Abstract
Carrier screening, a nearly half-century old practice, aims to provide individuals and couples with information about their risk of having children with serious genetic conditions. Traditionally, the conditions for which individuals were offered screening depended on their self-reported race or ethnicity and which conditions were seen commonly in that population. This process has led to disparities and inequities in care as the multi-racial population in the U.S. has grown exponentially, yet databases used to determine clinical practice guidelines are made up of primarily White cohorts. Technological advancements now allow for pan-ethnic expanded carrier screening (ECS), which screens for many conditions regardless of self-reported race or ethnicity. ECS presents a unique opportunity to promote equitable genetic testing practices in reproductive medicine. However, this goal can only be achieved if we acknowledge and appreciate the innumerable inequities evidenced in reproductive medicine and other socio-legal practices in the United States, and if we intentionally work in concert with healthcare providers, policy makers, advocates, and community health champions to reduce current and future reproductive health disparities. Herein, we provide a brief review of the way that US medical racism and genetic discrimination has shaped the current landscape of carrier screening.
1. Introduction
Counseling on reproductive genetic screening and testing options can be a complex process that many healthcare providers feel ill-equipped to execute [1,2,3]. The clinician’s consideration of which screens and tests to offer and, furthermore, to whom these evaluations should be offered, may be influenced by many factors, including limited genetics education within medical training, existing professional society guidelines, marketing initiatives from commercial laboratories, perceptions of a patient population’s desire for genetic information, the healthcare coverage status of the patient, and more. Even when genetic testing is offered to a patient considering reproduction, past and present atrocities within the United States’ history and healthcare research influence the understanding and desire of patients to partake in reproductive genetic testing, such as genetic carrier screening. Without a deeper appreciation of the history and implications of genetic screening as it pertains to human reproduction, a risk exists for inconsistent and, thus, inequitable provision of reproductive genetic testing, which, then, carries the distinct risk of preserving elements of systemic racism within the United States healthcare system. A paucity of literature exists at the intersection of clinical reproductive genetics, immigration policy, and scientific racism. This paper provides a macro lens on these issues and offers insight into the historical and sociological evolution of a specific application of reproductive genetic testing as it pertains to patients, providers, and payers alike.
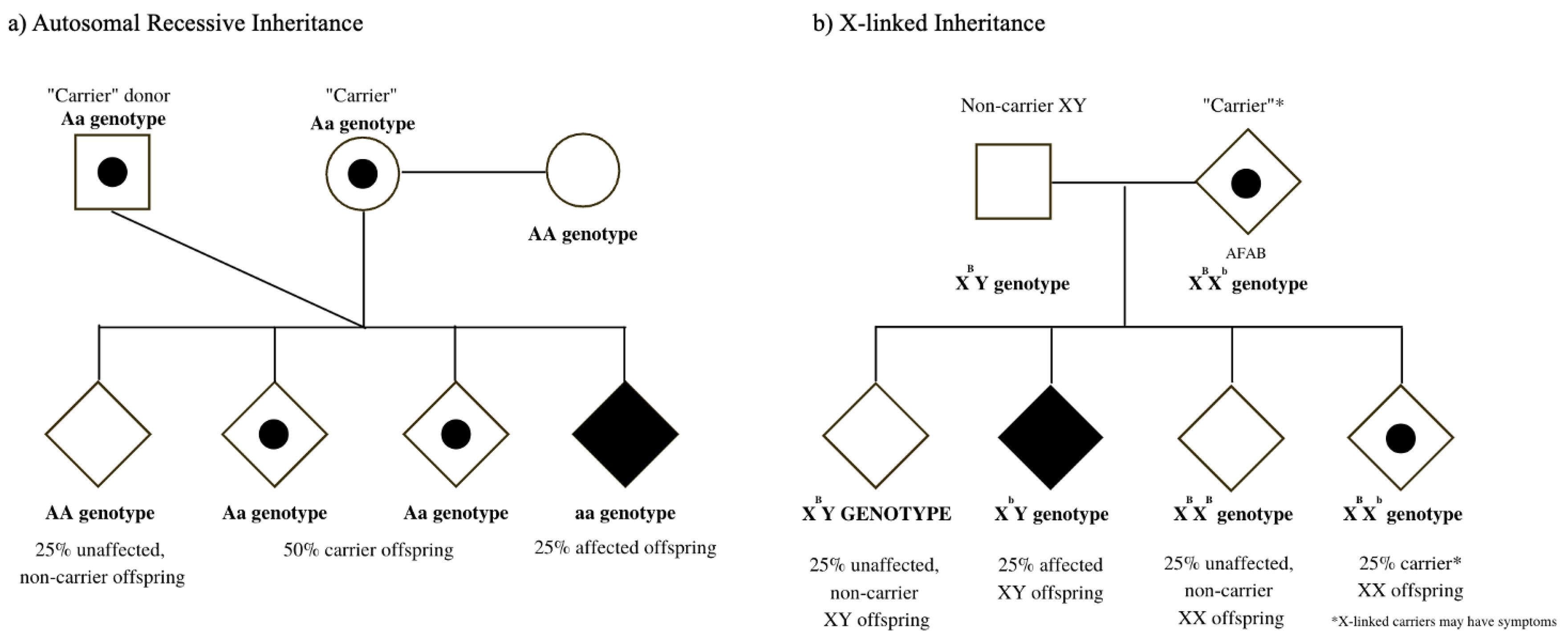
The goal of reproductive genetic evaluations, such as carrier screening, is to provide individuals with meaningful genetic health information related to their “carrier” status for inherited diseases that typically present in the infantile or early-childhood period. Persons undergoing carrier screening can, thereby, employ information from their test results to make reproductive decisions that align with their personal values, goals, and needs [4]. As birth is not the only outcome of pregnancy, carrier screening is most useful either as a preconceptual or early pregnancy tool for the individual whose goal is the conception and delivery of a liveborn child. Typically, carrier screening involves screening for both autosomal recessive diseases (in which an affected pregnancy/child inherits two non-functional gene copies, Figure 1a) as well as X-linked diseases (in which an offspring with XY chromosomes would manifest the disease, whereas offspring with XX chromosomes may be at risk for symptoms related to the disease, Figure 1b).
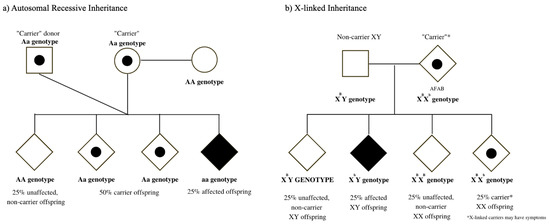
Figure 1.
(a) Autosomal recessive inheritance pattern: same-sex couple conceiving using a sperm donor (b) X-linked inheritance pattern: non-binary individual AFAB (assigned female at birth) who is a manifesting carrier.
Despite the rapid evolution of “expanded” or “universal” carrier screening panels since 2010, there is no carrier screening panel which can examine one’s reproductive risk for all autosomal recessive and X-linked diseases, given continual discoveries of new genes and ongoing gene/disease associations.
Those who are offered carrier screening often have limited familiarity with the concepts of autosomal recessive and X-linked inheritance patterns beyond what may be recalled from secondary education, when applicable. Sparse knowledge or recollection of human genetics concepts, together with societal stigma and misconceptions regarding what it can mean to carry a gene “mutation” or variant, can create or cement barriers in the patient/provider relationship upon discussion of the ubiquitous nature of genetic carrier status. Minority and/or oppressed populations in the United States are well documented to face disproportionate rates of disease due to existing health disparities [5]. Many years of medical mistreatment, extraction, and deceptions on these populations have understandably fostered a sense of distrust in many communities given the ramifications of these acts for many subsequent generations. Even today, a clinician’s offering of genetic carrier screening might still reasonably be perceived as an accusation of having “bad genes” and as such negatively influence a patient’s receptivity to a potentially useful clinical tool.
