks, J.F.; Wing, S.L.; Obradovich, J.D. High plant diversity in Eocene South America: Evidence from Patagonia. Science 2003, 300, 122–125. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Katz, M.E.; Milligan, A.J.; Fennel, K.; Cramer, B.S.; Aubry, M.P.; Berner, R.A.; Novacek, M.J.; Zapol, W.M. Mammals evolved, radiated, and grew in size as the concentration of oxygen in Earth’s atmosphere increased during the past 100 million years. Science 2005, 309, 2202–2204. [Google Scholar] [CrossRef]
- Agnini, C.; Muttoni, G.; Kent, D.V.; Rio, D. Eocene biostratigraphy and magnetic stratigraphy from Possagno, Italy: The calcareous nannofossils response to climate variability. Earth Planet. Sci. Lett. 2006, 241, 815–830. [Google Scholar] [CrossRef]
- Agnini, C.; Fornaciari, E.; Raffi, I.; Catanzariti, R.; Pälike, H.; Backman, J.; Rio, D. Biozonation and biochronology of Paleogene calcareous nannofossils from low to middle latitudes. Newsl. Strat. 2014, 47, 131–181. [Google Scholar] [CrossRef]
- Sims, P.A.; Mann, D.G.; Medlin, L.K. Evolution of the diatoms: Insights from fossil, biological and molecular data. Phycologia 2006, 45, 361–402. [Google Scholar] [CrossRef]
- Woodburne, M.O.; Gunnell, G.F.; Stucky, R.K. Climate directly influences Eocene mammal faunal dynamics in North America. Proc. Natl. Acad. Sci. USA 2009, 106, 13399–13403. [Google Scholar] [CrossRef]
- Schneider, L.J.; Bralower, T.J.; Kump, L.J. Response of nannoplankton to early Eocene ocean destratification. Palaeogeogr. Paleoeclimatol. Palaeoecol. 2011, 310, 152–162. [Google Scholar] [CrossRef]
- Shamrock, J.L.; Watkins, D.K.; Johnston, K.W. Eocene biogeochronology of ODP Leg 122 Hole 762C, Exmouth Plateau (northwest Australian Shelf). Stratigraphy 2012, 9, 55–76. [Google Scholar]
- Oreshkina, T.V. Evidence of late Paleocene—Early Eocene hyperthermal events in biosiliceous sediments of Western Siberia and adjacent areas. Aust. J. Earth Sci. 2012, 105, 145–153. [Google Scholar]
- Figueirido, B.; Janis, C.M.; Pérez-Claros, J.A.; De Renzi, M.; Palmqvist, P. Cenozoic climate change influences mammalian evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.D. Biased extinction and evolutionary trends. Paleobiology 1991, 17, 388–399. [Google Scholar] [CrossRef]
- Berggren, W.A.; Pearson, P.N.; Huber, B.T.; Wade, B.S. Taxonomy, biostratigraphy and phylogeny of Eocene Acarinina. In Atlas of Eocene Planktonic Foraminifera; Pearson, P.N., Olsson, R.K., Huber, B.T., Hemleben, C., Berggren, W.A., Eds.; Cushman Found. Special Publication for Foraminiferal Res.: Greenville, NC, USA, 2006; Volume 41, pp. 257–326. [Google Scholar]
- Pearson, P.N.; Olsson, R.K.; Hemblen, C.; Huber, B.T.; Berggren, W.A.; Pearson, P.N. Atlas of Eocene Planktonic Foraminifera; Pearson, P.N., Olsson, R.K., Hemblen, C., Huber, B.T., Berggren, W.A., Eds.; Cushman Found. Special Publication for Foraminiferal Res.: Greenville, NC, USA, 2006; Volume 41, p. 513. [Google Scholar]
- Aze, T.; Ezard, T.H.G.; Purvis, A.; Coxall, H.K.; Stewart, D.R.M.; Wade, B.S.; Pearson, P.N. A phylogeny of Cenozoic macroperforate planktonic foraminifera from fossil data. Biol. Rev. 2011, 86, 900–927. [Google Scholar] [CrossRef]
