Habitat Structure, Quality and Landscape Predict Species Richness and Communities of Collembola in Dry Grasslands in Austria
Abstract
1. Introduction
2. Material and Methods
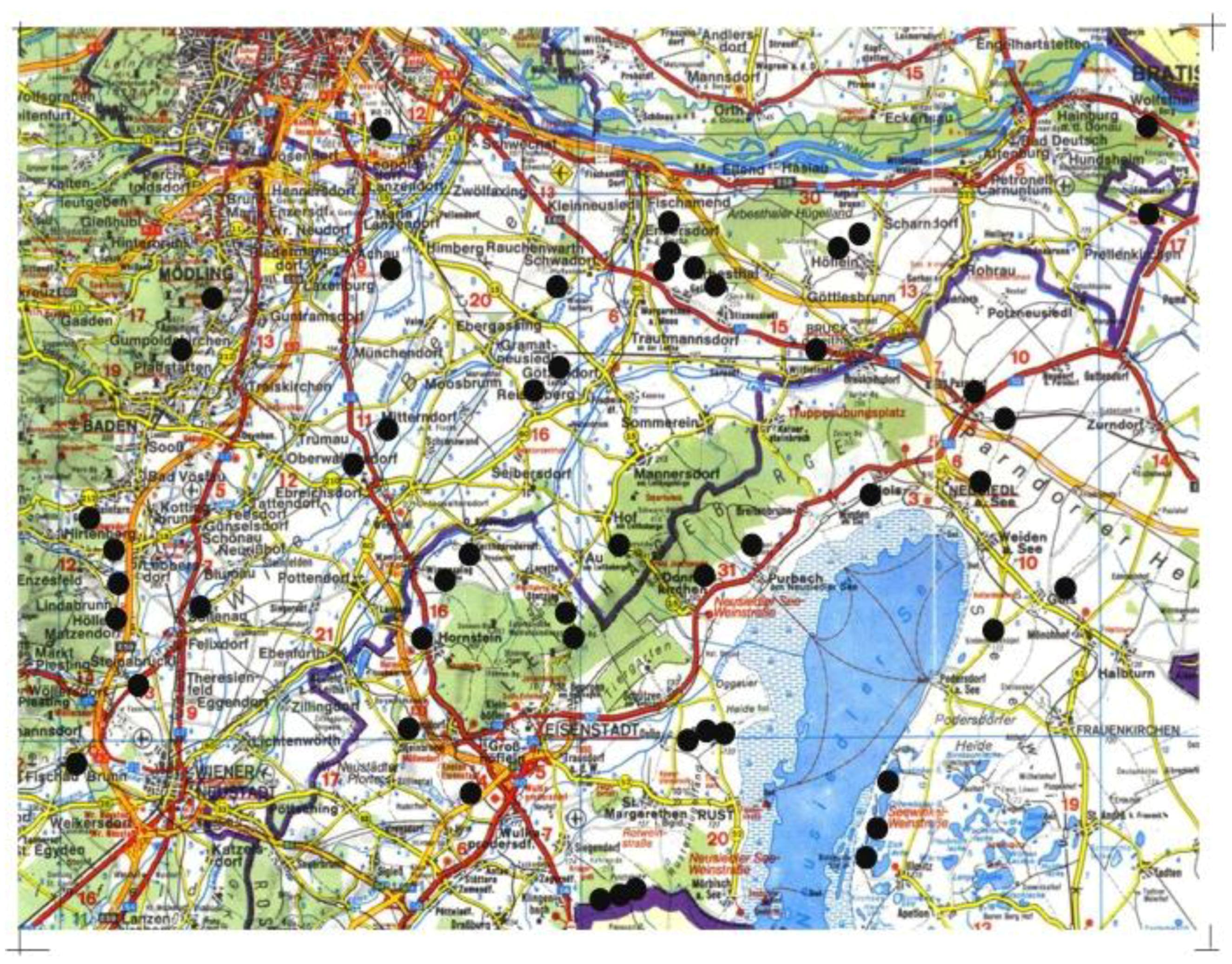
2.1. Study Area and Site Selection
2.2. Collembola Sampling
2.3. Collembola Determination and Classification
2.4. Patch Variables
2.5. Landscape Variables
- Fragmentation: To assess the influence of large dry grasslands in the proximity of the sites, the distance between site centre and the nearest large dry grassland area (>15 ha in size) was measured on a topographical map (scale 1:50,000).
- Land use intensity of the surrounding landscape: To obtain a simple measure for ‘landscape heterogeneity’ the number of land use types in the 1 × 1 km2 quadrat around the site was counted. Five combinations of element types and attributes were specified, (a) dry grassland elements; (b) elements with extensive agricultural use; (c) fallow-dominated elements; (d) short-grassed elements; and (e) linear elements of various kinds. The summed area within a 1 × 1 km2 quadrat area around each patch was calculated and used as a predictor for species richness in the patches. To quantify land use intensity the 62 pre-defined land use types in the 1 × 1 km2 quadrat around the patch were classified as high, low or neutral land use intensity. Natural forests or other grasslands are considered to reflect low land use intensity. In contrast, intensively used agricultural fields are associated with high land use intensity. An average land use intensity weighted by area was calculated to obtain a factor describing land use intensity of the landscape around each patch.