Herein, we discuss carrier screening, within the historical context of medical racism and genetic discrimination in the United States in order to review the manner in which past atrocities hold a mirror to the current inequities that further exacerbate healthcare disparities between those who do and do not receive the best care in reproductive medical care [6,7].
2. “Bad Genes” in the United States of America
Public attitudes regarding the possession of “bad genes” as well as broad skepticism or outright distrust in government-sponsored research targeting systematically marginalized communities, are undoubtedly rooted in the simultaneous emergence of the human genetics field alongside the United States’ eugenics movement in the early 1900s [7,8]. This period in our country’s history bore witness to numerous social “fitness” strategies such as forced sterilization, immigration restrictions, anti-miscegenation laws, and genetic discrimination.
In an effort to curb social problems and ailments such as poverty, “mental and temperamental” traits, or physical disability, eugenicists sought to prevent “unfit” individuals from having children [9]. Forced sterilizations through the removal of a person’s uterus without their consent and, at times, without their knowledge, were numerous and unapologetically performed, earning the nickname “Mississippi appendectomies” [10]. By World War II, over 60,000 individuals had undergone involuntary sterilization, and most of these individuals were poor people of color [11,12]. Suspiciously high rates of sterilization in both individuals experiencing incarceration and immigrants in custody at state detention centers have been observed even in recent years, over a century after the United States’ first compulsory sterilization law was enacted in Indiana [13,14].
Immigration control and marriage restrictions were two other important tools within the eugenics movement which, from the perspective of genomic ancestry, enormously shaped our present understanding of the genetic composition of United States citizens and residents. From colonial times onward, attitudes towards those perceived as “unfit” resulted in the passing of many xenophobic, racist, and classist laws. Examples of such legislation include the Chinese Exclusion Act of 1882, which barred all immigration from China save for select professionals, enacted in response to the immigration of Chinese workers during the California Gold Rush; the 1917 Immigration Act or Asiatic Barred Zone Act, which not only mandated literacy tests to all immigrants, but also prohibited immigration from nearly all of Asia and the Middle East, save for certain professionals; the 1921 Immigration Restriction Act, which imposed numerical limits so that the number of immigrants entering the country would not exceed 3% of the size of that foreign-born population residing in the United States as represented in the 1910 Census; and the 1924 Johnson–Reed Act, which amended the 1921 Immigration Act so that the new immigration quota would be set at 2% of the foreign-born population residing in the United States as represented in the 1890 Census [15]. This federal law, influenced by eugenic philosophy of the era, was revolutionary in its effective reversal of time, ensuring that the Baby Boomer generation would remember the U.S. of their childhood as one where those afforded the most privilege in the country were White Americans of Northern and Western European, Protestant descent [16]. American culture had long dictated that marriages must not occur across perceived racial and ethnic lines, resulting in generations of people with a single racial and/or ethnic identity. The Supreme Court ruling in Loving v. the Commonwealth of Virginia, which declared all anti-miscegenation laws unconstitutional, legitimized the unions of the few Americans at the time (3%) who were married to a person of a different race or ethnicity. Intermarriage rates have increased significantly since this ruling; in 2015, 17% of newlyweds reported being in an interracial marriage and, in 2020, the U.S. Census offered free-response lines to further describe the racial backgrounds of those who checked a box for Black or White [17].
Importantly, as is evidenced by the sheer amount of time between the arrival of the first enslaved Africans in the American colonies and the modern Civil Rights movement, the Black American population represents one primary U.S. demographic for whom medical, legal, and social protections and privileges have not extended across the majority of U.S. history [18]. For centuries, these individuals were forcibly displaced from their home countries and enslaved by colonizers, where the system of slavery was sustained in many ways, including through reproductive control [19,20,21].
Even when the Preliminary Emancipation Proclamation of 1862 freed those enslaved in rebellious states, the culture of enslaving blocked proper communication of the Thirteenth Amendment to the United States Constitution in 1865 to all enslaved peoples [21,22]. The true end of slavery did not occur until 19th June 1865 [23]. These instances serve as historical anchors to the repeated occurrences of the blocking of information and upholding of unequal status regardless of what laws and, as we later discuss, which guidelines, are in place.
Today, Black Americans continue to face disproportionate access to equal quality clinical care and may demonstrate suspicion towards available offerings due to flagrant ethical violations, such as those witnessed in the infamous, what we now refer to as, U.S. Public Health Service (USPHS) Syphilis Study at Tuskegee [24,25]. Advertisements circulated in Macon County, Alabama in 1932 seemed to present a community health initiative from government officials and healthcare providers, offering a “Free Blood Test; Free Treatment, By County Health Department and Government Doctors,” and cautioning that those eligible for study participation “MAY FEEL WELL AND STILL HAVE BAD BLOOD [sic]” [26]. As we now know, the goal of this study was to observe the course of syphilis in adult Black American males over the lifespan [27]. Despite funding and oversight from the U.S. Public Health Service, information regarding the syphilis diagnosis, as well as information regarding penicillin treatment once identified in 1943, was intentionally withheld from Tuskegee study participants and their partners, the sexual health of whom would have been directly impacted by this knowledge [28]. This health information withholding extended even to the procurement of federal exemptions from the World War II draft, for participants, as syphilis treatment would have been administered in the U.S. armed forces at that time [10].
When citing historically well-founded concerns centered on informed consent in healthcare, many in the Black community may also reasonably point to the case of Henrietta Lacks. A Black American mother from Baltimore, Ms. Lacks complained of vaginal bleeding in 1951 when she presented to the Johns Hopkins Hospital, one of the few local hospitals treating Black patients, albeit in segregated wards [29]. Following the diagnosis of epidermoid carcinoma of the cervix, tissue obtained from Ms. Lacks’ cervical biopsy was kept in a research lab and thrived, resulting in an immortal cell line now dubbed “HeLa” cells that was freely shared with researchers worldwide. The HeLa cell line has contributed to numerous medical achievements in the decades that followed, which include the discovery of Hydroxyurea for sickle cell anemia treatment, treatment for certain forms of cancer, Ebola and HIV research, as well as understanding SARS-CoV2 infections [30,31,32]. However, given the lack of informed consent practices of the 1950s, it is unclear if Ms. Lacks was aware that her biological specimen would be collected and used for medical and clinical research; furthermore, though individuals have won Nobel Prizes as a result of research conducted using HeLa cells, her family has not profited from the gains received by biotechnology companies that have developed profitable treatments as a result of work using HeLa cells [33]. Well-founded qualms surrounding informed consent in medical research and DNA testing are certainly not isolated to the Black community, however. Following the 2004 lawsuit Havasupai Tribe of the Havasupai Reservation v. Arizona Board of Regents, members of the Havasupai Tribe were awarded monetary compensation and their DNA samples were returned due to the unethical use of these samples for schizophrenia, inbreeding, and evolutionary genetics research unrelated to the original diabetes research for which participants had provided consent [34,35].
As was the expressed concern of the Havasupai Tribe, the inherently personal nature of genetic testing raises distinct concerns surrounding the possibility of discrimination based on genetic information. This has been very clearly exemplified through sickle cell testing in the past half-century. In 1971, millions of dollars were earmarked for the research and treatment of sickle cell disease following the community initiatives of the Black Panther Party (BPP) [36]. The BPP created 13 People’s Free Medical Clinics across the United States and provided community education and screening for sickle cell trait through their clinics as a means of championing healthcare as a human right first for Black Americans, and more broadly for the poor and oppressed [36]. Yet, by 1972, mandatory sickle cell testing had been implemented in some states and discriminatory practices were created, denying educational opportunities, employment, and insurance, all based on carrier status for sickle cell (hemoglobin S) trait—a genotype which is not typically associated with hemolytic symptoms beyond fatigue at very high altitudes [37,38,39]. In 1981, the U.S. Military was instructed to identify all personnel with sickle cell disease (hemoglobin SS), sickle cell trait (hemoglobin AS), and glucose-6-phosphate dehydrogenase (G6PD) deficiency and terminate their military careers [40]. This policy shaped occupational outcomes for an entire generation, as it was not until 2015 when the policy was modified and sickle cell screening became required only for specific situations, with employment no longer being automatically terminated upon screen-positive status; however, G6PD deficiency screening remains a universal requirement [41]. As of 2021, all U.S. Air Force personnel are required to answer questions regarding their status for sickle cell trait, and the U.S. Army has reinstated their mandatory testing policy for all recruits [42]. Mandatory sickle cell testing is also seen in civilian populations today through the NCAA’s 2010 eligibility requirement that all Division I athletes undergo screening due to concern for exertional deaths and subsequent lawsuits [43].