- Fraass, A.J.; Kelly, D.K.; Peters, S.E. Macroevolutionary history of the planktic foraminifera. Annu. Rev. Earth Planet. Sci. 2015, 43, 139–166. [Google Scholar] [CrossRef]
- Luciani, V.; D’Onofrio, R.; Dickens, G.R.; Wade, B.S. Did photosymbiont bleaching lead to the demise of planktic foraminifer Morozovella at the Early Eocene Climatic Optimum? Paleoceanography 2017, 32, 1115–1136. [Google Scholar] [CrossRef]
- Luciani, V.; D’Onofrio, R.; Dickens, G.R.; Wade, B.S. Planktic foraminiferal response to early Eocene carbon cycle perturbations in the southeast Atlantic Ocean (ODP site 1263). Glob. Planet. Chang. 2017, 158, 119–133. [Google Scholar] [CrossRef]
- Frontalini, F.; Coccioni, R.; Catanzariti, R.; Jovane, L.; Savian, J.F.; Sprovieri, M. The Eocene Thermal Maximum 3: Reading the environmental perturbations at Gubbio (Italy). In The Stratigraphic Record of Gubbio: Integrated Stratigraphy of the Late Cretaceous–Paleogene Umbria-Marche Pelagic Basin; Menichetti, M., Coccioni, R., Montanari, A., Eds.; Geological Society of America Special Papers: Boulder, Colorado, USA, 2016; Volume 524, pp. 161–326. [Google Scholar]
- Shipboard Scientific Party: Site 1258. In Proceedings of the Ocean Drilling Program, Init. Repts., /207; Erbacher, J., Mosher, D.C., Malone, M.J., Eds.; Ocean Drilling Program: College Station, TX, USA; pp. 1–117. [CrossRef]
- Van Hinsbergen, D.J.J.; de Groot, L.V.; van Schaik, S.J.; Spakman, W.; Bijl, P.K.; Sluijs, A.; Langereis, C.G.; Brinkhuis, H. A paleolatitude calculator for paleoclimate studies. PLoS ONE 2015, 10, e0126946. [Google Scholar] [CrossRef] [PubMed]
- Sexton, P.F.; Wilson, P.A.; Pearson, P.N. Microstructural and geochemical perspectives on planktic foraminiferal preservation: ‘Glassy’ versus ‘Frosty’. Geochem. Geophys. Geosyst. 2006, 7. [Google Scholar] [CrossRef]
- Martini, E. Standard Tertiary and Quaternary calcareous nannoplankton zonation. In Proceedings of the 2nd Planktonic Conference, Rome, Italy, 1970; Farinacci, A., Ed.; Tecnoscienza: Rome, Italy, 1971; Volume 2, pp. 739–785. [Google Scholar]
- Berggren, W.A.; Kent, D.V.; Swisher, C.C., III; Aubry, M.-P. A revised Cenozoic geochronology and chronostratigraphy. In Geochronology, Time Scales and Global Stratigraphic Correlation; Berggren, W.A., Kent, D.V., Aubry, M.P., Hardenbol, J., Eds.; SEPM Society for Sedimentary Geology Special Publication: Tysons, VA, USA, 1995; Volume 54, pp. 129–212. [Google Scholar] [CrossRef]
- Westerhold, T.; Röhl, U.; Frederichs, T.; Agnini, C.; Raffi, I.; Zachos, J.C.; Wilkens, R.H. Astronomical calibration of the Ypresian timescale: Implications for seafloor spreading rates and the chaotic behaviour of the solar system? Clim. Past 2017, 13, 1129–1152. [Google Scholar] [CrossRef]
- Westerhold, T.; Röhl, U.; Laskar, J. Time scale controversy: Accurate orbital calibration of the early Paleogene. Geochem. Geophys. Geosyst. 2012, 13. [Google Scholar] [CrossRef]
- Laskar, J.; Fienga, A.; Gastineau, M.; Manche, H. La2010: A new orbital solution for the long term motion of the Earth. Astron. Astrophys. 2011, 532. [Google Scholar] [CrossRef]
- Wade, B.S.; Pearson, P.N.; Berggren, W.A.; Pälike, H. Review and revision of Cenozoic tropical planktonic foraminiferal biostratigraphy and calibration to the geomagnetic polarity and astronomical time scale. Earth Sci. Rev. 2011, 104, 111–142. [Google Scholar] [CrossRef]
