The Effect of Neonicotinoid Insecticide and Fungicide on Sugar Responsiveness and Orientation Behavior of Honey Bee (Apis mellifera) in Semi-Field Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Agrochemicals
2.2. Insect Treatments
2.3. Orientation Behaviors
2.4. Sucrose Responsiveness
2.5. Data Analysis
3. Results
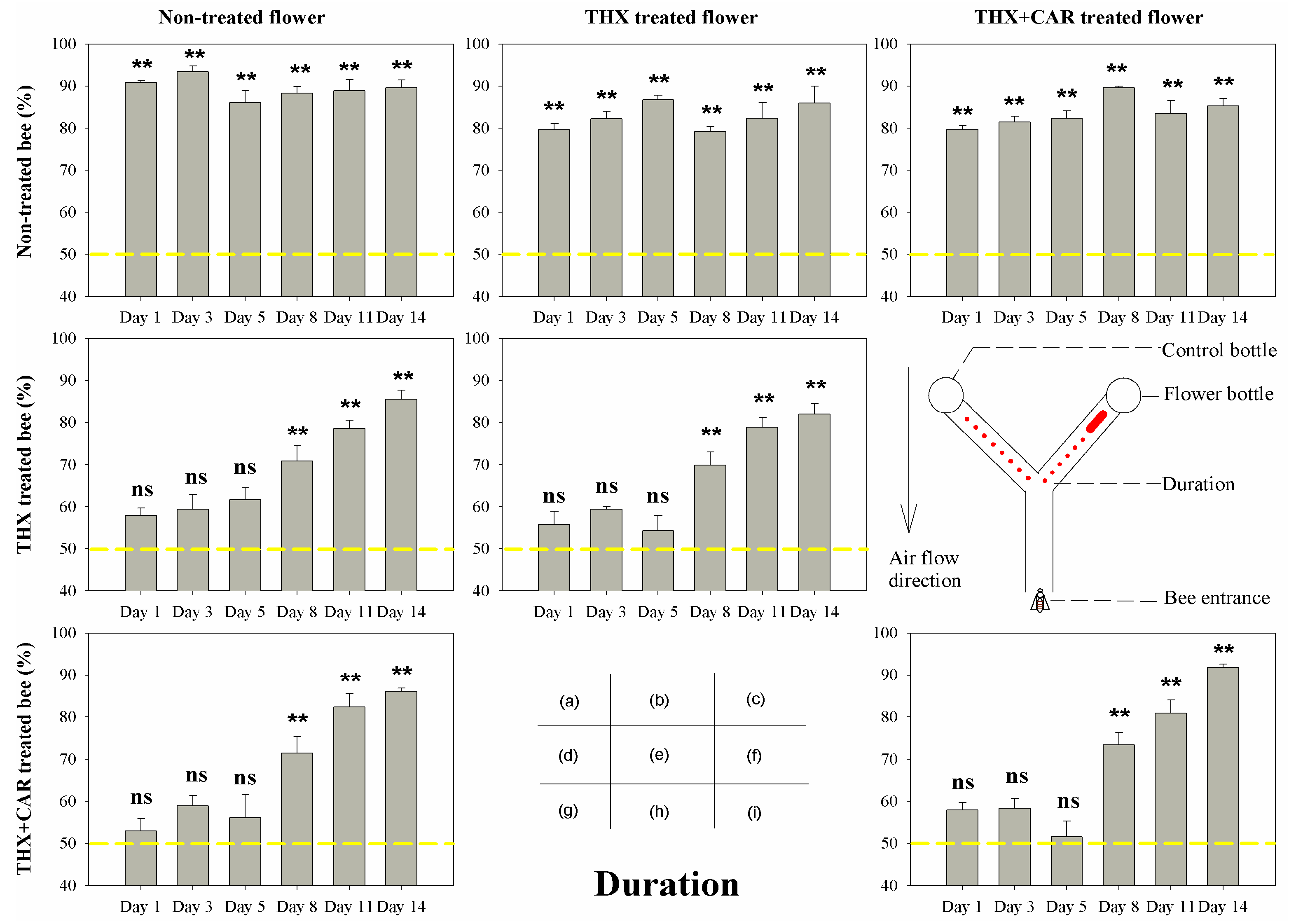
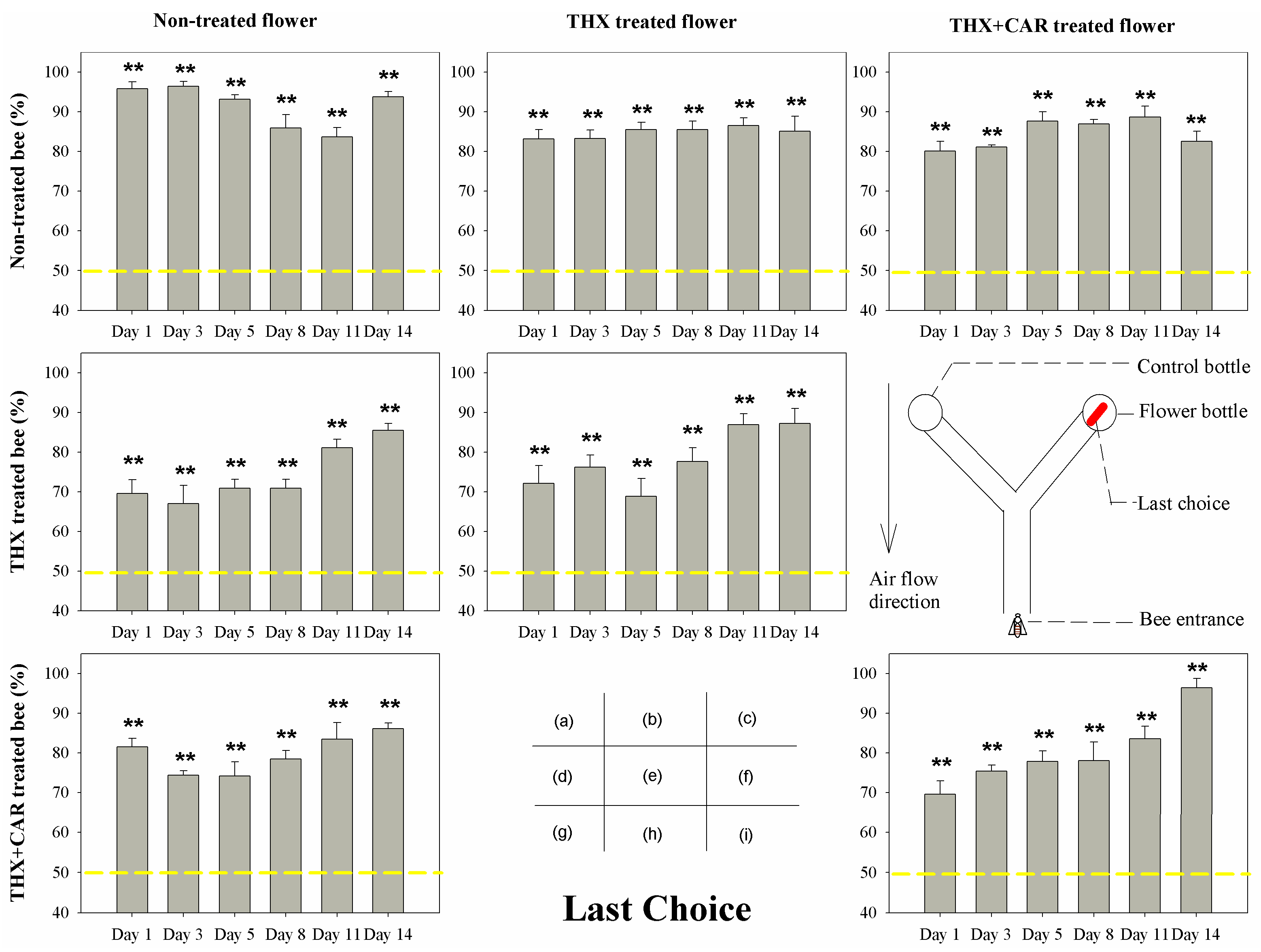
3.1. Orientation Behaviors
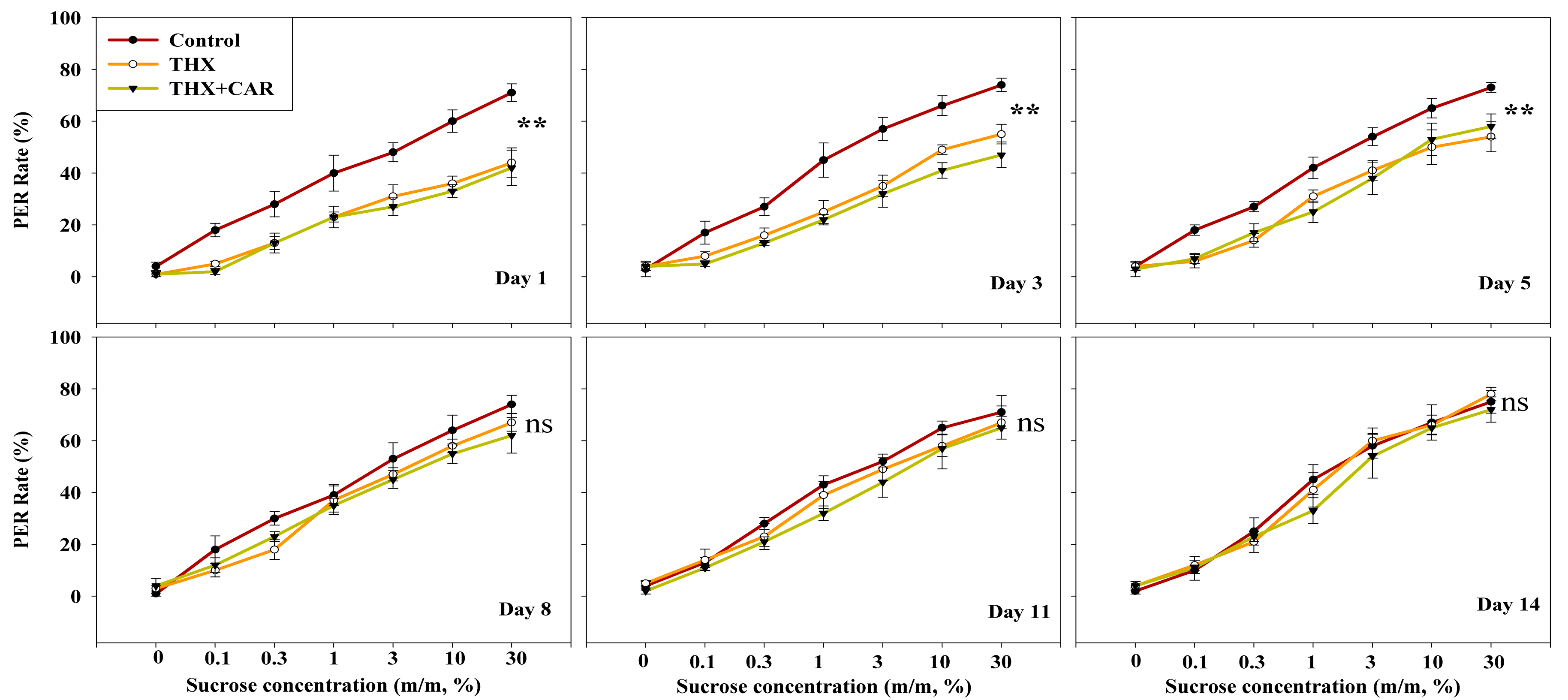
3.2. Sucrose Responsiveness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Mulhauser, B.; Mulot, M.; Mutabazi, A.; Glauser, G.; Aebi, A. A worldwide survey of neonicotinoids in honey. Science 2017, 358, 109. [Google Scholar] [CrossRef] [PubMed]
- Di, N.; Hladun, K.R.; Zhang, K.; Liu, T.X.; Trumble, J.T. Laboratory bioassays on the impact of cadmium, copper and lead on the development and survival of honeybee (Apis mellifera L.) larvae and foragers. Chemosphere 2016, 152, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Ware, G.W. Effects of Pesticides on Nontarget Organisms; Springer: New York, NY, USA, 1980; Volume 76, pp. 173–201. [Google Scholar]
- Hallmann, C.A.; Foppen, R.P.B.; van Turnhout, C.A.M.; de Kroon, H.; Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511, 341. [Google Scholar] [CrossRef] [PubMed]
- Gilburn, A.S.; Bunnefeld, N.; Wilson, M.V.; Botham, M.S.; Brereton, T.M.; Fox, R.; Goulson, D. Are neonicotinoid insecticides driving declines of widespread butterflies? PeerJ 2015, 3, e1402. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.; Blitzer, E.J.; Gibbs, J.; Losey, J.E.; Danforth, B.N. Negative effects of pesticides on wild bee communities can be buffered by landscape context. Proc. Biol. Sci. 2015, 282, 20150299. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.A.; Isaac, N.J.B.