Genetic testing has become more voluntarily sought out with the increasing availability and accessibility of genetic tests following the completion of the Human Genome Project. Though the 2008 Genetic Information Nondiscrimination Act (GINA) affords some protections against genetic discrimination in health insurance and employment, the protections of this federal law do not extend to protection from discrimination in obtaining life insurance, long term care insurance, or long-term disability insurance [44].
In the present day, discriminatory practices in genetic testing more broadly exist in the form of (1) whether a person is offered testing and informed consent for such testing, (2) what type of testing is offered, and (3) whether the person has access to adequate health insurance coverage or the financial resources necessary to complete any testing which may be offered. Given the shared applicability of genetic testing information to genetic relatives, the structural racism embedded within these discriminatory practices negatively impacts not just individual patients, but, potentially entire communities of individuals.
Obstetrician/gynecologist (OB/GYN) providers are typically the primary source of information for genetic testing as it may pertain to reproduction for many individuals, either prior to or during a pregnancy. According to the American College of Obstetricians & Gynecologists (ACOG) guidelines, if a patient reports that they are interested in becoming pregnant within the next year, counseling with recommendations on how to achieve a healthy pregnancy, including offering genetic carrier screening, should occur [45]. However, given that 45% of all pregnancies are unplanned, and no recommendations exist on what, if any, genetic testing may be appropriate to offer routine gynecological patients who are not otherwise planning a pregnancy, many future patients are immediately excluded from the opportunity to prospectively consider reproductive genetic risks [46].
Furthermore, many individuals residing within the United States do not have affordable access to health insurance outside of an ongoing pregnancy, as Medicaid qualification has historically required both very low income as well as pregnancy, disability, or motherhood of a child under 18 years old. While the 2014 Affordable Care Act allowed states to expand Medicaid eligibility to most individuals with incomes less than 138% of the federal poverty level, regardless of parenthood or disability status, many gaps in coverage still exist [47,48]. Without consistent access to health insurance, patients are unlikely to receive preconception counseling from a healthcare provider on the availability and potential utility of genetic carrier screening in advance of a pregnancy. Those who do receive counseling and education on carrier screening at any point in their reproductive lifespan may be inherently subjected to a provider’s implicit biases surrounding what type of carrier screening is appropriate, or what family planning actions are considered typical following the identification of new genetic information. Gatekeeping is not a phenomenon unique to reproductive medicine, yet, in this context, it presents an important opportunity for consideration of equitable access to genetic information that may shape family planning decisions [49].
3. The Evolution of Genetic Carrier Screening
The identification of prospective parents at risk to have a child with a serious inherited disorder via carrier screening is a long-standing core component of reproductive health care. Carrier screening can be performed during or before pregnancy and is typically ordered by providers of routine reproductive care, such as obstetrician-gynecologists, maternal-fetal medicine specialists, physician assistants, midwives, or nurse practitioners, as well as genetic counselors. However, with the availability of direct-to-consumer (DTC) testing, many individuals are also receiving carrier screening information through this mechanism though results may need to be confirmed in a clinical setting [50]. In distinct contrast to many other public health initiatives focused upon disease prevention, the broad goal of carrier screening is instead to promote autonomy in reproduction. Through the identification of at-risk individuals, couples, or other reproductive pairs, patients can receive education about their reproductive risks and available options and make informed decisions accordingly [4]. Today, more than ever before, reproductive options for those with access to appropriate resources may involve any combination of the following possibilities: undergoing in vitro fertilization (IVF) with pre-implantation genetic testing (PGT) to reduce the likelihood of an affected pregnancy through selective IVF transfer of only those embryos with normal/negative PGT results; conceiving using an oocyte donor or sperm donor who does not have the hereditary trait; pursuing prenatal diagnosis with or without plans for abortion, or with or without plans for in utero fetal therapy when applicable [51]; choosing to deliver at a tertiary care center with a high-level NICU in the event that the infant has the hereditary condition; commencing targeted work-up in the immediate postnatal period; electing to parent through fostering or through adoption; or choosing not to have children.
While the goal of carrier screening has not changed, laboratories now possess the ability to screen one blood or saliva sample for variants in multiple genes simultaneously due to advances in next-generation DNA sequencing. This process of efficient testing for many genes at once has historically been called expanded carrier screening (ECS), and in future years may even be the concept to which the phrase “carrier screening” is intended to refer in place of carrier screening as offered based on a person’s self-reported racial or ethnic background [52].
Despite the inaccuracy, both the general public as well as clinicians and researchers tend to use the concepts of race, ethnicity, and ancestry interchangeably. As defined by Popejoy et al., the first two of these concepts, race and ethnicity, are considered to be social constructs and not indicative of genetic risk factors for disease, while the latter, ancestry, is what refers to the genetic inheritance of variants from global ancestral populations [53]. Although race and ethnicity are both social constructs, they are used prominently within medical care, a practice which negatively impacts all elements of medicine from scientific research to clinical guidelines and clinical practice [54].
In the 1970s, prior to the availability of molecular-based DNA testing, limited carrier screening options were available for members of racial or ethnic groups in which certain conditions are observed with a high incidence, such as Tay–Sachs disease in the Ashkenazi Jewish population and sickle-cell disease in the African and Black American populations [55]. This approach set the stage for carrier screening based on an individual’s self-reported race or ethnicity. The first challenge to this proposed race/ethnicity-based approach came shortly after, when CFTR, the gene associated with cystic fibrosis (CF), was identified and testing became readily available. In 2001, professional medical societies recommended CF screening be offered to individuals of European, non-Hispanic descent [56,57]. However, within only five years, professional guidance changed so that CF screening should be offered on a “pan-ethnic” basis, given consensus on the difficulty of assigning a single race or ethnicity to every patient presenting for care in the U.S. [58,59].
In order for race/ethnicity-based screening to be effective, a person’s racial and ethnic background must be easily obtained, accurate, and well-studied within the medical literature so that carrier frequency and disease incidence data are known. In practice, however, a patient’s race and/or ethnicity may not be disclosed and is often an imperfect indicator of genetic ancestry [60]. Each known inherited disease has not necessarily been described in every racial or ethnic group ethnicity, impeding the ability to calculate allele frequency in all populations. Many commercial genetic testing laboratories have disease risk information for some racial/ethnic groups but revert to a global risk for ethnicities in which specific data is not available.
Historically, medical society guideline recommendations have been inconsistent and driven by a patient’s self-reported race or ethnicity. Individuals are tested for different conditions depending on their race or ethnicity and/or the medical society recommendation that their provider followed. However, the 2020 United States Census demonstrated that the multiracial population in the United States increased by 276% since the census prior [61]. One out of every six new marriages are between individuals of different ethnic backgrounds [17]. Forty percent of Americans cannot correctly identify the ancestry of all four grandparents [62]. Furthermore, even when an individual self-reports their race and/or ethnicity, 1 in 10 individuals had a 50% or greater genetic ancestry different from what they self-reported [60]. Additionally, direct to consumer testing has played a major role in informing individuals of their genetic ancestry though how this information is used to direct clinic care remains unseen. Medical guidelines currently have no recommendations for management of individuals who self-report blended ancestry, who are adopted, or who are otherwise uncertain of their ancestry, highlighting the manner in which current guidelines and practices do not reflect the diversity of the patient population served.
