Molecular Phylogeny, Diversity and Zoogeography of Net-Winged Beetles (Coleoptera: Lycidae)
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Laboratory Methods
2.3. Sequence Handling and Phylogenetic Analyses
2.4. Geographical Distribution and Ancestral State Reconstruction
3. Results
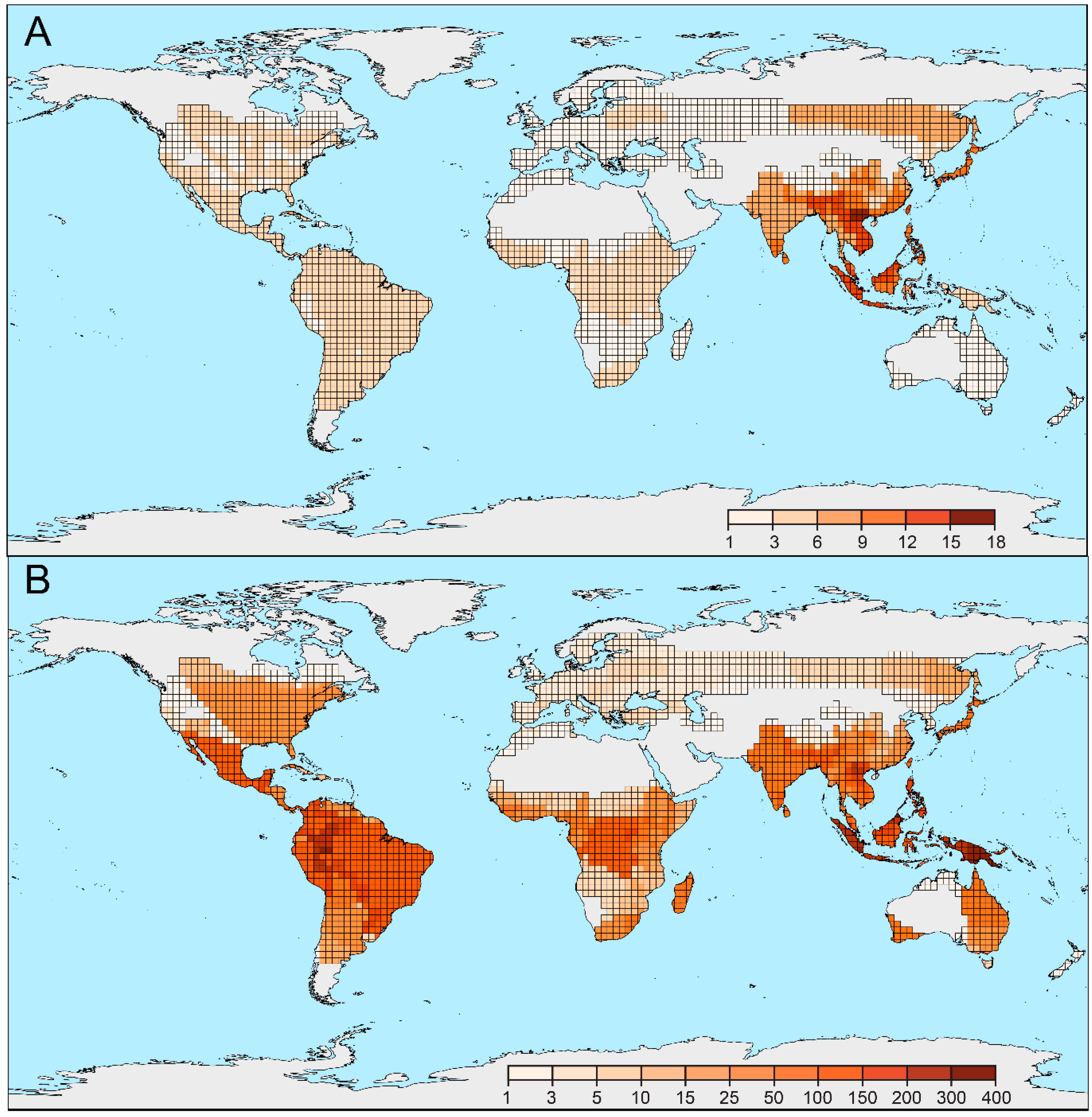
3.1. Sampling of the Diversity
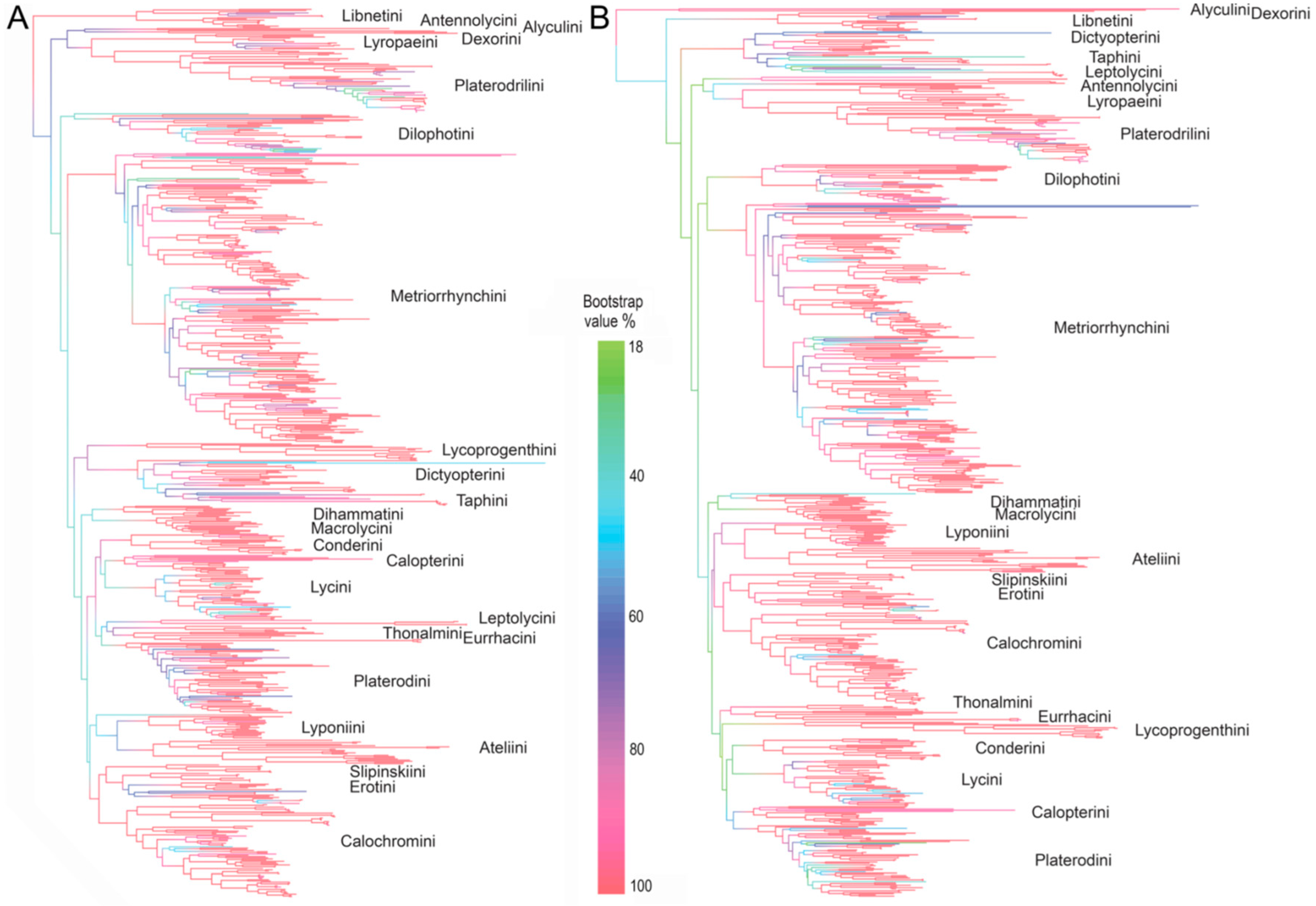
3.2. The Tree Topology
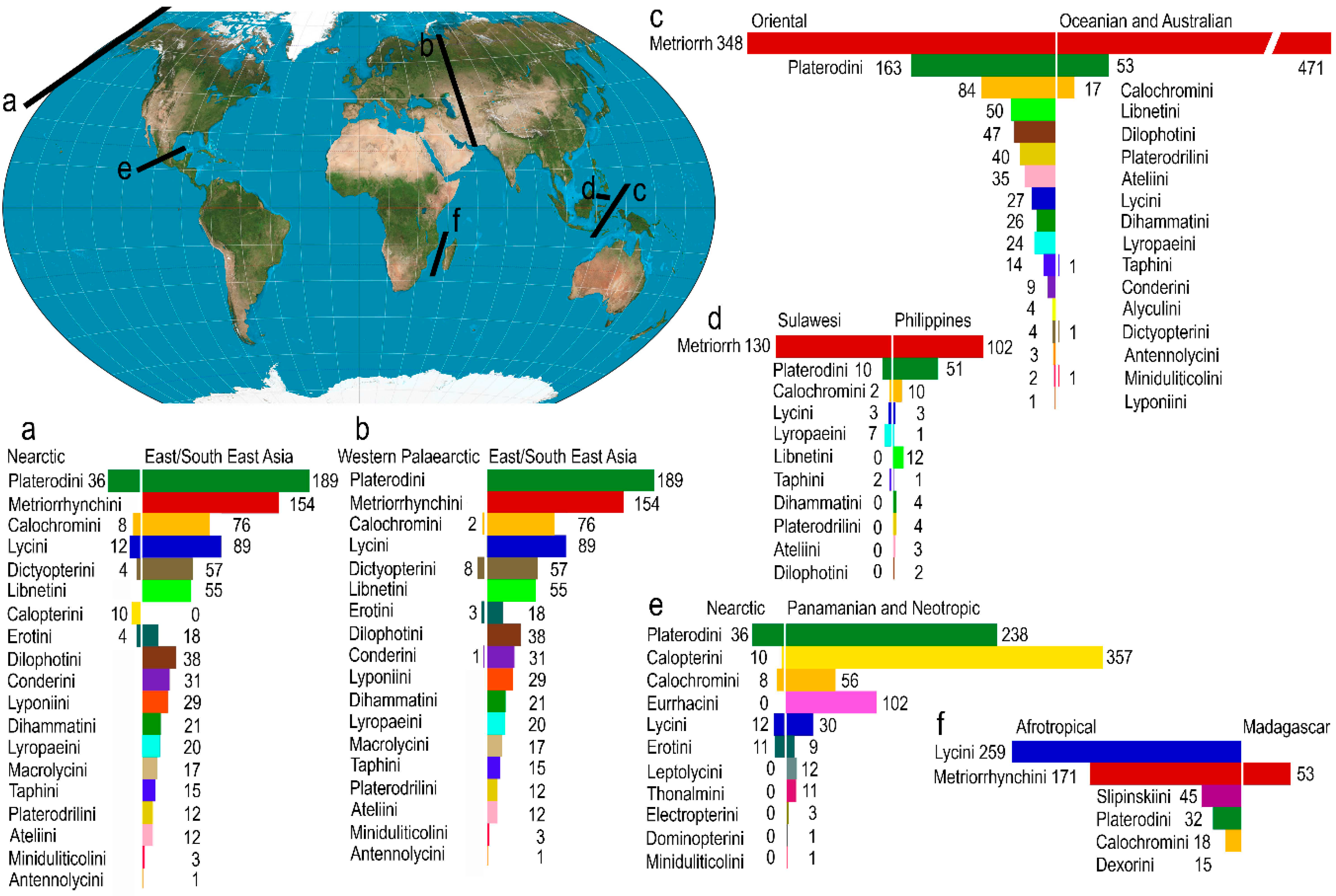
3.3. Geographical Structure of Diversity
4. Discussion
4.1. Higher Level Phylogeny and Classification of Net-Winged Beetles
4.2. Diversity Centers, Ancestral Areas and Dispersal Routes
4.3. Major Dispersal Barriers
4.4. Mozambique Strait
4.5. Bering Strait
4.6. Isthmus of Panama (Former Atrato Seaway)
4.7. Central Asia Dry Gap
4.8. North Africa Dry Gap
4.9. Wallace and Huxley Lines
4.9.1. Borneo/Sulawesi Sector
4.9.2. Sulawesi/Mindanao Sector
4.9.3. Huxley Line (the Philippines/Borneo Sector)
4.10. Sea of Japan
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruaud, A.; Jabbour-Zahab, R.; Genson, G.; Couloux, A.; Yan-Qiong, P.; Rong, Y.D.; Ubaidillah, R.; Augusto, R.; Pereira, S.; Kjellberg, F.; et al. Out of Australia and back again: The world-wide historical biogeography of non-pollinating fig wasps (Hymenoptera: Sycophaginae). J. Biogeogr. 2011, 38, 209–225. [Google Scholar] [CrossRef]
- Fabre, P.H.; Moltensen, M.; Fjeldså, J.; Irestedt, M.; Lessard, J.P.; Jønsson, K.A. Multiple waves of colonization by monarch flycatchers (Myiagra, Monarchidae) across the Indo-Pacific and their implications for coexistence and speciation. J. Biogeogr. 2014, 41, 274–286. [Google Scholar] [CrossRef]
- Gomez-Rodriguez, C.; Freijeiro, A.; Baselga, A. Dispersal and ecological traits explain differences in beta diversity patterns of European beetles. J. Biogeogr. 2015, 42, 1526–1537. [Google Scholar] [CrossRef]