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E. Neonicotinoids and Other Insect Nicotinic Receptor Competitive Modulators: Progress and Prospects. Ann. Rev. Entomol. 2018, 63, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A common pesticide decreases foraging success and survival in honey bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Bloomquist, J.R.; Ellis, J.D. Chronic toxicity of clothianidin, imidacloprid, chlorpyrifos, and dimethoate to Apis mellifera L. larvae reared in vitro. Pest Manag. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Burgio, G.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, impairs honey bee flight ability. Sci. Rep. 2017, 7, 1201. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Nieh, J.C. A common neonicotinoid pesticide, thiamethoxam, alters honey bee activity, motor unctions, and movement to light. Sci. Rep. 2017, 7, 15132. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.J.; Ramosrodriguez, O.; Raine, N.E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 2012, 491, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 2017, 356, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, A.; Limayrios, V.; Baute, T.; Smith, J.; Xue, Y. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in southwestern Ontario. PLoS ONE 2015, 10, e0118139. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of wild plants near neonicotinoid seed-treated crops, and implications for non-target insects. Sci. Total Environ. 2016, 566–567, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.; Roat, T.C.; Carvalho, S.M.; Malaspina, O. Side-effects of thiamethoxam on the brain andmidgut of the africanized honeybee Apis mellifera (Hymenopptera: Apidae). Environ. Toxicol. 2014, 29, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pang, Q.; Zhang, W.W.; Ting, J.I. Effects of sublethal doses of carbendazim on the growth and detoxifying enzyme activities of honeybee(Apis mellifera ligustica) larvae. Acta Entomol. Sin. 2017, 60, 642–649. [Google Scholar]