Despite recognition of its flaws, race/ethnicity-based carrier screening paradigms persisted well into the 2010s, primarily due to the limitations of costly and slow screening assays. While multi-gene panels had already been incorporated for hereditary cancer evaluations in the early 2000s, the first multi-gene panel for carrier screening was not introduced commercially until 2010, when a panel including over 100 autosomal recessive and X-linked disorders was made available for any person desirous of this information [63]. Initially, carrier screening panels analyzed a unique set of known variants for each gene—a process referred to as genotyping. While the multi-gene analysis afforded through ECS offered an improvement over single-gene testing, genotyping assays of carrier screening still carried distinct limitations in genetic testing equity, as variants included were most common in White populations (as these are the populations that have been the most studied). Eventually, next-generation high-throughput sequencing replaced genotyping, thereby, significantly expanding detection of disease-causing variants across all ancestries [64,65,66,67]. While panels may vary between labs, professional medical societies have issued criteria for the types of diseases that should and should not be included, as well as guidance regarding classification of genetic variants [4,52].
Decisions regarding whether and how to use information about genetic carrier status relies on individuals having access to obtaining this information. When ECS is offered during pregnancy, the uptake rate is around 50% and may be as high as 77% if offered before pregnancy [68,69,70]. Studies of at-risk carrier couples highlight that most (77%) used the information learned through ECS to make changes to their reproductive planning when they learned it prior to pregnancy; over a third pursued prenatal diagnosis when they learned the news during pregnancy [70]. Yet, many prospective parents who might elect for more comprehensive screening are not being informed that such screening is an option. Less than 30% of reproductive care providers surveyed in 2018 reported routinely offering ECS, and 90% reported that they routinely offered race/ethnicity-based carrier screening, despite the limitations of this screening being well described [71]. The tendency to offer minimal screening options in place of alternatives with more comprehensive coverage is likely even more pronounced for minority populations, who are known to utilize genetic testing at disproportionately lower rates [72]. Societal guidelines, medical practice, and insurance coverage all impact the ability for individuals to access information that they may find useful prior to reproductive decision-making. The goal of prenatal genetic screening is to “provide individuals with meaningful information they can use to guide pregnancy planning based on their personal values,” but, unfortunately, information on the availability and potential utility of ECS is not uniformly provided to all who may benefit [4].
It is vital that we recognize, acknowledge, and understand the role that race and ethnicity-based constructs have played in upholding structural racism not only in society but also within healthcare. While these constructs may still be helpful as part of assessments to understand health differences, it is important that clinicians examine how race and ethnicity are used to impact the care they provide. We cannot eliminate racial determinants of health when examining historical reproductive care access, utilization, and barriers. However, using historical ethnicity and race in forming clinical guidelines will further restrict universal and equitable access to carrier screening and informed reproductive planning.
4. Professional Society Guidelines
As utilization of ECS has become more widespread, professional medical societies have responded by issuing position statements and practice resources to guide both ordering providers and laboratories. While such guidelines aim to provide specificity and practicality in daily use, inconsistencies and gaps remain, particularly as perceived by payers.
The National Institutes of Health (NIH) issued one of the first carrier screening recommendations when, in 1997, it recommended that genetic testing for cystic fibrosis should be offered to “all couples currently planning a pregnancy” and to “couples seeking prenatal care” [73]. ACOG and the American College of Medical Genetics and Genomics (ACMG) agreed with NIH in their 2001 guidelines recommending cystic fibrosis (CF) carrier screening, although, while the NIH recommended this screening regardless of race or ethnicity, both ACOG and ACMG recommended that CF screening be offered to non-Jewish Caucasians and Ashkenazi Jews [56]. Individuals of other ethnic and racial groups were recommended “to be informed of CF variant detectability through educational brochures, the informed consent process, and/or other efficient methods” [56]. In 2011, ACOG updated guidelines to state that, as it was becoming “increasingly difficult to assign a single ethnicity to all people with CF”, it was reasonable to offer CF carrier screening to all patients [74]. Over the years, some updates were made regarding the propriety of testing for various conditions, with spinal muscular atrophy (SMA) recommended for pan-ethnic screening by the ACMG [75]. Additionally, both ACOG and ACMG recommended screening for different conditions depending on an individual’s self-reported race or ethnicity (Table 1). A history of carrier screening guidelines can be found in Figure 2.

Table 1.
American College of Obstetricians and Gynecologists (ACOG) and American College of Medical Genetics and Genomics (ACMG) Single-Gene Carrier Screening Guidelines Prior to 2017 *.


Figure 2.
A History of Carrier Screening Guidelines in the United States [4,52,56,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94].
In 2015, a joint statement entitled “Expanded Carrier Screening in Reproductive Medicine—Points to Consider” was issued by ACMG, ACOG, the National Society of Genetic Counselors (NSGC), Perinatal Quality Foundation (PQF), and the Society for Maternal-Fetal Medicine (SMFM) [93]. This early document acknowledged that ECS provides a more comprehensive risk assessment for our increasingly multiethnic society, summarized existing single-gene screening guidance, sought to define the ECS paradigm, and listed critical and unique components of ECS. It also highlighted the need for more thorough and rigorously curated variant databases, more educational resources for providers and patients, and data around cost. While this statement highlighted the benefits of ECS, it stopped short of recommending ECS as an acceptable screening strategy until additional data were available [93].
In 2017, ACOG released two new committee opinions on carrier screening: one that consolidated and replaced all previous single-gene carrier screening guidance and one that stated expanded carrier screening was an acceptable screening strategy and provided guidance on implementing ECS into practice [4,94]. While some providers had already started offering ECS in many parts of the country, this committee opinion reaffirmed that OB-GYN’s and other gynecological providers should offer carrier screening to all couples who were pregnant or planning a pregnancy regardless of race/ethnicity and validated that there were other acceptable screening options beyond race/ethnicity-based screening [4,94]. Another highlight of this guideline is that it included consensus-based criteria for panel development, similar to ACMG’s panel development criteria from four years prior [92]. For a disease to be included on a panel, prenatal diagnosis must be possible, and the condition should meet several of the following criteria: have a heterozygous carrier frequency of at least 1 in 100, have a well-defined phenotype, have a detrimental effect on quality of life, cause cognitive or physical impairment, require surgical or medical intervention, or have onset early in life [4]. Most recently, ACMG published a consensus-based carrier screening practice resource that is more ambitious and in-depth than any prior published guidance on the topic [52]. Through analysis of analytic validity, clinical validity, and clinical utility, ACMG recommended a tier-based approach to carrier screening in which race/ethnicity-based carrier screening paradigms were slotted into lower tiers deemed inappropriate for use in the general population due to well documented limitations. ACMG’s practice resource goes beyond the advice provided by ACOG in recommending that diseases with a carrier frequency of ≥1 in 200 should be routinely included for anyone who is pregnant or considering reproduction. While ACMG went to greater lengths to evaluate and define carrier frequency, inherent limitations exist from the underrepresentation of minority groups within large genomic datasets used to establish carrier frequency for rare disease genes.
On the whole, providers and laboratories alike seem to appreciate the formal recognition and standardization of ECS, but the provision of various professional guidelines has not solved all health equity concerns pertaining to ECS. Importantly, the lack of consistent ECS guidelines across professional societies, together with commercial interest, has resulted in U.S.-based labs offering ECS panels ranging in size from only a few genes to as many as >500 genes. Furthermore, ACOG’s guideline has not been sufficient to drive payers to adopt favorable medical policies; it is too early to determine how payers will respond to ACMG’s 2021 practice resource calling for ECS for all.