- Peters, R.S.; Meyer, B.; Krogmann, L.; Borner, J.; Meusemann, K.; Schutte, K.; Niehuis, O.; Misof, B. The taming of an impossible child: A standardized all-in approach to the phylogeny of Hymenoptera using public database sequences. BMC Biol. 2011, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Bocak, L.; Barton, C.; Crampton-Platt, A.; Chesters, D.; Ahrens, D.; Vogler, A.P. Building the Coleoptera tree-of-life for >8000 species: Composition of public DNA data and fit with Linnaean classification. Syst. Entomol. 2014, 39, 97–110. [Google Scholar] [CrossRef]
- Zhou, X.; Frandsen, P.B.; Holzenthal, R.W.; Beet, C.R.; Bennett, K.R.; Blahnik, R.J.; Bonada, N.; Cartwright, D.; Chuluunbat, S.; Cocks, G.V.; et al. The Trichoptera barcode initiative: A strategy for generating a species-level tree of life. Philos. Trans. R. Soc. B 2016, 371, 20160025. [Google Scholar] [CrossRef] [PubMed]
- Linard, B.; Crampton-Platt, A.; Moriniere, J.; Timmermans, M.J.T.N.; Andujar, C.; Arribas, P.; Miller, K.E.; Lipecki, J.; Favreau, E.; Hunter, A.; et al. The contribution of mitochondrial metagenomics to large-scale data mining and phylogenetic analysis of Coleoptera. Mol. Phylogenet. Evol. 2018, 128, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bocak, L.; Matsuda, K. Review of the immature stages of the family Lycidae (Insecta: Coleoptera). J. Nat. Hist. 2003, 37, 1463–1507. [Google Scholar] [CrossRef]
- Bray, T.C.; Bocak, L. Slowly dispersing neotenic beetles can speciate on a penny coin and generate space-limited diversity in the tropical mountains. Sci. Rep. 2016, 6, 33579. [Google Scholar] [CrossRef] [PubMed]