- Wang, Z.; Qu, Y.; Dong, S.; Wen, P.; Li, J.; Tan, K.; Menzel, R. Honey bees modulate their olfactory learning in the presence of hornet predators and alarm component. PLoS ONE 2016, 11, e0150399. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, R.; Kuritz-Kaiser, A.; Menzel, R.; Erber, J. Sensory responsiveness and the effects of equal subjective rewards on tactile learning and memory of honeybees. Learn. Mem. 2005, 12, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tan, K. Comparative analysis of olfactory learning of Apis cerana and Apis mellifera. Apidologie 2014, 45, 45–52. [Google Scholar] [CrossRef]
- Kessler, S.C.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Stout, J.C.; Wright, G.A. Bees prefer foods containing neonicotinoid pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Démares, F.J.; Pirk, C.W.W.; Nicolson, S.W.; Human, H. Neonicotinoids decrease sucrose responsiveness of honey bees at first contact. J. Insect Physiol. 2018, 108, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Duan, J.; Wu, Y.; Liu, Q.; He, Q.; Shi, Y.; Yu, L.; Cao, H. A survey of multiple pesticide residues in pollen and beebread collected in China. Sci. Total Environ. 2018, 640–641, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Székács, A.; Mörtl, M.; Darvas, B. Monitoring pesticide residues in surface and ground water in Hungary: Surveys in 1990–2015. J. Chem. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [PubMed]

| First Choice | Duration | Last Choice | |||||
|---|---|---|---|---|---|---|---|
| χ2 | p | χ2 | p | χ2 | p | ||
| Non-treated bee | Non-treated flower | 7.731 | 0.172 | 2.281 | 0.809 | 20.22 | <0.001 |