5. Payer Coverage of Carrier Screening
Proving most influential for payers thus far is the favorable determination ECS received from the BlueCross BlueShield Association’s evidence review arm, Evidence Street, in late 2020. After reviewing the topic for several years, Evidence Street finally concluded that “the evidence was sufficient to determine that [ECS] results in a meaningful improvement in health outcomes for individuals at increased risk or population risk of having an offspring with an inherited recessive disorder” [95]. Per Policy Reporter, as of August 2021, select commercial payers have medical policies that cover ECS, including Florida Blue, Blue Shield of California, BCBS of Idaho, BCBS of North Carolina, BCBS of South Carolina, BCBS of Nebraska, Highmark of Western New York, BCBS of Kansas City, BCBS of Minnesota, and Health Partners [96]. With the exception of Health Partners, which does require the test to be ordered by a genetic specialist, there are no provider type requirements included in these medical policies. Most other national and regional health plans cover race/ethnicity-based carrier screening only.
Within state Medicaid programs (50 states + DC), 29 state programs cover carrier screening, although this is typically localized to CF and SMA for any prenatal/preconception patient and hemoglobinopathies and/or Ashkenazi Jewish screening if indicated by race/ethnicity. Although there are no state Medicaid programs in which written medical policy endorses ECS, states have 81443, the unique CPT code associated with ECS, open on their fee schedules. Despite some positive momentum, fewer than 5% of commercially covered lives and 2.5% of Medicaid-covered lives have access to ECS [96]. It is worth noting that, in the US, there is disproportionate reliance by people of color on Medicaid and other publicly funded health programs [97,98]. Due to a history of structural racism in the US that has resulted in restricted education and employment opportunities, people of color are overrepresented in the types of jobs that do not provide employer-sponsored health insurance.
As is typical, a primary concern among payers is whether a new paradigm will be cost-effective. Using modeled data, preconception ECS has been shown to reduce the rate of affected births and, therefore, is cost-effective when compared to screening for CF and SMA alone [99]. The increased cost of ECS is also expected to be justified when testing is performed during pregnancy, but these studies are lacking at this time. Yet, the fact that pediatric genetic diseases present a significant and increasing financial burden is one that is widely accepted, particularly given the continued development of new and costly “orphan drugs” which exist solely to treat ultra-rare disorders. One study seeking to quantify this economic impact analyzed national data and found that genetic diseases have a significant and disproportionate impact on resources in the US healthcare system, accounting for up to 46% of the “national bill” for pediatric patients [100]. Another study found that pediatric costs associated with rare disease diagnosis codes accounted for $34 billion more per year than the diagnosis codes associated with common conditions, and that a rare disease diagnosis code in a medical record is the single biggest predictor of healthcare utilization (type, duration, and cost) [101]. While additional cost-effectiveness studies are necessary and important, when existing data are considered through the lens of equity in reproductive care, ECS appears to be a cost-effective tool that can offer broad benefits to all US populations.
6. Carrier Screening as a Public Health Equity Initiative: A Call to Action
The implementation of ECS into routine prenatal care has been nuanced and complex, but this has been and will remain true of any large-scale public health initiative. Population-wide genetic testing deployments present distinct concerns, including (1) what group of diseases is appropriate for screening, (2) whether reproductive healthcare providers are equipped to deliver pre-test counseling and informed consent for genetic testing, particularly in light of concerns pertaining to genetic discrimination, (3) whether reproductive healthcare providers are equipped to deliver post-test counseling for rare diseases, and (4) the manner in which clinics and health systems will be burdened by partner testing and other follow-up after the initial patient’s testing [102]. While these and other clinic- and/or population-specific concerns are legitimate and should be addressed, sufficient consensus exists regarding the need for provider education, and, most importantly, the clinical utility of ECS for any person considering reproduction.
A 2021 publication using a data-driven approach to interpret carrier screening panel design criteria from ACOG and ACMG revealed two different sized panels depending on the societal guideline followed [103]. Following ACOG criteria led to a guidelines-consistent panel of 37 genes, while following ACMG criteria led to a panel of 74 genes [103]. While both panels are technically consistent with medical societal guidelines, they can lead to disparities in care depending on what guideline a provider may follow. This study highlights the need for consistency, clarity, and collaboration when developing guidelines across multiple medical societies. Reproductive healthcare providers, commercial laboratories, medical policy decision makers, community health leaders, and disability justice advocates (including both parent and patient advocates whenever possible) must work in collaboration in order for ECS to be offered equitably in the United States. Many payers, professional organizations, and individual authors have described a need for additional research exploring the clinical utility of ECS in the general population. While such research is undoubtedly integral to a more comprehensive understanding of the benefits, limitations, and applications of ECS in various groups, clinicians should challenge the notion that offering ECS only to those who know to ask for it is an acceptable and equitable practice until the availability of further research previously recommended.
The design and implementation of accessible carrier screening paradigms is important, but it will not erase centuries of health equity issues surrounding reproductive justice in our country in one fell swoop. Equitable carrier screening relies on the input of those with lived experience of inherited disease during laboratory panel development. It must prioritize the inclusion of all racial/ethnic groups, rather than focus on genetic variants observed most commonly in Europeans and their descendants. It must promote data sharing between laboratories, and demonstrate quality variant curation as a chief objective, particularly with regard to variant interpretation for those in systematically marginalized communities. Equitable carrier screening models, furthermore, will promote the ability of all current and future obstetrical patients to be informed of ECS as an optional reproductive health tool. These models must acknowledge the origins of carrier screening in race-based medicine, and even more so the origins of the medical genetics discipline in a troublesome sociological era of United States history that must not be repeated. They must incorporate informed consent templates that allow space for patients to carefully consider their concerns related to disability perceptions and justice; the insinuation of having “bad genes,” the internal reactions accordingly based on historical precedent; genetic discrimination; and the possibility of incidental diagnoses from this testing.
As allies and advocates for reproductive justice, carrier screening stakeholders should work together to promote equitable access to the reproductive options that are theoretically available to at-risk reproductive pairs, but all but out of reach for many in the United States (such as IVF with PGT, a process which requires not only enormous financial resources but also intangible resources such as time away from work for multiple medical appointments). Collaborative efforts should be directed to promoting accessible gynecological care for the under- or uninsured, as well as to ensuring access to comprehensive contraceptive options when desired, and access to abortion as a safe and legal human right.
7. Conclusions
We have illustrated some of the egregious atrocities faced by Black descendants of enslaved people in the United States regarding the pursuit of equitable medical care, and many other minority and Indigenous populations have no doubt encountered similar unequal care upon entry to the US and forced displacement across the US, respectively. With respect to reproductive medicine, expanded carrier screening presents a unique opportunity to promote equitable genetic testing practices. This goal can only be achieved through, first, an appreciation for the innumerable inequities evidenced in reproductive medicine and other socio-legal practices in the United States and, second, the intentional efforts amongst healthcare providers, policy makers, advocates, and community health champions working in concert to reduce current and future reproductive health disparities.
Author Contributions
Conceptualization, K.G.S. and A.B.S.; writing—original draft preparation, A.B.S., D.R.D., A.A., K.G.S.; writing—review and editing, A.B.S., D.R.D., A.A., K.G.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to extend their gratitude to Sally Rodriguez for her review of this manuscript.
Conflicts of Interest
Authors #2 and #3 are clinical reproductive genetic counselors and each order expanded carrier screening products from a variety of commercial laboratories. Authors #1 and #4. are current and former employees of a genetic testing laboratory which offers expanded carrier screening.