- Luciani, V.; Giusberti, L. Reassessment of the early–middle Eocene planktic foraminiferal biomagnetochronology: New evidence from the Tethyan Possagno section (NE Italy) and Western North Atlantic Ocean ODP Site 1051. J. Foraminifer. Res. 2014, 44, 187–201. [Google Scholar] [CrossRef]
- Zachos, J.C.; Röhl, U.; Schellenberg, S.A.; Sluijs, A.; Hodell, D.A.; Kelly, D.C.; Thomas, E.; Nicolo, M.; Raffi, I.; Lourens, L.J.; et al. Rapid acidification of the ocean during the Paleocene–Eocene thermal maximum. Science 2005, 308, 1611–1615. [Google Scholar] [CrossRef]
- Agnini, C.; Macrì, P.; Backman, J.; Brinkhuis, H.; Fornaciari, E.; Giusberti, L.; Luciani, V.; Rio, D.; Sluijs, A.; Speranza, F. An early Eocene carbon cycle perturbation at _52.5 Ma in the Southern Alps: Chronology and biotic response. Paleoceanography 2009, 24. [Google Scholar] [CrossRef]
- Stap, L.; Sluijs, A.; Thomas, E.; Lourens, L.J. Patterns and magnitude of deep sea carbonate dissolution during Eocene Thermal Maximum 2 and H2, Walvis Ridge, Southeastern Atlantic Ocean. Paleoceanography 2009, 24. [Google Scholar] [CrossRef]
- Leon-Rodriguez, L.; Dickens, G.R. Constraints on ocean acidification associated with rapid and massive carbon injections: The early Paleogene record at ocean drilling program site 1215, equatorial Pacific Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 298, 409–420. [Google Scholar] [CrossRef]
- Berger, W.H. Planktonic foraminifera—Selective solution and lysocline. Mar. Geol. 1970, 8, 111–138. [Google Scholar] [CrossRef]
- Hancock, H.J.L.; Dickens, G.R. Carbonate dissolution episodes in Paleocene and Eocene sediment, Shatsky Rise, west-central Pacific. In Proceedings of the Ocean Drilling Program, Scientific Results; Bralower, T.J., Premoli Silva, I., Malone, M.J., Eds.; Texas A&M University: College Station, TX, USA, 2005; Volume 198, pp. 1–24. Available online: http://www-odp.tamu.edu/publications/198_SR/116/116.htm (accessed on 27 February 2020). [CrossRef]
- Nguyen, T.M.P.; Speijer, R.P. A new procedure to assess dissolution based on experiments on Pliocene–Quaternary foraminifera (ODP Leg 160, Eratosthenes Seamount, Eastern Mediterranean). Mar. Micropaleontol. 2014, 106, 22–39. [Google Scholar] [CrossRef]
- Green, O.R. A Manual of Practical Laboratory and Field Techniques in Palaeobiology; Kluwer Academic: London, UK, 2001; Volume 538. [Google Scholar]
- Boersma, A.; Premoli Silva, I.; Shackleton, N. Atlantic Eocene planktonic foraminiferal biogeography and stable isotopic paleoceanography. Paleoceanography 1987, 2, 287–331. [Google Scholar] [CrossRef]
- Pearson, P.N.; Shackleton, N.J.; Hall, M.A. Stable isotope paleoecology of middle Eocene planktonic foraminifera and multispecies isotope stratigraphy, DSDP Site 523, South Atlantic. J. Foraminifer. Res. 1993, 23, 123–140. [Google Scholar] [CrossRef]
- Luciani, V.; D’Onofrio, R.; Filippi, G.; Moretti, S. Which was the habitat of Early Eocene planktic foraminifer Chiloguembelina? Stable isotope paleobiology from Atlantic Ocean and implication for paleoceanographic reconstructions. Glob. Planet. Chang. Under review.