2.6. Statistical Analysis
3. Results
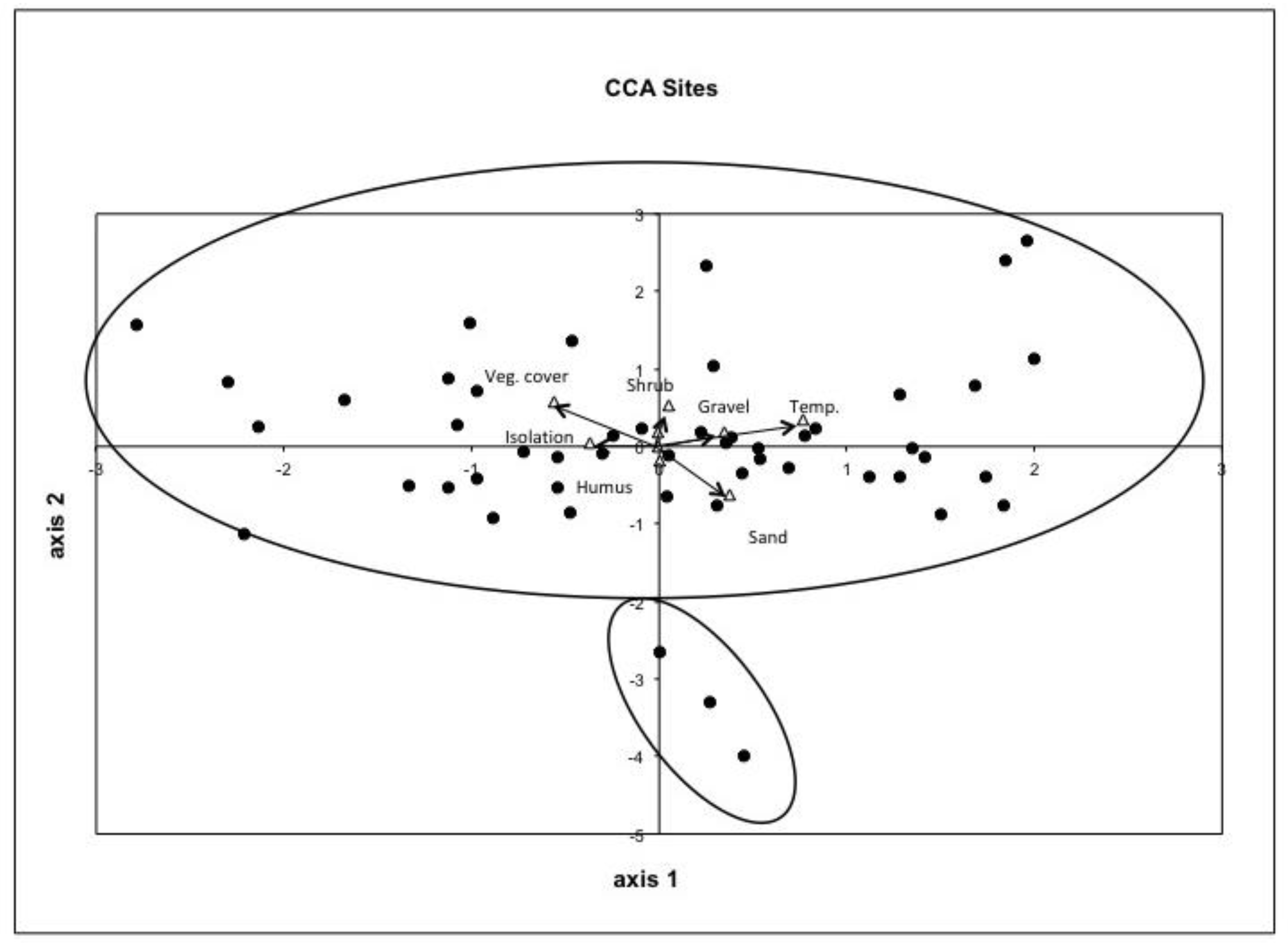
3.1. CCA
3.2. Single Correlations
3.3. Multivariate Regression Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petersen, H. A review of collembolan ecology in ecosystem context. Acta Zool. Fenn. 1994, 195, 111–118. [Google Scholar]
- Petersen, H.; Luxton, M. A comparative analyses of soil fauna population and their role in decomposition processes. Oikos 1982, 39, 287–388. [Google Scholar] [CrossRef]
- Rusek, J. Die bodenbildende Funktion von Collembolen und Acarina. Pedobiologia 1975, 15, 299–308. [Google Scholar]
- Rusek, J. Soil microstructures-contributions on specific soil organisms. Quest. Entomol. 1985, 21, 497–514. [Google Scholar]
- Copley, J. Ecology goes underground. Nature 2000, 406, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H. General aspects of collembolan ecology at the turn of the millennium. Pedobiologia 2002, 46, 246–260. [Google Scholar] [CrossRef]
- Hopkin, S.P. Biology of the springtails (Insecta: Collembola); Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Betsch, J.M. Effets de la privation des apports annuels de litière sur les collemboles symphypléones épigés d’une forêt sur rendzine. Rev. Ecol. Biol. Sol 1991, 28, 41–49. [Google Scholar]
- Betsch, J.M.; Cancela da Fonseca, P. Changes in edaphic factors and microarthropod communities after clearing and burning in a tropical rain forest in French Guyana. Acta Zool. Fenn. 1995, 196, 142–145. [Google Scholar]
- Hashimoto, H.; Tamura, H. Change in collembolan community during litter breakdown. Acta Zool. Fenn. 1994, 195, 67–68. [Google Scholar]
- Klironomos, J.N.; Kendrick, W.B. Stimulative effects of arthropods on endomycorrhizas of sugar maple in the presence of decaying litter. Funct. Ecol. 1995, 9, 528–536. [Google Scholar] [CrossRef]
- Kovác, L. Effects of soil type on collembolan communities in agroecosystems. Acta Zool. Fenn. 1994, 195, 89–93. [Google Scholar]
- Ponge, J.F.; Arpin, P.; Vannier, G. Collembolan response to experimental perturbations of litter supply in a temperate forest ecosystem. Eur. J. Soil Biol. 1993, 29, 141–153. [Google Scholar]
- Ponge, J.F. Biocenoses of Collembola in Atlantic temperate grass-woodland ecosystems. Pedobiologia 1993, 37, 223–244. [Google Scholar]
- Ponge, J.F.; Prat, B. Collembola, indicators of the humification process in the resinous, deciduous and mixed plantings: Results obtained in the forest d’Orleans, France. Rev. Ecol. Biol. Sol 1982, 19, 237–250. [Google Scholar]
- Querner, P. Collembola (Insecta). Checkliste der Fauna Österreichs No. 3; Akademie der Wissenschaften: Vienna, Austria, 2008. [Google Scholar]
- Querner, P.; Bruckner, A. The landscape ecology of Collembola. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2009, 17, 139–141. [Google Scholar]
- Yoshida, T.; Tanaka, K. Land-use diversity index: A new means of detecting diversity at landscape level. Landsc. Ecol. Eng. 2005, 1, 201–206. [Google Scholar] [CrossRef]
- Chust, G.; Lek, S.; Deharveng, L.; Ventura, D.; Ducrot, D.; Pretus, J. The effects of the landscape pattern on arthropod assemblages: An analysis of scale-dependence using satellite data. Belg. J. Entomol. 2000, 2, 99–110. [Google Scholar]
- Chust, G.; Pretus, J.L.; Ducrot, D.; Bedòs, A.; Deharveng, L. Response of soil fauna to landscape heterogeneity: Determining optimal scales for biodiversity modelling. Conserv. Biol. 2003, 17, 1712–1723. [Google Scholar] [CrossRef]