References
- Kathrens-Gallardo, A.; Propst, L.; Linn, E.; Pothast, E.; Wicklund, C.; Arjunan, A. OB/GYN residents’ training, attitudes, and comfort level regarding genetics. J. Assist. Reprod. Genet. 2021, 38, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Mikat-Stevens, N.A.; Larson, I.A.; Tarini, B.A. Primary-care providers’ perceived barriers to integration of genetics services: A systematic review of the literature. Genet. Med. 2015, 17, 169–176. [Google Scholar] [CrossRef]
- Mainous, A.G.; Johnson, S.P.; Chirina, S.; Baker, R. Academic family physicians’ perception of genetic testing and integration into practice: A CERA Study. Fam. Med. 2013, 45, 257–262. [Google Scholar] [PubMed]
- American College of Obstetrics and Gynecologists Committee on Genetics. Committee Opinion No. 690: Carrier Screening in the Age of Genomic Medicine. Obstet. Gynecol. 2017, 129, e35–e40. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. The State of Health Disparities in the United States. Communities in action: Pathways to Health Equity; The National Academies Press: Washington, DC, USA, 2017; pp. 2–12. [Google Scholar]
- Hall, M.; Olopade, O.I. Confronting genetic testing disparities: Knowledge is Power. JAMA. 2005, 293, 1783–1785. [Google Scholar] [CrossRef]
- Suther, S.; Kiros, G.E. Barriers to the use of genetic testing: A study of racial and ethnic disparities. Genet. Med. 2009, 11, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.K. Bad habits and bad genes: Early 20th-century eugenic attempts to eliminate syphilis and associated “defects” from the United States. Can. Bull. Med. Hist. 2003, 20, 11–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eugenics Record Office, Cold Spring Harbor Laboratory. Eugenics Seeks to Improve the Natural, Physical, Mental, and Temperamental Qualities of Man. 1927. Available online: http://www.eugenicsarchive.org/eugenics/image_header.pl?id=248&printable=1&detailed=0 (accessed on 21 September 2021).
- Washington, H.A. Medical Apartheid: The Dark History of Medical Experimentation on Black Americans from Colonial Times to the Present; Doubleday: New York, NY, USA, 2006; p. 165. [Google Scholar]
- Reilly, P.R. Eugenics and Involuntary Sterilization: 1907–2015. Annu. Rev. Genomics Hum. Genet. 2015, 16, 351–368. [Google Scholar] [CrossRef]
- Novak, N.L.; Lira, N.; O’Connor, K.E.; Harlow, S.D.; Kardia, S.L.R.; Stern, A.M. Disproportionate Sterilization of Latinos Under California’s Eugenic Sterilization Program, 1920–1945. Am. J. Public Health 2018, 108, 611–613. [Google Scholar] [CrossRef]
- Idle Wild Films Inc., Independent Television Service, Black Public Media. Belly of the Beast; USA. 2021. Available online: https://www.bellyofthebeastfilm.com/ (accessed on 21 September 2021).
- Project South. Re: Lack of Medical Care, Unsafe Work Practices, and Absence of Adequate Protection against COVID-19 for Detained Immigrants and Employees Alike at the Irwin County Detention Center; 2020. Available online: https://projectsouth.org/wp-content/uploads/2020/09/OIG-ICDC-Complaint-1.pdf (accessed on 21 September 2021).
- The 1619 Project. Available online: https://www.nytimes.com/interactive/2019/08/14/magazine/1619-america-slavery.html (accessed on 8 February 2022).
- Joshi, K.Y. White Christian Privilege: The Illusion of Religious Equality in America; NYU Press: New York, NY, USA, 2020. [Google Scholar]
- Livingston, G.; Brown, A. Pew Research Center: Intermarriage in the U.S. 50 Years after Loving v. Virginia. Available online: https://www.pewresearch.org/social-trends/2017/05/18/intermarriage-in-the-u-s-50-years-after-loving-v-virginia/ (accessed on 21 September 2021).
- Roberts, D. Killing the Black Body; Vintage Books: New York, NY, USA, 1998; pp. 21–28. [Google Scholar]
- Taylor, J.K. Structural Racism and Maternal Health Among Black Women. J. Law Med. Ethics 2020, 48, 506–517. [Google Scholar] [CrossRef]
- Tunc, T.E. The mistress, the midwife, and the medical doctor: Pregnancy and childbirth on the plantations of the antebellum American South, 1800–1860. Women’s Hist. Rev. 2010, 19, 395–419. [Google Scholar] [CrossRef] [PubMed]
- National Archives. The Preliminary Emancipation Proclamation & Emancipation Proclamation. Available online: https://www.archives.gov/exhibits/featured-documents/emancipation-proclamation (accessed on 5 November 2021).
- Smithsonian National Museum of African American History & Culture. History of Juneteenth. Available online: https://nmaahc.si.edu/blog-post/historical-legacy-juneteenth (accessed on 5 November 2021).
- Bailey, Z.D.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef]
- Taylor, J. Racism, Inequality, and Health Care for African Americans. The Century Foundation. 2019. Available online: https://tcf.org/content/report/racism-inequality-health-care-african-americans/ (accessed on 5 November 2021).
- Brown, D.L. ‘You’ve Got Bad Blood’: The Horror of the Tuskegee Syphilis Experiment. Washington Post. May 2017. Available online: https://www.washingtonpost.com/news/retropolis/wp/2017/05/16/youve-got-bad-blood-the-horror-of-the-tuskegee-syphilis-experiment/ (accessed on 21 September 2021).
- Park, J. Historical Origins of the Tuskegee Experiment: The Dilemma of Public Health in the United States. Uisahak 2017, 26, 545–578. [Google Scholar] [CrossRef] [PubMed]
- Washington, D.A. Examining the “Stick” of Accreditation for Medical Schools Through Reproductive Justice Lens: A Transformative Remedy for Teaching the Tuskegee Syphilis Study. Available online: https://racism.org/articles/basic-needs/health-and-health-care/health-care/quality-of-health-care/103-cultural-competent-care/1634-tuskegeesylliusstudy01?start=7 (accessed on 8 February 2022).
- Alsan, M.; Wanamaker, M.; Hardeman, R.R. The Tuskegee Study of Untreated Syphilis: A Case Study in Peripheral Trauma with Implications for Health Professionals. J. Gen. Intern Med. 2020, 35, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins Medicine. The Legacy of Henrietta Lacks. Available online: https://www.hopkinsmedicine.org/henriettalacks/index.html (accessed on 21 September 2021).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Significant Research Advances Enabled by HeLa Cells. Available online: https://osp.od.nih.gov/scientific-sharing/hela-cells-timeline/ (accessed on 5 November 2021).
- British Society for Immunology. HeLa Cells. 1951. Available online: https://www.immunology.org/hela-cells-1951 (accessed on 5 November 2021).
- Garrison, N.A. Genomic Justice for Native Americans: Impact of the Havasupai Case on Genetic Research. Sci. Technol. Hum. Values. 2013, 38, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Mello, M.M.; Wolf, L.E. The Havasupai Indian tribe case—Lessons for research involving stored biologic samples. N. Engl. J. Med. 2010, 363, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R. The American Presidency Project. Special Message to the Congress Proposing a National Health Strategy. 1971. Available online: https://www.presidency.ucsb.edu/documents/special-message-the-congress-proposing-national-health-strategy (accessed on 8 September 2021).
- Bassett, M.T. Beyond Berets: The Black Panthers as Health Activists. Am. J. Public Health 2016, 106, 1741–1743. [Google Scholar] [CrossRef]
- Rutkow, I.M.; Lipton, J.M. Some negative aspects of state health departments’ policies related to screening for sickle cell anemia. Am. J. Public Health 1974, 64, 217–221. [Google Scholar] [CrossRef]
- Naik, R.P.; Haywood, C., Jr. Sickle cell trait diagnosis: Clinical and social implications. Hematol. Am. Soc. Hematol. Educ. Program. 2015, 1, 160–167. [Google Scholar] [CrossRef]
- Mitchell, B.L. Sickle cell trait and sudden death—Bringing it home. J. Nat. Med. Assoc. 2007, 99, 300–305. [Google Scholar]
- Department of Defense Instruction. Subject: Hemoglobin S and Erythrocyte Glucose-6-Phosphate Dehydrogenase Deficiency Testing. July 1981. Available online: https://apps.dtic.mil/sti/pdfs/ADA272798.pdf (accessed on 8 September 2021).