- Malohlava, V.; Bocak, L. Evidence of extreme habitat stability in a Southeast Asian biodiversity hotspot based on the evolutionary analysis of neotenic net-winged beetles. Mol. Ecol. 2010, 19, 4800–4811. [Google Scholar] [CrossRef] [PubMed]
- Sklenarova, K.; Chesters, D.; Bocak, L. Phylogeography of poorly dispersing net-winged beetles: A role of drifting India in the origin of Afrotropical and Oriental fauna. PLoS ONE 2013, 8, e67957. [Google Scholar] [CrossRef] [PubMed]
- Masek, M.; Ivie, M.A.; Palata, V.; Bocak, L. Molecular phylogeny and classification of Lyropaeini (Coleoptera: Lycidae) with description of larvae and new species of Lyropaeus. Raffles Bull. Zool. 2014, 62, 136–145. [Google Scholar]
- Masek, M.; Palata, V.; Bray, T.C. Bocak, L. Molecular phylogeny reveals high diversity and geographic structure in Asian neotenic net-winged beetles Platerodrilus (Coleoptera: Lycidae). PLoS ONE 2015, 10, e0123855. [Google Scholar] [CrossRef] [PubMed]
- Bocak, L.; Bocakova, M. Phylogeny and classification of the family Lycidae (Insecta: Coleoptera). Ann. Zool. 2008, 58, 695–720. [Google Scholar] [CrossRef]
- Kleine, R. Coleopterorum Catalogus Auspiciis et Auxilio W; Pars 128; Lycidae. W. Junk: Berlin, Germany, 1933. [Google Scholar]
- Cicero, J.M. Composite, haustellate mouthparts in netwinged beetle and firefly larvae (Coleoptera, Cantharoidea: Lycidae, Lampyridae). J. Morph. 1994, 219, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kazantsev, S.V. Morphology of Lycidae with some considerations on evolution of the Coleoptera. Elytron 2005, 17, 49–226. [Google Scholar]
- Motyka, M.; Masek, M.; Bocak, L. Congruence between morphology and molecular phylogeny: The reclassification of Calochromini (Coleoptera: Lycidae) and their dispersal history. Zool. J. Linn. Soc. 2017, 180, 47–65. [Google Scholar] [CrossRef]