| Non-treated bee | THX treated flower | 1.608 | 0.9 | 5.118 | 0.402 | 0.997 | 0.963 |
| Non-treated bee | THX + CAR treated flower | 3.525 | 0.62 | 4.992 | 0.417 | 5.649 | 0.342 |
| THX treated bee | Non-treated flower | 31.227 | <0.001 | 27.448 | <0.001 | 13.196 | 0.022 |
| THX treated bee | THX treated flower | 43.708 | <0.001 | 32.7 | <0.001 | 11.957 | 0.035 |
| THX + CAR treated bee | Non-treated flower | 34.549 | <0.001 | 45.313 | <0.001 | 7.49 | 0.187 |
| THX + CAR treated bee | THX + CAR treated flower | 46.722 | <0.001 | 57.691 | <0.001 | 24.39 | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Wang, Z.; He, Q.; Liu, Q.; Li, X.; Yu, L.; Cao, H. The Effect of Neonicotinoid Insecticide and Fungicide on Sugar Responsiveness and Orientation Behavior of Honey Bee (Apis mellifera) in Semi-Field Conditions. Insects 2018, 9, 130. https://doi.org/10.3390/insects9040130
Jiang X, Wang Z, He Q, Liu Q, Li X, Yu L, Cao H. The Effect of Neonicotinoid Insecticide and Fungicide on Sugar Responsiveness and Orientation Behavior of Honey Bee (Apis mellifera) in Semi-Field Conditions. Insects. 2018; 9(4):130. https://doi.org/10.3390/insects9040130
Chicago/Turabian StyleJiang, Xingchuan, Zhengwei Wang, Qibao He, Qiongqiong Liu, Xinyang Li, Linsheng Yu, and Haiqun Cao. 2018. "The Effect of Neonicotinoid Insecticide and Fungicide on Sugar Responsiveness and Orientation Behavior of Honey Bee (Apis mellifera) in Semi-Field Conditions" Insects 9, no. 4: 130. https://doi.org/10.3390/insects9040130
APA StyleJiang, X., Wang, Z., He, Q., Liu, Q., Li, X., Yu, L., & Cao, H. (2018). The Effect of Neonicotinoid Insecticide and Fungicide on Sugar Responsiveness and Orientation Behavior of Honey Bee (Apis mellifera) in Semi-Field Conditions. Insects, 9(4), 130. https://doi.org/10.3390/insects9040130