- Olsson, R.K.; Hemleben, C.; Berggren, W.A.; Huber, B.T. Atlas of Paleocene Planktonic Foraminifera. In Smithsonian Contribution to Paleobiology; Smithsonian Institution Press: Washington, DC, USA, 1999; Volume 85, p. 225. [Google Scholar]
- Molina, E.; Alegret, L.; Apellaniz, E.; Bernaola, G.; Caballero, F.; Dinarès-Turell, J.; Hardenbol, J.; Heilmann-Clausen, C.; Larrasoaña, J.C.; Luterbacher, H.; et al. The Global Stratotype Section and Point (GSSP) for the base of the Lutetian Stage at the Gorrondatxe section, Spain. Episodes 2011, 34, 86–108. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Zeebe, R.E.; Dickens, G.R. Understanding long-term carbon cycle trends: The late Paleocene through the early Eocene. Paleoceanography 2013, 28, 650–662. [Google Scholar] [CrossRef]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Olivarez Lyle, A.; Lyle, M.W. Missing organic carbon in Eocene marine sediments: Is metabolism the biological feedback that maintains end-member climates? Paleoceanography 2006, 21. [Google Scholar] [CrossRef]
- O’Connor, M.; Piehler, M.F.; Leech, D.M.; Anton, A.; Bruno, J.F. Warming and resource availability shift food web structure and metabolism. PLoS Biol. 2009, 7. [Google Scholar] [CrossRef]
- John, E.H.; Pearson, P.N.; Coxall, H.K.; Birch, H.; Wade, B.S.; Foster, G.L. Warm ocean processes and carbon cycling in the Eocene. Philos. Trans. R. Soc. A 2013, 371. [Google Scholar] [CrossRef] [PubMed]
- John, E.H.; Pearson, P.N.; Coxall, H.K.; Birch, H.; Wade, B.S.; Foster, G.L. Temperature-dependent remineralization and carbon cycling in the warm Eocene oceans. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 413, 158–166. [Google Scholar] [CrossRef]
- Petrizzo, M.-R.; Leoni, G.; Speijer, R.P.; De Bernardi, B.; Felletti, F. Dissolution susceptibility of some Paleogene planktonic foraminifera from ODP Site 1209 (Shatsky Rise, Pacific Ocean). J. Foraminifer. Res. 2008, 38, 357–371. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Petrizzo, M.-R.; Speijer, R.P. Experimental dissolution of a fossil foraminiferal assemblage (Paleocene–Eocene Thermal Maximum, Dababiya, Egypt): Implications for paleoenvironmental reconstructions. Mar. Micropaleontol. 2009, 73, 241–258. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Petrizzo, M.-R.; Stassen, P.; Speijer, R.P. Dissolution susceptibility of Paleocene–Eocene planktic foraminifera: Implications for palaeoceanographic reconstructions. Mar. Micropaleontol. 2011, 81, 1–21. [Google Scholar] [CrossRef]
- D’Onofrio, R.; Luciani, V.; Giusberti, L.; Fornaciari, E.; Sprovieri, M. Tethyan planktic foraminiferal record of the early Eocene hyperthermal events ETM2, H2 and I1 (Terche section, northeastern Italy). Rend. Online Soc. Geol. Ital. 2014, 31, 66–67. [Google Scholar] [CrossRef]
- D’Onofrio, R.; Luciani, V.; Fornaciari, E.; Giusberti, L.; Boscolo Galazzo, F.; Dallanave, E.; Westerhold, T.; Sprovieri, M.; Telch, S. Environmental perturbations at the early Eocene ETM2, H2, and I1 events as inferred by Tethyan calcareous plankton (Terche section, northeastern Italy). Paleoceanography 2016, 31, 1225–1247. [Google Scholar] [CrossRef]
- Ruddy, G. An overview of carbon and sulfur cycling in marine sediments. In Biogeochemistry of Intertidal Sediments; Jickells, T.D., Rae, J.E., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 98–118. [Google Scholar]
- Berggren, W.A.; Pearson, P.N. A revised tropical to subtropical Paleogene planktic foraminiferal zonation. J. Foraminifer. Res. 2005, 35, 279–298. [Google Scholar] [CrossRef]
- Luciani, V.; Giusberti, L.; Agnini, C.; Backman, J.; Fornaciari, E.; Rio, D. The Paleocene–Eocene Thermal Maximum as recorded by Tethyan planktonic foraminifera in the Forada section (northern Italy). Mar. Micropaleontol. 2007, 64, 189–214. [Google Scholar] [CrossRef]