- Chust, G.; Pretus, J.L.; Ducrot, D.; Bedòs, A.; Deharveng, L. Identification of landscape units from an insect perspective. Ecography 2003, 26, 257–268. [Google Scholar] [CrossRef]
- Martins da Silva, P.; Berg, M.P.; Serrano, A.R.M.; Dubs, F.; Sousa, J.P. Environmental factors at different spatial scales governing soil fauna community patterns in fragmented forests. Landsc. Ecol. 2012, 27, 1337–1349. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Berg, M.P.; da Silva, A.A.; Dias, S.; Leitão, P.J.; Chamberlain, D.; Niemela, J.; Serrano, A.R.M.; Sousa, J.P. Soil fauna through the landscape window: Factors shaping surface-and soil-dwelling communities across spatial scales in cork-oak mosaics. Landsc. Ecol. 2015, 30, 1511–1526. [Google Scholar] [CrossRef]
- Martins da Silva, P.; Carvalho, F.; Dirilgen, T.; Stone, D.; Creamer, R.; Bolger, T.; Sousa, J.P. Traits of collembolan life-form indicate land use types and soil properties across an European transect. Appl. Soil Ecol. 2016, 97, 69–77. [Google Scholar] [CrossRef]
- Ponge, J.F.; Gillet, S.; Dubs, F.; Fedoroff, E.; Haese, L.; Sousa, J.P.; Lavelle, P. Collembolan communities as bioindicators of land use intensification. Soil Biol. Biochem. 2003, 35, 813–826. [Google Scholar] [CrossRef]
- Ponge, J.F.; Dubs, F.; Gillet, S.; Sousa, J.P.; Lavelle, P. Decreased biodiversity in soil springtail communities: The importance of dispersal and landuse history in heterogeneous landscapes. Soil Biol. Biochem. 2006, 38, 1158–1161. [Google Scholar] [CrossRef]
- Querner, P.; Bruckner, A.; Drapela, T.; Moser, D.; Zaller, J.G. Effects of site and landscape parameters on Collembola diversity in 29 winter oilseed rape fields. Agric. Ecosyst. Environ. 2013, 164, 145–154. [Google Scholar] [CrossRef]
- Sousa, J.P.; da Gama, M.M.; Pinto, C.; Keating, A.; Calhôa, F.; Lemos, M.; Castro, C.; Luz, T.; Leitão, P.; Dias, S. Effects of land-use on Collembola diversity patterns in a Mediterranean landscape. Pedobiologia 2004, 48, 609–622. [Google Scholar] [CrossRef]
- Sousa, J.P.; Bolger, T.; da Gama, M.M.; Lukkari, T.; Ponge, J.F.; Simón, C.; Traser, G.; Vanbergen, A.J.; Brennan, A.; Dubs, F.; et al. Changes in Collembola richness and diversity along a gradient of land-use intensity: A pan European study. Pedobiologia 2006, 50, 147–156. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Watt, A.D.; Mitchell, R.; Truscott, A.M.; Palmer, S.C.F.; Ivits, E.; Eggleton, P.; Jones, T.H.; Sousa, J.P. Scale-specific correlations between habitat heterogeneity and soil fauna diversity along a landscape structure gradient. Oecologia 2007, 153, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Gepp, J. Trockenrasen in Österreich als schützenwürdige Refugien wärmeliebender Tierarten. In Österreichische Trockenrasenkatalog. “Steppen”, “Heiden”, Trockenwiesen, Magerwiesen: Bestand, Gefährdung, Möglichkeiten ihrer Erhaltung; Holzner, W., Horvatic, E., Köllner, E., Köppl, W., Pokorny, M., Scharfetter, E., Schramayr, G., Strudl, M., Eds.; Grüne Reihe des Bundesministeriums für Gesundheit und Umweltschutz; Bundesministeriums für Gesundheit und Umweltschutz: Styria, Graz, Austria, 1986; Band 6; pp. 15–28. Available online: https://www.zobodat.at/pdf/Gruene-Reihe-Lebensministerium_AS_6_0001-0380.pdf (accessed on 5 July 2018). (In German)