- Department of Defense Instruction. Subject: Erythrocyte Glucose-6-Phosphate Dehydrogenase Deficiency (G6PD) and Sickle Cell Testing, July 2015. Available online: https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/646501p.pdf (accessed on 8 September 2021).
- South, T. Army Now Testing New Recruits, Soldiers for Sickle Cell Trait. Army Times. December 2020. Available online: https://www.armytimes.com/news/your-army/2020/12/01/army-now-testing-new-recruits-soldiers-for-sickle-cell-trait/ (accessed on 8 September 2021).
- Tarini, B.A.; Brooks, M.A.; Bundy, D.G. A policy impact analysis of the mandatory NCAA sickle cell trait screening program. Health Serv. Res. 2012, 47, 446–461. [Google Scholar] [CrossRef] [PubMed]
- U.S. Equal Employment Opportunity Commission. Genetic Information Discrimination. Available online: https://www.eeoc.gov/genetic-information-discrimination (accessed on 5 November 2021).
- American College of Obstetrics and Gynecologists. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet. Gynecol. 2019, 133, e78–e89. [Google Scholar] [CrossRef] [PubMed]
- Guttmacher Institute. Unintended Pregnancy in the United States. January 2019 Fact Sheet. Available online: https://www.guttmacher.org/fact-sheet/unintended-pregnancy-united-states (accessed on 5 November 2021).
- 2014 Affordable Care Act Patient Protection and Affordable Care Act of 2010, Pubic Law No. 111–148. 23 March 2010. Available online: https://www.congress.gov/111/plaws/publ148/PLAW-111publ148.pdf (accessed on 21 September 2021).
- Kaiser Family Foundation. Women’s Health Insurance Coverage. 2019. Available online: https://www.kff.org/womens-health-policy/fact-sheet/womens-health-insurance-coverage/ (accessed on 21 September 2021).
- Buchbinder, M. Keeping out and getting in: Reframing emergency department gatekeeping as structural competence. Sociol. Health Illn. 2017, 39, 1166–1179. [Google Scholar]
- Delaney, S.K.; Christman, M.F. Direct-to-consumer genetic testing: Perspectives on its value in healthcare. Clin. Pharmacol. Ther. 2016, 99, 146–148. [Google Scholar] [CrossRef]
- Kreger, E.M.; Singer, S.T.; Witt, R.G.; Sweeters, N.; Lianoglou, B.; Lal, A.; Mackenzie, T.C.; Vichinsky, E. Favorable outcomes after in utero transfusion in fetuses with alpha thalassemia major: A case series and review of the literature. Prenat. Diagn. 2016, 36, 1242–1249. [Google Scholar] [CrossRef]
- Gregg, A.R.; Aarabi, M.; Klugman, S.; Leach, N.; Bashford, M.; Goldwaser, T.; Chen, E.; Sparks, T.N.; Reddi, H.V.; Rajkovic, A.; et al. Screening for autosomal recessive and X-linked conditions during pregnancy and preconception: A practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 1793–1806. [Google Scholar] [CrossRef]
- Popejoy, A.B.; Crooks, K.R.; Fullerton, S.M.; Hindorff, L.A.; Hooker, G.W.; Koenig, B.A.; Pino, N.; Ramos, E.; Ritter, D.I.; Wand, H.; et al. Clinical Genetics Lacks Standard Definitions and Protocols for the Collection and Use of Diversity Measures. Am. J. Hum. Genet. 2020, 107, 72–82. [Google Scholar] [CrossRef]
- Hunt, L.M.; Truesdell, N.D.; Kreiner, M.J. Genes, Race, and Culture in Clinical Care: Racial Profiling in the Management of Chronic Illness. Med. Anthropol. Q. 2013, 27, 253–271. [Google Scholar] [CrossRef]
- Baja, K.; Gross, S.J. Carrier Screening: Past, Present, and Future. J. Clin. Med. 2014, 3, 1033–1042. [Google Scholar] [CrossRef]
- Grody, W.W.; Cutting, G.R.; Klinger, K.W.; Richards, C.S.; Watson, M.S.; Desnick, R.J.; Subcommittee on Cystic Fibrosis Screening, Accreditation of Genetic Services Committee, American College of Medical Genetics. Laboratory standards and guidelines for population-based cystic fibrosis carrier screening. Genet. Med. 2001, 3, 149–154. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists and American College of Medical Genetics. Preconception and Prenatal Carrier Screening for Cystic Fibrosis: Clinical and Laboratory Guidelines; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2001. [Google Scholar]
- American College of Obstetricians and Gynecologists, Committee on Genetics. ACOG Committee Opinion Number 325: Update on carrier screening for cystic fibrosis. Obstet. Gynecol. 2011, 106, 1465–1468, Retraction in Obstet. Gynecol. 2011, 117, 995. [Google Scholar]
- Watson, M.S.; Cutting, G.R.; Desnick, R.J.; Driscoll, D.A.; Klinger, K.; Mennuti, M.; Palomaki, G.E.; Popovich, B.W.; Pratt, V.W.; Rohlfs, E.M.; et al. Cystic fibrosis population carrier screening: 2004 revision of American College of Medical Genetics mutation panel. Genet. Med. 2004, 6, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Kaseniit, K.E.; Haque, I.S.; Goldberg, J.D.; Shulman, L.P.; Muzzey, D. Genetic ancestry analysis on >93,000 individuals undergoing expanded carrier screening reveals limitations of ethnicity-based medical guidelines. Genet. Med. 2020, 22, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- U.S. Census Bureau Improved Race and Ethnicity Measures Reveal, U.S. Population Is Much More Multiracial. 2021. Available online: https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html (accessed on 24 September 2021).
- Condit, C.; Templeton, A.; Bates, B.R.; Bevan, J.L.; Harris, T.M. Attitudinal barriers to delivery of race-targeted pharmacogenomics among informed lay persons. Genet. Med. 2003, 5, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A. Firm Brings Gene Tests to Masses. New York Times. 28 January 2010. Available online: https://www.nytimes.com/2010/01/29/business/29gene.html (accessed on 8 February 2022).
- Beauchamp, K.A.; Muzzey, D.; Wong, K.K.; Hogan, G.J.; Karimi, K.; Candille, S.I.; Mehta, N.; Mar-Heyming, R.; Kaseniit, K.E.; Kang, H.P.; et al. Systematic design and comparison of expanded carrier screening panels. Genet. Med. 2018, 20, 55–63. [Google Scholar] [CrossRef]
- Hogan, G.H.; Vysotskaia, V.S.; Beauchamp, K.A.; Seisenberger, S.; Grauman, P.V.; Haas, K.R.; Hong, S.H.; Jeon, D.; Kash, S.; Lai, H.H.; et al. Validation of an Expanded Carrier Screen that Optimizes Sensitivity via Full-Exon Sequencing and Panel-wide Copy Number Variant Identification. Clin. Chem. 2018, 64, 1063–1073. [Google Scholar] [CrossRef]
- Arjunan, A.; Bellerose, H.; Torres, R.; Ben-Shachar, R.; Hoffman, J.; Angle, B.; Slotnick, R.N.; Simpson, B.N.; Lewis, A.M.; Magoulas, P.L.; et al. Evaluation and classification of severity for 176 genes on an expanded carrier screening panel. Prenat. Diagn. 2020, 40, 1246–1257. [Google Scholar] [CrossRef]
- Westemeyer, M.; Saucier, J.; Wallace, J.; Prins, S.; Shetty, A.; Malhotra, M.; Demko, Z.; Eng, C.; Weckstein, L.; Boostanfar, R.; et al. Clinical experience with carrier screening in a general population: Support for a comprehensive pan-ethnic approach. Genet. Med. 2020, 22, 1320–1328. [Google Scholar] [CrossRef]
- Propst, L.; Connor, G.; Hinton, M.; Poorvu, T.; Dungan, J. Pregnant Women’s Perspectives on Expanded Carrier Screening. J. Genet. Couns. 2018, 27, 1148–1156. [Google Scholar] [CrossRef]
- Rabkina, L.; Swanson, A.; Aufox, S.; Propst, L.; Fiddler, M.; Wagner, A.; Arjunan, A. What women want: General population perspectives and access to preconception expanded carrier screening. Prenat. Diag. 2021, 41, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Johansen Taber, K.A.; Beauchamp, K.A.; Lazarin, G.A.; Muzzey, D.; Arjunan, A.; Goldberg, J.D. Clinical utility of expanded carrier screening: Results-guided actionability and outcomes. Genet. Med. 2019, 21, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Haque, I.S.; Lazarin, G.A.; Kang, H.P.; Evans, E.A.; Goldberg, J.D.; Wapner, R.J. Modeled Fetal Risk of Genetic Diseases Identified by Expanded Carrier Screening. JAMA 2016, 316, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.S.; Norton, M.; Nakagawa, S.; Bishop, J.; Pena, S.; Gregorich, S.; Kuppermann, M. Variation in Women’s Understanding of Prenatal Testing. Obstet. Gynecol. 2015, 125, 1306–1312. [Google Scholar] [CrossRef]
- National Institutes of Health Consensus Development Conference Statement, Genetic Testing for Cystic Fibrosis. 1997. Available online: https://consensus.nih.gov/1997/1997genetictestcysticfibrosis106html.htm (accessed on 24 September 2021).