- Shackleton, N.J.; Corfield, R.M.; Hall, M.A. Stable isotope data and the ontogeny of Paleocene planktonic foraminifera. J. Foraminifer. Res. 1985, 15, 321–336. [Google Scholar] [CrossRef]
- Pearson, P.N.; Ditchfield, P.W.; Singano, J.; Harcourt-Brown, K.G.; Nicholas, C.J.; Olsson, R.K.; Shackleton, N.J.; Hall, M.A. Warm tropical sea surface temperatures in the Late Cretaceous and Eocene epochs. Nature 2001, 413, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Quillévéré, F.; Norris, R.D.; Moussa, I.; Berggren, W.A. Role of photosymbiosis and biogeography in the diversification of early Paleogene acarininids (planktonic foraminifera). Paleobiology 2001, 27, 311–326. [Google Scholar] [CrossRef]
- Arenillas, I.; Molina, E.; Schmitz, B. Planktic foraminiferal and 13C isotopic changes across the Paleocene/Eocene boundary at Possagno (Italy). Int. J. Earth Sci. 1999, 88, 352–364. [Google Scholar] [CrossRef]
- Molina, E.; Arenillas, I.; Pardo, A. High resolution planktic foraminiferal biostratigraphy and correlation across the Palaeocene Palaeocene/Eocene boundary in the Tethys. Bull. Soc. Géol. Fr. 1999, 170, 521–530. [Google Scholar]
- Ernst, S.R.; Guasti, E.; Dupuis, C.; Speijer, R.P. Environmental perturbation in the southern Tethys across the Paleocene/Eocene boundary (Dababiya, Egypt): Foraminiferal and clay mineral records. Mar. Micropaleontol. 2006, 60, 89–111. [Google Scholar] [CrossRef]
- Guasti, E.; Speijer, R.P. The Paleocene–Eocene thermal maximum in Egypt and Jordan: An overview of the planktic foraminiferal record. Geol. Soc. Spec. Pap. 2007, 424, 53–67. [Google Scholar]
- Schmitz, B.; Pujalte, V. Abrupt increase in seasonal extreme precipitation at the Paleocene-Eocene boundary. Geology 2007, 35, 215–218. [Google Scholar] [CrossRef]
- Giusberti, L.; Rio, D.; Agnini, C.; Backman, J.; Fornaciari, E.; Tateo, E.; Oddone, M. Mode and tempo of the Paleocene–Eocene thermal maximum in an expanded section from the Venetian pre-Alps. Geol. Soc. Am. Bull. 2007, 119, 391–412. [Google Scholar] [CrossRef]
- Schulte, P.; Scheibner, C.; Speijer, R.C. Fluvial discharge and sea-level changes controlling black shale deposition during the Paleocene–Eocene Thermal Maximum in the Dababiya Quarry section, Egypt. Chem. Geol. 2011, 285, 167–183. [Google Scholar] [CrossRef]
- Slotnick, B.S.; Dickens, G.R.; Nicolo, M.J.; Hollis, C.J.; Crampton, J.S.; Zachos, J.C.; Sluijs, A. Large-amplitude variations in carbon cycling and terrestrial weathering during the latest Paleocene and earliest Eocene: The Record at Mead Stream, New Zealand. J. Geol. 2012, 120, 487–505. [Google Scholar] [CrossRef]
- Pujalte, V.; Baceta, J.I.; Schmitz, B. A massive input of coarsegrainedsiliciclastics in the Pyrenean Basin during the PETM: The missing ingredient in a coeval abrupt change in hydrological regime. Clim. Past 2015, 11, 1653–1672. [Google Scholar] [CrossRef]
- Hallock, P. Fluctuations in the trophic resource continuum: A factor in global diversity cycles? Paleoceanography 1987, 2, 457–471. [Google Scholar] [CrossRef]
- Schmidt, D.N.; Thierstein, H.R.; Bollmann, J. The evolutionary history of size variation of planktic foraminiferal assemblages in the Cenozoic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 212, 159–180. [Google Scholar] [CrossRef]
- Schmidt, D.N.; Lazarus, D.; Young, J.R.; Kucera, M. Biogeography and evolution of body size in marine plankton. Earth Sci. Rev. 2006, 78, 239–266. [Google Scholar] [CrossRef]
- Atkinson, D.; Ciotti, B.J.; Montagnes, D.J.S. Protists decrease in size linearly with temperature ca. 2.5 %°C−1. Proc. R. Soc. Lond. B 2003, 270, 2605–2611. [Google Scholar] [CrossRef]