- Holzner, W.; Horvatic, E.; Köllner, E.; Köppl, W.; Pokorny, M.; Scharfetter, E.; Schramayr, G.; Strudl, M. Österreichische Trockenrasenkatalog: “Steppen”, “Heiden”, Trockenwiesen, Magerwiesen: Bestand, Gefährdung, Möglichkeiten ihrer Erhaltung; Grüne Reihe des Bundesministeriums für Gesundheit und Umweltschutz; Bundesministeriums für Gesundheit und Umweltschutz: Styria, Graz, Austria, 1986; Band 6; pp. 15–28. Available online: https://www.zobodat.at/pdf/Gruene-Reihe-Lebensministerium_AS_6_0001-0380.pdf (accessed on 5 July 2018). (In German)
- Poschlod, P.; WallisDeVries, M.F. The historical and socioeconomic perspective of calcareous grasslands - lessons from the distant and recent past. Biol. Conserv. 2002, 104, 361–376. [Google Scholar] [CrossRef]
- Hobohm, C.; Härdtle, W. Zur Bedeutung einiger ökologischer Parameter für die Artenvielfalt innerhalb von Pflanzengesellschaften Mitteleuropas. Tuexenia 1997, 17, 19–52. [Google Scholar]
- Van Swaay, C.A.M. The importance of calcareous grasslands for butterflies in Europe. Biol. Conserv. 2002, 104, 315–318. [Google Scholar] [CrossRef]
- Willner, W.; Jakomini, C.; Sauberer, N.; Zechmeister, H.G. Zur Kenntnis kleiner Trockenraseninseln im Osten Österreichs. Tuexenia 2004, 24, 215–226. [Google Scholar]
- Waitzbauer, W. Die Naturschutzgebiete der Hundsheimer Berge in Niederösterreich. Entwicklung, Gefährdung, Schutz. Abhandlungen Zoologisch-Botanischen Gesellschaft 1990, 24, 1–88. [Google Scholar]
- Franz, H.; Beier, M. Zur Kenntnis der Bodenfauna im pannonischen Klimagebiet Österreichs. Annalen Naturhistorischen Museums Wien 1948, 56, 440–549. [Google Scholar]
- Baumgartner-Gamauf, M. Zur Kenntnis der Collembolenfauna des Neusiedlersees. Wissenschaftliche Arbeiten Burgenland 1959, 23, 144–146. [Google Scholar]
- Baumgartner-Gamauf, M. Einige ufer- und wasserbewohnende Collembolen des Seewinkels, Sitzungsberichte der Österreichischen Akademie der Wissenschaft. Mathematische Naturwissenschaftliche Abteilung 1959, 168, 363–369. [Google Scholar]
- Gunhold, P.; Pschorn-Wacher, H. Untersuchung über die Mikrofauna von Verlandungs-, Steppen- und Waldböden im Neusiedlersee-Gebiet. Wissenschaftliche Arbeiten Burgenland 1956, 12, 1–24. [Google Scholar]
- Kampichler, C. Community structure and composition of Collembola and Cryptostigmata in a dry-turf cushion plant. Biol. Fert. Soils 1990, 9, 130–134. [Google Scholar] [CrossRef]
- Kampichler, C. Zur Collembolenfauna der Trockenrasen im Naturschutzgebiet des Hundsheimer Berges (Niederösterreich). Verhandlungen Zoologisch-Botanischen Gesellschaft Österreich 1991, 128, 145–155. [Google Scholar]
- Kampichler, C. Community structure and phenology patterns of epedaphic Collembola in a dry-turf grassland. Zoologische Jahrbücher Systematik 1992, 119, 369–381. [Google Scholar]
- Christian, E.; Kampichler, C. On the zoogeography of some epedaphic Collembola from eastern Lower Austria. Annalen Naturhistorischen Museums Wien 1984, 86, 133–139. [Google Scholar]
- Winklehner, R.; Winkler, H.; Kampichler, C. Estimating local species richness of epigeic Collembola in temperate dry grassland. Pedobiologia 1997, 41, 154–158. [Google Scholar]
- Winkler, H.; Kampichler, C. Species saturation in communities of epigeic Collembola in temperate dry grassland. Verhandlungen Gesellschaft Ökologie 1999, 29, 161–167. [Google Scholar]