- Crowson, R.A. A review of the classification of Cantharoidea (Coleoptera), with definition of two new families Cneoglossidae and Omethidae. Rev. Univ. Madrid 1972, 21, 35–77. [Google Scholar]
- Lawrence, J.F.; Slipinski, A.; Seago, A.E.; Thayer, M.K.; Newton, A.F.; Marvaldi, A.E. Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Ann. Zool. 2011, 61. [Google Scholar] [CrossRef]
- Kundrata, R.; Bocakova, M.; Bocak, L. The comprehensive phylogeny of the superfamily Elateroidea (Coleoptera: Elateriformia). Mol. Phylogenet. Evol. 2014, 76, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Bocakova, M.; Bocak, L.; Hunt, T.; Teravainen, M.; Vogler, A.P. Molecular phylogenetics of Elateriformia (Coleoptera): Evolution of bioluminescence and neoteny. Cladistics 2007, 23, 477–496. [Google Scholar] [CrossRef]
- Bocak, L.; Bocakova, M.; Hunt, T.; Vogler, A.P. Multiple ancient origins of neoteny in Lycidae (Coleoptera): Consequences for ecology and macroevolution. Proc. R. Soc. B Biol. Sci. 2008, 275, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Bocak, L.; Motyka, M.; Bocek, M.; Bocakova, M. Incomplete sclerotization and phylogeny: The phylogenetic classification of Plastocerus (Coleoptera: Elateroidea). PLoS ONE 2018, 13, e0194026. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, G.A. The control of water loss in desert tenebrionid beetles. J. Exp. Biol. 1970, 53, 573–595. [Google Scholar] [PubMed]
- Motyka, M.; Kampova, L.; Bocak, L. Phylogeny and evolution of Müllerian mimicry in aposematic Dilophotes: Evidence for advergence and size-constraints in evolution of mimetic sexual dimorphism. Sci. Rep. 2018, 8, 3744. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bocak, L.; Pang, H. Molecular phylogeny of Macrolycus (Coleoptera: Lycidae) with description of new species from China. Entomol. Sci. 2015, 18, 319–329. [Google Scholar] [CrossRef]