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 486: Update on carrier screening for cystic fibrosis. Obstet. Gynecol. 2011, 117, 1028–1031. [Google Scholar] [CrossRef]
- Prior, T.; The Professional Practice and Guidelines Committee. Carrier screening for spinal muscular atrophy. Genet. Med. 2008, 10, 840–842. [Google Scholar] [CrossRef]
- Committee Opinion 93: Committee on Obstetrics: Maternal and Fetal Medicine. Screening for Tay-Sachs disease. Int. J. Gynaecol. Obstet. 1992, 38, 64. [CrossRef]
- Committee Opinion 161: Committee on Genetics: Fragile X syndrome. Int. J. Gynaecol. Obstet. 1996, 52, 209–210.
- Committee Opinion 162: Committee on Genetics: Screening for Tay-Sachs disease. Int. J. Gynaecol. Obstet. 1996, 52, 311–312.
- Committee Opinion 168: Committee on Genetics: Genetic screening for hemoglobinopathies. Int. J. Gynaecol. Obstet. 1996, 53, 195–196.
- Genetic testing for cystic fibrosis. National Institutes of Health Consensus Development Conference Statement on genetic testing for cystic fibrosis. Arch. Intern Med. 1999, 159, 1529–1539.
- Committee Opinion 212: Committee on Genetics: Screening for Canavan disease. Int. J. Gynaecol. Obstet. 1999, 65, 91–92.
- Committee Opinion 238: Committee on Genetics: Screening for hemoglobinopathies. Int. J. Gynaecol. Obstet. 2001, 74, 309–310. [CrossRef]
- Committee Opinion #298. Obstet. Gynecol. 2004, 104, 425–428.
- Sherman, S.; Pletcher, B.; Driscoll, D. Fragile X syndrome: Diagnostic and carrier testing. Genet. Med. 2005, 7, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Committee on Genetics. ACOG Committee Opinion. Number 318, October 2005. Screening for Tay-Sachs Disease. Obstet. Gynecol. 2005, 106, 893–894. [Google Scholar] [CrossRef]
- Committee on Obstetrics. ACOG Practice Bulletin No. 78: Hemoglobinopathies in pregnancy. Obstet. Gynecol. 2007, 109, 229–237. [Google Scholar] [CrossRef]
- Gross, S.J.; Pletcher, B.A.; Monaghan, K.G.; Professional Practice and Guidelines Committee. Carrier screening in individuals of Ashkenazi Jewish descent. Genet. Med. 2008, 10, 54–56. [Google Scholar] [CrossRef]
- Committee Opinion, No. 432: Spinal muscular atrophy. Obstet. Gynecol. 2009, 113, 1194–1196. [CrossRef]
- Committee Opinion, No. 442: Preconception and prenatal carrier screening for genetic diseases in individuals of Eastern European Jewish descent. Obstet. Gynecol. 2009, 114, 950. [CrossRef]
- Committee Opinion, No. 469: Carrier screening for fragile X syndrome. Obstet. Gynecol. 2010, 116, 1008–1010. [CrossRef] [PubMed]
- Committee Opinion, No. 486: Update on Carrier Screening for Cystic Fibrosis. Obstet. Gynecol. 2011, 117, 1028–1031. [CrossRef]
- Grody, W.W.; Thompson, B.H.; Gregg, A.R.; Bean, L.H.; Monaghan, K.G.; Schneider, A.; Lebo, R.V. ACMG position statement on prenatal/preconception expanded carrier screening. Genet. Med. 2013, 15, 482–483. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.G.; Feldman, G.; Goldberg, J.; Gregg, A.R.; Norton, M.E.; Rose, N.C.; Schneider, A.; Stoll, K.; Wapner, R.; Watson, M.S. Expanded carrier screening in reproductive medicine-points to consider: A joint statement of the American College of Medical Genetics and Genomics, American College of Obstetricians and Gynecologists, National Society of Genetic Counselors, Perinatal Quality Foundation, and Society for Maternal-Fetal Medicine. Obstet. Gynecol. 2015, 125, 653–662. [Google Scholar] [CrossRef]
- Committee Opinion, No. 691: Carrier Screening for Genetic Conditions. Obstet. Gynecol. 2017, 129, e41–e55. [CrossRef]
- Blue Cross Blue Shield Evidence Street Opinion 2.04.107, Carrier Screening for Genetic Diseases. 2020. Available online: https://www.blueshieldca.com/bsca/bsc/public/common/PortalComponents/provider/StreamDocumentServlet?fileName=PRV_Carrier_Screening_GenDis.pdf (accessed on 4 February 2022).
- Policy Reporter. Available online: www.policyreporter.com (accessed on 26 September 2021).
- MACPAC Fact sheet. Racial and Ethnic Disparities in Medicaid: An Annotated Bibliography. 2021. Available online: https://www.macpac.gov/wp-content/uploads/2021/04/Racial-and-Ethnic-Disparities-in-Medicaid-An-Annotated-Bibliography.pdf (accessed on 8 February 2022).
- Artiga, S.; Hill, L.; Orega, K.; Damico, A. Health Coverage by Race and Ethnicity, 2010–2019. Available online: https://www.kff.org/racial-equity-and-health-policy/issue-brief/health-coverage-by-race-and-ethnicity/ (accessed on 8 February 2022).
- Beauchamp, K.A.; Johansen Taber, K.A.; Muzzey, D. Clinical impact and cost-effectiveness of a 176-condition expanded carrier screen. Genet. Med. 2019, 21, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Gonzaludo, N.; Belmont, J.W.; Gainullin, V.G.; Taft, R.J. Estimating the burden and economic impact of pediatric genetic disease. Genet. Med. 2019, 21, 1781–1789. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.A.; Singh, M.; Tisdale, A.; Cutillo, C.M.; Garrison, S.R. Can you hear us now? The impact of health-care utilization by rare disease patients in the United States. Genet. Med. 2021, 23, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Norton, M.E. Expanded Carrier Screening and the Complexity of Implementation. Obstet. Gynecol. 2021, 137, 345–350. [Google Scholar] [CrossRef]
- Johansen Taber, K.; Ben-Shachar, R.; Torres, R.; Arjunan, A.; Muzzey, D.; Kaseniit, K.E.; Goldberg, J.; Brown, H. A guidelines consistent carrier screening panel that supports equity across diverse populations. Genet. Med. 2021, 24, 201–213. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).