- Winkler, H.; Kampichler, C. Local and regional species richness in communities of surface-dwelling grassland Collembola: Indication of species saturation. Ecography 2000, 23, 385–392. [Google Scholar] [CrossRef]
- Querner, P. Epigäische Springschwänze (Collembola) von Trockenrasenstandorten in Wien, Niederösterreich und Burgenland. Beiträge Entomofaunistik 2004, 4, 17–26. [Google Scholar]
- Zulka, K.P.; Abensperg-Traun, M.; Milasowszky, N.; Bieringer, G.; Gereben-Krenn, B.-A.; Holzinger, W.; Hölzler, G.; Rabitsch, W.; Reischütz, A.; Querner, P.; et al. Species richness in dry grassland patches of eastern Austria: A multi-taxon study on the role of local, landscape and habitat quality variables. Agric. Ecosyst. Environ. 2015, 182, 25–36. [Google Scholar] [CrossRef]
- Paar, M.; Tiefenbach, M.; Winkler, I. Trockenrasen in Österreich. Rep. Umweltbundesamtes 1994, 94/107, 86. Available online: http://www.umweltbundesamt.at/fileadmin/site/publikationen/R107.pdf (accessed on 5 July 2018). (In German).
- Gisin, H. Collembolenfauna Europas; Museum d’Histoire Naturelle: Genève, Switzerland, 1960. [Google Scholar]
- Babenko, A.B.; Chernova, N.M.; Potapov, M.B.; Stebaeva, S.K. Collembola of Russia and Adjacent Countries: Family Hypogastruridae; Nauka: Moscow, Russia, 1994. [Google Scholar]
- Bretfeld, G. Synopses on Palaearctic Collembola, Volume 2: Symphypleona; State Museum of the Natural History Museum of Gorlitz: Gorlitz, Germany, 1999. [Google Scholar]
- Stach, J. The Apterygotan Fauna of Poland in Relation to the World-Fauna of this Group of Insects. Tribe: Orchesellini; Polska Akademia Nauk: Kraków, Poland, 1960. [Google Scholar]
- Stach, J. The Apterygotan Fauna of Poland in Relation to the World-Fauna of this Group of Insects. Tribe: Entomobryini; Polska Akademia Nauk: Kraków, Poland, 1963. [Google Scholar]
- Zimdars, B.; Dunger, W. Synopses on Palaearctic Collembola, Volume 1: Tullberginae; State Museum of the Natural History Museum of Gorlitz: Gorlitz, Germany, 1994. [Google Scholar]
- Pomorski, R.J. Onychiurinae of Poland (Collembola: Onychiuridae); Taxonomical Society: Wrocklaw, Poland, 1998. [Google Scholar]
- Christian, E. Catalogus Faunae Austriae: Collembola (Springschwänze); Österreichische Akademie der Wissenschaften: Vienna, Austria, 1987. [Google Scholar]
- Pallmann, H.; Eichenberger, E.; Hasler, A. Eine neue Methode der Temperaturmessung bei ökologischen und bodenkundlichen Untersuchungen. Ber. Schweiz. Bot. Ges. 1940, 50, 337–362. [Google Scholar]
- Bransby, D.I.; Matches, A.G.; Krause, G.F. Disc meter for rapid estimation of herbage yield in grazing trials. Agron. J. 1977, 69, 393–396. [Google Scholar] [CrossRef]
- Bransby, D.I.; Tainton, N.M. The disc pasture meter: Possible applications in grazing management. Proc. Grassl. Soc. South. Afr. 1977, 12, 115–118. [Google Scholar] [CrossRef]
- Rabotnov, T.A. On the Development of Scales in Vegetation Science, with Emphasis on Drude’s Scale. In Sampling Methods and Taxon Analysis in Vegetation Science; Handbook of Vegetation Science; Junk, W., Knapp, R., Eds.; Springer: Berlin, Germany, 1984; Volume 4, pp. 55–59. [Google Scholar]
- Zechmeister, H.G. Annual growth of four pleurocarpous moss species and their applicability for biomonitoring heavy metals. Environ. Monit. Assess. 1998, 52, 441–451. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Microcomputer Power: Ithaca, NY, USA, 1998. [Google Scholar]