- Li, Y.; Gunter, N.; Pang, H.; Bocak, L. DNA-based species delimitation separates highly divergent populations within morphologically coherent clades of poorly dispersing beetles. Zool. J. Linn. Soc. 2015, 175, 59–72. [Google Scholar] [CrossRef]
- Li, Y.; Pang, H.; Bocak, L. The taxonomy of neotenic net-winged beetles from China based on morphology and molecular data (Coleoptera: Lycidae). Ann. Zool. 2017, 67, 679–687. [Google Scholar] [CrossRef]
- Miller, R.S. A Revision of the Leptolycini (Coleoptera: Lycidae) with a Discussion of Paedomorphosis. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 1991. [Google Scholar]
- Wong, A.T.C. A new species of neotenous beetle, Duliticola hoiseni (Insecta: Coleoptera: Cantharoidea: Lycidae) from Peninsular Malaysia and Singapore. Raffles Bull. Zool. 1996, 44, 173–187. [Google Scholar]
- Levkanicova, Z.; Bocak, L. Identification of net-winged beetle larvae (Coleoptera: Lycidae) using three mtDNA fragments: A comparison of their utility. Syst. Entomol. 2009, 34, 210–221. [Google Scholar] [CrossRef]
- Bocak, L.; Grebennikov, V.V.; Masek, M. A new species of Dexoris (Coleoptera: Lycidae) and parallel evolution of brachyptery in the soft-bodied elateroid beetles. Zootaxa 2013, 3721, 495–500. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Hayward, A. Why grow up? A perspective on insect strategies to avoid metamorphosis. Ecol. Entomol. 2016, 41, 505–515. [Google Scholar] [CrossRef]
- Kazantsev, S.V. New and little known taxa of neotenic Lycidae (Coleoptera), with discussion of their phylogeny. Russ. Entomol. J. 2013, 22, 9–31. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Haeseler, A.V.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree 1.4.2. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 23 July 2017).