- Henehan, M.J.; Evans, D.; Shankle, M.; Burke, J.; Foster, G.L.; Durrant, J.; Anagnostou, E.; Chalk, T.B.; Stewart, J.A.; Claudia, H.S.; et al. Size-dependent response of foraminiferal calcification to seawater carbonate chemistry. Biogeosceinces 2017, 14, 3287–3308. [Google Scholar] [CrossRef]
- Zeebe, R.E.; Zachos, J.C.; Dickens, G.R. Carbon dioxide forcing alone insufficient to explain Palaeocene–Eocene Thermal Maximum warming. Nat. Geosci. 2009, 2, 576–580. [Google Scholar] [CrossRef]
- Premoli Silva, I.; Boersma, A. Atlantic Eocene planktonic foraminiferal historical biogeography and paleohydrographic indices. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1988, 67, 315–356. [Google Scholar] [CrossRef]
- Pearson, P.N.; Coxall, H.K. Origin of the Eocene planktonic foraminifer Hantkenina by gradual evolution. Palaeontology 2014, 57, 243–267. [Google Scholar] [CrossRef]
- Kast, E.R.; Stopler, D.A.; Auderset, A.; Higgins, J.A.; Ren, H.; Wang, X.T.; Martinez-Garcìa, A.; Haug, G.H.; Sigman, D.M. Nitrogen isotope evidence for expanded suboxia in the early Cenozoic. Science 2019, 364, 386–389. [Google Scholar] [CrossRef]
| Sample Depth (rmcd) | Morozovellids >300 µm (%) | Morozovellids 250−300 µm (%) | Morozovellids 200–250 µm (%) | Events |
|---|---|---|---|---|
| 77.8 | 47.7 | 42.8 | 8.3 | |
| 86 | 27.6 | 60.4 | 12 | |
| 98.53 | 32.3 | 60.1 | 7.6 | |
| 100.06 | 24 | 52 | 24 | |
| 106.06 | 24 | 53 | 24 | |
| 106.37 | 7.4 | 69.6 | 23 | J-2 |
| 106.87 | 22.1 | 56.5 | 21.4 | |
| 107.27 | 9.5 | 21.6 | 68.9 | J-1 |
| 107.87 | 16.8 | 54.5 | 28.7 | morozovellid abundance decline |
| 108.37 | 22.4 | 37.3 | 40.3 | |
| 112.37 | 4.1 | 19.5 | 76.4 | |
| 116.63 | 27.3 | 37.2 | 35.3 | |
| 123.21 | 47.9 | 31 | 21.1 | |
| 130.58 | 29.1 | 36 | 34.9 | |
| 262.24 | 14 | 44 | 42 | |
| 265.43 | 4 | 25 | 71 | |
| 267.83 | 14 | 58 | 28 | L-2 |
| 269.33 | 41 | 32 | 27 | L-1 |
| 271.43 | 36 | 53 | 11 | |
| 274.813 | 11 | 45.5 | 44.5 | K/X |
| 277.12 | 13 | 58 | 27 | morozovellid abundance decline |
| 278.128 | 5 | 32 | 63 | |
| 280.77 | 24 | 32 | 43 | J-1 |
| 283.272 | 24.5 | 42 | 33.5 | |
| 287.309 | 22 | 48 | 30 | I-2 |
| 287.76 | 8 | 40 | 52 | I-1 |
| 292.19 | 17 | 44 | 39 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Onofrio, R.; Luciani, V.; Dickens, G.R.; Wade, B.S.; Kirtland Turner, S. Demise of the Planktic Foraminifer Genus Morozovella during the Early Eocene Climatic Optimum: New Records from ODP Site 1258 (Demerara Rise, Western Equatorial Atlantic) and Site 1263 (Walvis Ridge, South Atlantic). Geosciences 2020, 10, 88. https://doi.org/10.3390/geosciences10030088
D’Onofrio R, Luciani V, Dickens GR, Wade BS, Kirtland Turner S. Demise of the Planktic Foraminifer Genus Morozovella during the Early Eocene Climatic Optimum: New Records from ODP Site 1258 (Demerara Rise, Western Equatorial Atlantic) and Site 1263 (Walvis Ridge, South Atlantic). Geosciences. 2020; 10(3):88. https://doi.org/10.3390/geosciences10030088
Chicago/Turabian StyleD’Onofrio, Roberta, Valeria Luciani, Gerald R. Dickens, Bridget S. Wade, and Sandra Kirtland Turner. 2020. "Demise of the Planktic Foraminifer Genus Morozovella during the Early Eocene Climatic Optimum: New Records from ODP Site 1258 (Demerara Rise, Western Equatorial Atlantic) and Site 1263 (Walvis Ridge, South Atlantic)" Geosciences 10, no. 3: 88. https://doi.org/10.3390/geosciences10030088
APA StyleD’Onofrio, R., Luciani, V., Dickens, G. R., Wade, B. S., & Kirtland Turner, S. (2020). Demise of the Planktic Foraminifer Genus Morozovella during the Early Eocene Climatic Optimum: New Records from ODP Site 1258 (Demerara Rise, Western Equatorial Atlantic) and Site 1263 (Walvis Ridge, South Atlantic). Geosciences, 10(3), 88. https://doi.org/10.3390/geosciences10030088