- Deharveng, L. Soil Collembola diversity, endemism, and reforestation: A case study in the Pyrenees (France). Conserv. Biol. 1996, 10, 74–84. [Google Scholar] [CrossRef]
- Menta, C.; Leoni, A.; Gardi, C.; Conti, F.D. Are grasslands important habitats for soil microarthropod conservation? Biodivers. Conserv. 2011, 20, 1073–1087. [Google Scholar] [CrossRef]
- Greenslade, P. Are Collembola useful as indicators of the conservation value of native grasslands? Pedobiologia 1997, 41, 215–220. [Google Scholar]
- Sabais, A.C.W.; Scheu, S.; Eisenhauer, N. Plant species richness drives the density and diversity of Collembola in temperate grassland. Acta Oecol. 2011, 37, 195–202. [Google Scholar] [CrossRef]
- Glick, P.A. The distribution of insects, spiders and mites in the air. In Technical Bulletin No. 673; U.S. Department of Agriculture: Washington, DC, USA, 1939. [Google Scholar]
- Freeman, J.A. Occurence of Collembola in the air. In Proceedings of the Royal Entomological Society of London. Series A, General Entomology; Blackwell Publishing Ltd.: Oxford, UK, 1952; Volume 27, p. 28. [Google Scholar]
- Greenslade, P.; Convey, P. Exotic Collembola on subantarctic islands: Pathways, origins and biology. Biol. Invasion 2012, 14, 405–417. [Google Scholar] [CrossRef]
- Gressitt, J.L.; Leech, R.E.; Leech, T.S.; Sedlacek, J.; Wise, K.A.J. Trapping of air-borne insects in the Antarctic area (Part 2). Pac. Insects 1961, 3, 559–562. [Google Scholar]
- Johnson, C.G. The distribution of insects in the air and the empirical relation of density to height. J. Anim. Ecol. 1957, 26, 479–494. [Google Scholar] [CrossRef]
| Species | Category * | Site Occupancy, % |
|---|---|---|
| Order Poduromorpha Hypogastrura assimilis | 3 | 24 |
| Ceratophysella bengtssoni | 3 | 18 |
| Ceratophysella sigillata | 3 | 4 |
| Ceratophysella succinea | 3 | 36 |
| Schoettella ununguiculata | § | 12 |
| Choreutinula inermis | § | 2 |
| Xenylla grisea | 3 | 6 |
| Xenylla maritima | § | 4 |
| Brachystomella parvula | 3 | 8 |
| Microgastrura duodecimoculata | 3 | 2 |
| Pseudachorutes dubius | § | 6 |
| Pseudachorutes parvulus | 3 | 72 |
| Pseudachorutes subcrassus | § | 4 |
| Pseudachorutes palmiensis | § | 8 |
| Deutonura conjuncta | § | 20 |
| Neanura alba | § | 4 |
| Neanura muscorum | § | 2 |
| Odontella empodialis | § | 2 |
| Protaphorura armata | 3 | 8 |
| Protaphorura gisini | § | 6 |
| Protaphorura subfimata | 1 | 2 |
| Metaphorura affinis | § | 2 |
| Metaphorura riozoi | 1 | 2 |
| Order Entomobryomorpha Folsomia quadrioculata | 3 | 18 |
| Folsomides angularis | 3 | 4 |
| Proisotoma crassicauda | § | 2 |
| Proisotoma minuta | 3 | 6 |
| Proisotoma sp. | § | 48 |
| Isotoma olivacea | 3 | 26 |
| Isotoma sp. | § | 16 |
| Isotoma notabilis | 3 | 54 |
| Isotoma viridis | 3 | 90 |
| Entomobrya atrocincta | 2 | 4 |
| Entomobrya multifasciata | 1 | 80 |
| Entomobrya handschini | 1 | 80 |
| Entomobrya marginata | 2 | 8 |
| Entomobrya quinquelineata | 2 | 2 |
| Entomobrya sp. 1 | § | 42 |
| Entomobrya sp. 2 | § | 14 |
| Orchesella cincta | 3 | 98 |
| Orchesella flavescens | § | 2 |
| Orchesella multifasciata | § | 14 |
| Orchesella spectabilis | 1 | 2 |
| Orchesella pannonica | 1 | 2 |
| Orchesella villosa | 3 | 2 |
| Orchesella taurica | § | 18 |
| Orchesella xerothermica | 2 | 2 |
| Seira dollfusi | 2 | 14 |
| Heteromurus major | 2 | 60 |
| Lepidocyrtus cyaneus | 3 | 96 |
| Lepidocyrtus languinosus | 3 | 68 |