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A Software Platform for Bayesian Evolutionary Analysis. PLoS Comp. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Bouckaert, R.R. Bayesian Evolutionary Analysis with Beast; Cambridge University Press: Cambridge, UK, 2015; ISBN 978-1-107-01965-2. [Google Scholar]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef] [PubMed]
- Bielejec, F.; Rambaut, A.; Suchard, M.A.; Lemey, P. SPREAD: Spatial phylogenetic reconstruction of evolutionary dynamics. Bioinformatics 2011, 27, 2910–2912. [Google Scholar] [CrossRef] [PubMed]
- Bocak, L.; Bocakova, M. Revision of the genus Dexoris C. O. Waterhouse (Coleoptera, Lycidae). Acta Entomol. Bohemoslov. 1988, 85, 194–204. [Google Scholar]
- Bocak, L.; Bocakova, M. Revision of the supergeneric classification of the family Lycidae (Coleoptera). Polskie Pismo Entomologiczne 1990, 59, 623–676. [Google Scholar]
- Holt, B.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araujo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jonsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the world. Science 2013, 339, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Bocakova, M. Lolodorfus, a new genus of net-winged beetles (Coleoptera: Lycidae: Dexorinae) from Cameroon. Zootaxa 2014, 3811, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Kundrata, R.; Bocak, L. The phylogeny and limits of Elateridae (Insecta, Coleoptera): Is there a common tendency of click beetles to soft-bodiedness and neoteny? Zool. Scr. 2011, 40, 364–378. [Google Scholar] [CrossRef]
- Bocak, L.; Grebennikov, V.V.; Sklenarova, K. Cautires apterus, a new species and the first record of wingless male Lycidae (Coleoptera) discovered in the North Pare Mountains, Tanzania. Ann. Zool. 2014, 64, 1–7. [Google Scholar] [CrossRef]
- Bocak, L.; Kundrata, R.; Andújar-Fernández, C.; Vogler, A.P. The discovery of Iberobaeniidae (Coleoptera: Elateroidea): A new family of beetles from Spain, with immatures detected by environmental DNA sequencing. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152350. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.D.; Wild, A.L.; Kanda, K.; Bellamy, C.L.; Beutel, R.G.; Caterino, M.S.; Farnum, C.W.; Hawks, C.D.; Ivie, M.A.; Jameson, M.L.; et al. The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 2015, 40, 835–880. [Google Scholar] [CrossRef]
- Toussaint, E.F.A.; Seidel, M.; Arriaga-Varela, E.; Hajek, J.; Kral, D.; Sekerka, L.; Short, A.E.Z.; Fikacek, M. The peril of dating beetles. Syst. Entomol. 2017, 42, 1–10. [Google Scholar] [CrossRef]
- Kusy, D.; Motyka, M.; Andujar, C.; Bocek, M.; Masek, M.; Sklenarova, K.; Kokas, F.; Bocakova, M.; Vogler, A.P.; Bocak, L. Genome sequencing of Rhinorhipus Lawrence exposes an early branch of the Coleoptera. Front. Zool. 2018, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Seton, M.; Müller, R.D.; Zahirovic, S.; Gaina, C.; Torsvik, T.; Shephard, G.; Talsma, A.; Gurnis, M.; Turner, M.; Maus, S.; et al. Global continental and ocean basin reconstructions since 200 Ma. Earth Sci. Rev. 2012, 113, 212–270. [Google Scholar] [CrossRef]
- Bocakova, M. Phylogenetic analysis of the tribe Libnetini with establishment of a new genus (Coleoptera, Lycidae). Dtsch. Entomol. Z. 2004, 51, 53–64. [Google Scholar] [CrossRef]
- Bocakova, M. Review of the tribe Lyropaeini (Coleoptera: Lycidae). Eur. J. Entomol. 2006, 103, 127–136. [Google Scholar] [CrossRef]
- Smith, A.G.; Smith, D.G.; Funnell, B.M. Atlas of Cenozoic and Mesozoic Coastlines; Cambridge University Press: Cambridge, UK, 2004; ISBN 9780521602877. [Google Scholar]
- Zarcone, G.; Petti, F.M.; Cillari, A.; Nicosia, U. A possible bridge between Adria and Africa: New palaeobiogeographic and stratigraphic constraints of the Mesozoic palaeogeography of the Central Mediterranean area. Earth Sci. Rev. 2010, 103, 154–162. [Google Scholar] [CrossRef]
- Li, Y.; Pang, H.; Bocak, L. A review of the neotenic genus Atelius Waterhouse, 1878 from China (Coleoptera: Lycidae). Ann. Zool. 2018, 68, 351–356. [Google Scholar] [CrossRef]
- Bocakova, M. Revision and phylogenetic analysis of the subfamily Platerodinae (Coleoptera: Lycidae). Eur. J. Entomol. 2001, 98, 53–85. [Google Scholar] [CrossRef]
- Bocakova, M. Phylogeny and classification of the tribe Calopterini (Coleoptera, Lycidae). Ind. Syst. Evol. 2005, 35, 437–447. [Google Scholar] [CrossRef]
- Neall, V.E.; Trew, S.A. The age and origin of the Pacific islands: A geological overview. Philos. Trans. R. Soc. B 2008, 363, 3293–3308. [Google Scholar] [CrossRef] [PubMed]
- Yoder, A.D.; Nowak, M.D. Has Vicariance or Dispersal Been the Predominant Biogeographic Force in Madagascar? Only Time Will Tell. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 405–431. [Google Scholar] [CrossRef]
- Tiffney, B.H.; Manchester, S.R. The use of geological and paleontological evidence in evaluating plant phylogeographic hypotheses in the Northern Hemisphere Tertiary. Int. J. Plant Sci. 2001, 162, 3–17. [Google Scholar] [CrossRef]
- Sota, T.; Bocak, L.; Hayashi, M. Molecular phylogeny and historical biogeography of the Holarctic wetland leaf beetle of the genus Plateumaris. Mol. Phylogenet. Evol. 2008, 46, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Li, J.T.; Wang, J.S.; Nian, H.H.; Litvinchuk, S.N.; Wang, J.C.; Li, Y.; Rao, D.Q.; Klaus, S. Amphibians crossing the Bering Land Bridge: Evidence from holarctic treefrogs (Hyla, Hylidae, Anura). Mol. Phylogenet. Evol. 2015, 87, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.R. Three new genera of fossil Lycidae from Baltic Amber (Coleoptera, Lycidae). Mitt. Munch. Entomol. Ges. 1987, 77, 61–78. [Google Scholar]
- Kazantsev, S.V. A new fossil genus of net-winged beetles, with a brief review of amber Lycidae (Insecta: Coleoptera). Zootaxa 2013, 3608, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kosuch, J.; Vences, M.; Dubois, A.; Ohler, A.; Bohme, W. Out of Asia: Mitochondrial DNA evidence for an oriental origin of tiger frogs, genus Hoplobatrachus. Mol. Phylogenet. Evol. 2001, 21, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Bocak, L.; Yagi, T. Evolution of mimicry patterns in Metriorrhynchus (Coleoptera: Lycidae): The history of dispersal and speciation in South East Asia. Evolution 2010, 64, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Kubecek, V.; Dvorak, M.; Bocak, L. The phylogenetic structure of Metriorrhynchini fauna of Sulawesi, (Coleoptera: Lycidae) with description of a new genus. Zool. Stud. 2011, 50, 645–656. [Google Scholar]
- Bocak, L.; Matsuda, K.; Yagi, T. A revision of Metriorrhynchus from the Philippines with molecular evidence of an Australian origin of the Oriental Metriorrhynchus fauna (Coleoptera: Lycidae). Eur. J. Entomol. 2006, 103, 115–126. [Google Scholar] [CrossRef]
- Hall, R. Late Jurassic-Cenozoic reconstructions of the Indonesian region and the Indian Ocean. Tectonophysics 2012, 570–571, 1–41. [Google Scholar] [CrossRef]
- Maruyama, S.; Isozaki, Y.; Kimura, G.; Terabayashi, M. Paleogeographic maps of the Japanese Islands: Plate tectonic synthesis from 750 Ma to the present. Isl. Arc 1997, 6, 121–142. [Google Scholar] [CrossRef]
- Barnes, G.L. Origins of the Japanese Islands: The new ‘big picture’. Jpn. Rev. 2003, 15, 3–50. [Google Scholar]