| Lepidocyrtus lignorum | 3 | 78 |
| Lepidocyrtus paradoxus | 3 | 82 |
| Lepidocyrtus nigrescens | 1 | 37 |
| Pseudosinella alba | § | 14 |
| Pseudosinella decipiens | 3 | 24 |
| Pseudosinella octopunctata | § | 6 |
| Pseudosinella sexoculata | 3 | 4 |
| Pseudosinella imparipunctata | 3 | 2 |
| Pseudosinella petterseni | 3 | 6 |
| Willowsia buski | 3 | 2 |
| Willowsia nigromaculata | 3 | 2 |
| Tomocerus flavescens | § | 34 |
| Tomocerus vulgaris | 3 | 2 |
| Cyphoderus albinos | § | 14 |
| Cyphoderus bidenticulatus | 2 | 2 |
| Order Symphypleona Sphaeridia pumilis | 3 | 14 |
| Sminthurinus elegans | 2 | 58 |
| Sminthurinus niger | 3 | 38 |
| Sminthurinus aureus | 3 | 38 |
| Sminthurinus bimaculatus | 2 | 2 |
| Sminthurinus sp. | § | 2 |
| Stenognathellus denisi | 2 | 2 |
| Dicyrtoma fusca | § | 4 |
| Sminthurus multipunctatus | 1 | 60 |
| Sminthurus viridis | 3 | 92 |
| Caprainea marginata | 3 | 2 |
| Sminthurus maculatus | 1 | 4 |
| Bourletiella viridescens | 3 | 2 |
| Deuterosminthurus bicinctus | 3 | 2 |
| Deuterosminthurus sulphureus sulphureus | 2 | 6 |
| Deuterosminthurus pallipes | 1 | 70 |
| Deuterosminthurus sp. | § | 2 |
| Heterosminthurus bilineatus | 3 | 2 |
| Heterosminthurus insignis | 3 | 2 |
| Fasciosminthurus sp. | § | 2 |
| Site | Area, m2 | Total Abundance, No. Individuals | Total | Number of Species | |||
|---|---|---|---|---|---|---|---|
| Dry Grassland Specialists | Grassland Species | Total Grassland Species | Generalists | ||||
| 01 | 2158 | 371 | 19 | 4 | 2 | 6 | 11 |
| 02 | 3503 | 681 | 23 | 5 | 3 | 8 | 9 |
| 03 | 2579 | 1025 | 19 | 3 | 2 | 5 | 11 |
| 04 | 9202 | 1400 | 19 | 3 | 2 | 5 | 13 |
| 05 | 97,271 | 493 | 18 | 3 | 3 | 6 | 11 |
| 06 | 13,338 | 834 | 20 | 3 | 2 | 5 | 9 |
| 07 | 7130 | 2013 | 16 | 3 | 1 | 4 | 8 |
| 08 | 13,902 | 1614 | 15 | 2 | 1 | 3 | 9 |
| 09 | 1638 | 556 | 23 | 3 | 1 | 4 | 15 |
| 10 | 2905 | 472 | 19 | 4 | 2 | 6 | 11 |
| 11 | 870 | 530 | 17 | 1 | 2 | 3 | 11 |
| 12 | 5588 | 1615 | 21 | 1 | 2 | 3 | 12 |
| 13 | 3117 | 777 | 21 | 4 | 2 | 6 | 13 |
| 14 | 1713 | 516 | 17 | 5 | 3 | 8 | 9 |
| 15 | 4699 | 1200 | 22 | 5 | 1 | 6 | 11 |
| 16 | 6737 | 549 | 16 | 3 | 1 | 4 | 11 |
| 17 | 878 | 624 | 16 | 5 | 1 | 6 | 10 |
| 18 | 63,538 | 5690 | 18 | 5 | 3 | 8 | 8 |
| 19 | 7816 | 990 | 16 | 4 | 0 | 4 | 10 |
| 20 | 7214 | 206 | 16 | 3 | 1 | 4 | 10 |
| 21 | 28,342 | 424 | 19 | 4 | 3 | 7 | 9 |
| 22 | 7934 | 1112 | 19 | 4 | 2 | 6 | 9 |
| 23 | 1556 | 786 | 24 | 4 | 2 | 6 | 11 |
| 24 | 60,701 | 1064 | 17 | 3 | 1 | 4 | 10 |
| 25 | 3808 | 1200 | 15 | 2 | 0 | 2 | 10 |
| 26 | 1257 | 1023 | 21 | 4 | 1 | 5 | 10 |
| 27 | 5224 | 578 | 15 | 4 | 2 | 6 | 6 |
| 28 | 391 | 865 | 18 | 3 | 1 | 4 | 10 |
| 29 | 2016 | 3637 | 18 | 4 | 0 | 4 | 12 |
| 30 | 1155 | 1321 | 23 | 4 | 3 | 7 | 10 |
| 31 | 13,215 | 2463 | 19 | 3 | 1 | 4 | 10 |
| 32 | 23,492 | 937 | 18 | 3 | 4 | 7 | 8 |
| 33 | 89,674 | 1036 | 17 | 4 | 0 | 4 | 10 |
| 34 | 4297 | 394 | 17 | 5 | 2 | 7 | 10 |
| 35 | 11,777 | 980 | 23 | 4 | 2 | 6 | 14 |
| 36 | 1842 | 274 | 13 | 3 | 3 | 6 | 7 |
| 37 | 6073 | 1569 | 20 | 2 | 1 | 3 | 9 |
| 38 | 450 | 1917 | 20 | 2 | 1 | 3 | 12 |
| 39 | 5368 | 204 | 14 | 1 | 0 | 1 | 10 |
| 40 | 7078 | 310 | 20 | 5 | 2 | 7 | 10 |
| 41 | 1027 | 487 | 21 | 3 | 2 | 5 | 11 |
| 42 | 21,454 | 5576 | 17 | 3 | 1 | 4 | 12 |
| 43 | 3154 | 1493 | 17 | 1 | 1 | 2 | 10 |
| 44 | 53,203 | 496 | 16 | 3 | 2 | 5 | 9 |
| 45 | 1043 | 704 | 17 | 3 | 1 | 4 | 12 |