- Dobson, M.; Kawamura, Y. Origin of the Japanese land mammal fauna: Allocation of extant species to historically-based categories. Quat. Res. 1998, 37, 385–395. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Che, L.H.; Li, Y.; Dan, L.; Pang, H.; Slipinski, A.; Zhang, P. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Gravely, F.H. The larvae and pupae of some beetles from Cochin. Rec. Ind. Mus. 1915, 11, 354–366. [Google Scholar]
- Mjöberg, E. The mystery of the so called “Trilobite” or “Perty’s larvae” definitely solved. Psyche 1925, 22, 119–153. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. A framework for delineating biogeographical regions based on species distributions. J. Biogeogr. 2010, 37, 2029–2053. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. Comment on “An Update of Wallace’s Zoogeographic Regions of the World”. Science 2013, 341, 343. [Google Scholar] [CrossRef] [PubMed]

| Subfamily | Tribe | No of Species | Distribution | Hypothesized Ancestral Area | |
|---|---|---|---|---|---|
| Described/Sequenced | |||||
| LIBNETINAE | Libnetini | 112 | 17 | 2–7, 11 | Oriental (Figure S30A) |
| LEPTOLYCINAE | Leptolycini n | 12 | 4 | 15 | Panamanian (endemic) |
| DEXORINAE | Dexorini n | 15 | 2 | 12 | Afrotropical (endemic) |
| LYROPAEINAE | Lyropaeini n | 43 | 9 | 4–8, 11 | Oriental (Figure S30B) |
| Alyculini n | 3 | 1 | 6–7 | Oriental (endemic) | |
| Antennolycini n | 3 | 2 | 6 | Oriental (endemic) | |
| Platerodrilini n | 49 | 32 | 2, 4–7 | Oriental (endemic) | |
| DICTYOPTERINAE | Dictyopterini | 73 | 25 | 1–7, 14 | Sino-Japanese (Figure S30C) |
| Lycoprogenthini | 7 | 9 | 2, 4–7 | Oriental (endemic) | |
| Taphini | 31 | 9 | 2, 4–10 | Oriental (endemic except one sp.) | |
| LYCINAE | Ateliini n | 45 | 19 | 2, 4–7 | Oriental (endemic [10]) |
| Metriorrhynchini n | 1403 | 161 | 2–13 | Australian [11] | |
| Dilophotini | 81 | 30 | 2–7 | Oriental [26] | |
| Calochromini | 288 | 47 | 1–12, 14–16 | Oriental [18] | |
| Calopterini n | 367 | 15 | 14–16 | Neotropical (endemic, a few species in the Nearctic realm) | |
| Conderini | 42 | 14 | 1–7 | Oriental (Figure S31C) | |
| Dihammatini | 44 | 9 | 2–7 | Oriental (Figure S31B) | |
| Erotini | 54 | 18 | 1–3, 14 | Sino-Japanese (Figure S31A) | |
| Slipinskiini | 46 | 4 | 12 | Afrotropical (endemic) | |
| Eurrhacini | 102 | 8 | 15–16 | Neotropical (endemic) | |
| Lycini | 413 | 34 | 1–8, 12, 14–16 | unresolved: Nearctic or Afrotropical | |
| Lyponiini | 45 | 17 | 2–3, 5, 7, 11 | Sino-Japanese | |
| Macrolycini | 69 | 14 | 2–3, 5, 11 | Sino-Japanese | |
| Platerodini | 861 | 48 | 1–12, 14–15 | Oriental (Figure S30E) | |
| Thonalmini | 11 | 3 | 15 | Panamanian (endemic) | |
| LYCIDAE | 4230 | 551 | 1–16 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masek, M.; Motyka, M.; Kusy, D.; Bocek, M.; Li, Y.; Bocak, L. Molecular Phylogeny, Diversity and Zoogeography of Net-Winged Beetles (Coleoptera: Lycidae). Insects 2018, 9, 154. https://doi.org/10.3390/insects9040154
Masek M, Motyka M, Kusy D, Bocek M, Li Y, Bocak L. Molecular Phylogeny, Diversity and Zoogeography of Net-Winged Beetles (Coleoptera: Lycidae). Insects. 2018; 9(4):154. https://doi.org/10.3390/insects9040154
Chicago/Turabian StyleMasek, Michal, Michal Motyka, Dominik Kusy, Matej Bocek, Yun Li, and Ladislav Bocak. 2018. "Molecular Phylogeny, Diversity and Zoogeography of Net-Winged Beetles (Coleoptera: Lycidae)" Insects 9, no. 4: 154. https://doi.org/10.3390/insects9040154
APA StyleMasek, M., Motyka, M., Kusy, D., Bocek, M., Li, Y., & Bocak, L. (2018). Molecular Phylogeny, Diversity and Zoogeography of Net-Winged Beetles (Coleoptera: Lycidae). Insects, 9(4), 154. https://doi.org/10.3390/insects9040154