| 46 | 487 | 659 | 20 | 3 | 4 | 7 | 11 |
| 47 | 1076 | 676 | 16 | 3 | 1 | 4 | 11 |
| 48 | 22,972 | 328 | 18 | 5 | 1 | 6 | 11 |
| 49 | 7189 | 259 | 14 | 2 | 0 | 2 | 11 |
| 50 | 381 | 1437 | 21 | 5 | 1 | 6 | 10 |
| Response Parameter | Independent Variable | r | P |
|---|---|---|---|
| Dry grassland specialist species richness | Dry grassland plant species richness | 0.48 | <0.01 |
| Soil gravel content | 0.353 | <0.05 | |
| Soil temperature | 0.282 | <0.05 | |
| Distance to the nearest source area, mainland | 0.379 | <0.01 | |
| Generalist species richness | Total plant species richness | 0.35 | <0.05 |
| Species Group/Background Variable | Unstandardized | Standardized | |||
|---|---|---|---|---|---|
| B | Standardised Beta | β | Student’s t | P | |
| Dry grassland specialist species richness | −0.1539 | 0.8649 | −0.1779 | 0.8596 | |
| All plant species richness | 0.0527 | 0.0123 | 0.5015 | 4.2738 | 0.0001 |
| MPAR | 0.0005 | 0.0002 | 0.2656 | 2.2611 | 0.0286 |
| Soil temperature | 0.0769 | 0.0275 | 0.3167 | 2.7980 | 0.0075 |
| Distance to nearest large dry grassland | 0.0001 | 0.0000 | −0.2777 | −2.4736 | 0.0172 |
| Grassland species richness | −1.6670 | 1.2521 | −1.3314 | 0.1896 | |
| All plant species richness | 0.0818 | 0.0189 | 0.5244 | 4.3217 | 0.0001 |
| MPAR | 0.0006 | 0.0003 | 0.2432 | 1.9881 | 0.0528 |
| Soil temperature | 0.1433 | 0.0429 | 0.3977 | 3.3355 | 0.0017 |
| Generalist species richness | 12.7616 | 0.7648 | 16.6866 | 0.0000 | |
| All plant species richness | −0.0924 | 0.0218 | −0.6119 | −4.2367 | 0.0001 |
| Humus | 0.1549 | 0.0454 | 0.4930 | 3.4136 | 0.0013 |
| Total species richness | 21.7679 | 2.0429 | 10.6552 | 0.0000 | |
| MPAR | 0.0012 | 0.0005 | 0.2899 | 2.2315 | 0.0309 |
| Humus | 0.3596 | 0.1171 | 0.7137 | 3.0712 | 0.0037 |
| Gravel | −0.0453 | 0.0189 | −0.3421 | −2.1315 | 0.0388 |
| Clay | −0.2259 | 0.0654 | −1.0045 | −3.4558 | 0.0012 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Querner, P.; Milasowszky, N.; Zulka, K.P.; Abensperg-Traun, M.; Willner, W.; Sauberer, N.; Jakomini, C.; Wrbka, T.; Schmitzberger, I.; Zechmeister, H.G. Habitat Structure, Quality and Landscape Predict Species Richness and Communities of Collembola in Dry Grasslands in Austria. Insects 2018, 9, 81. https://doi.org/10.3390/insects9030081
Querner P, Milasowszky N, Zulka KP, Abensperg-Traun M, Willner W, Sauberer N, Jakomini C, Wrbka T, Schmitzberger I, Zechmeister HG. Habitat Structure, Quality and Landscape Predict Species Richness and Communities of Collembola in Dry Grasslands in Austria. Insects. 2018; 9(3):81. https://doi.org/10.3390/insects9030081
Chicago/Turabian StyleQuerner, Pascal, Norbert Milasowszky, Klaus Peter Zulka, Max Abensperg-Traun, Wolfgang Willner, Norbert Sauberer, Christine Jakomini, Thomas Wrbka, Ingrid Schmitzberger, and Harald G. Zechmeister. 2018. "Habitat Structure, Quality and Landscape Predict Species Richness and Communities of Collembola in Dry Grasslands in Austria" Insects 9, no. 3: 81. https://doi.org/10.3390/insects9030081
APA StyleQuerner, P., Milasowszky, N., Zulka, K. P., Abensperg-Traun, M., Willner, W., Sauberer, N., Jakomini, C., Wrbka, T., Schmitzberger, I., & Zechmeister, H. G. (2018). Habitat Structure, Quality and Landscape Predict Species Richness and Communities of Collembola in Dry Grasslands in Austria. Insects, 9(3), 81. https://doi.org/10.3390/insects9030081





