Determination of Genetic Diversity in Chilo partellus, Busseola fusca, and Spodoptera frugiperda Infesting Sugarcane in Southern Malawi Using DNA Barcodes
Abstract
1. Introduction
2. Materials and Methods
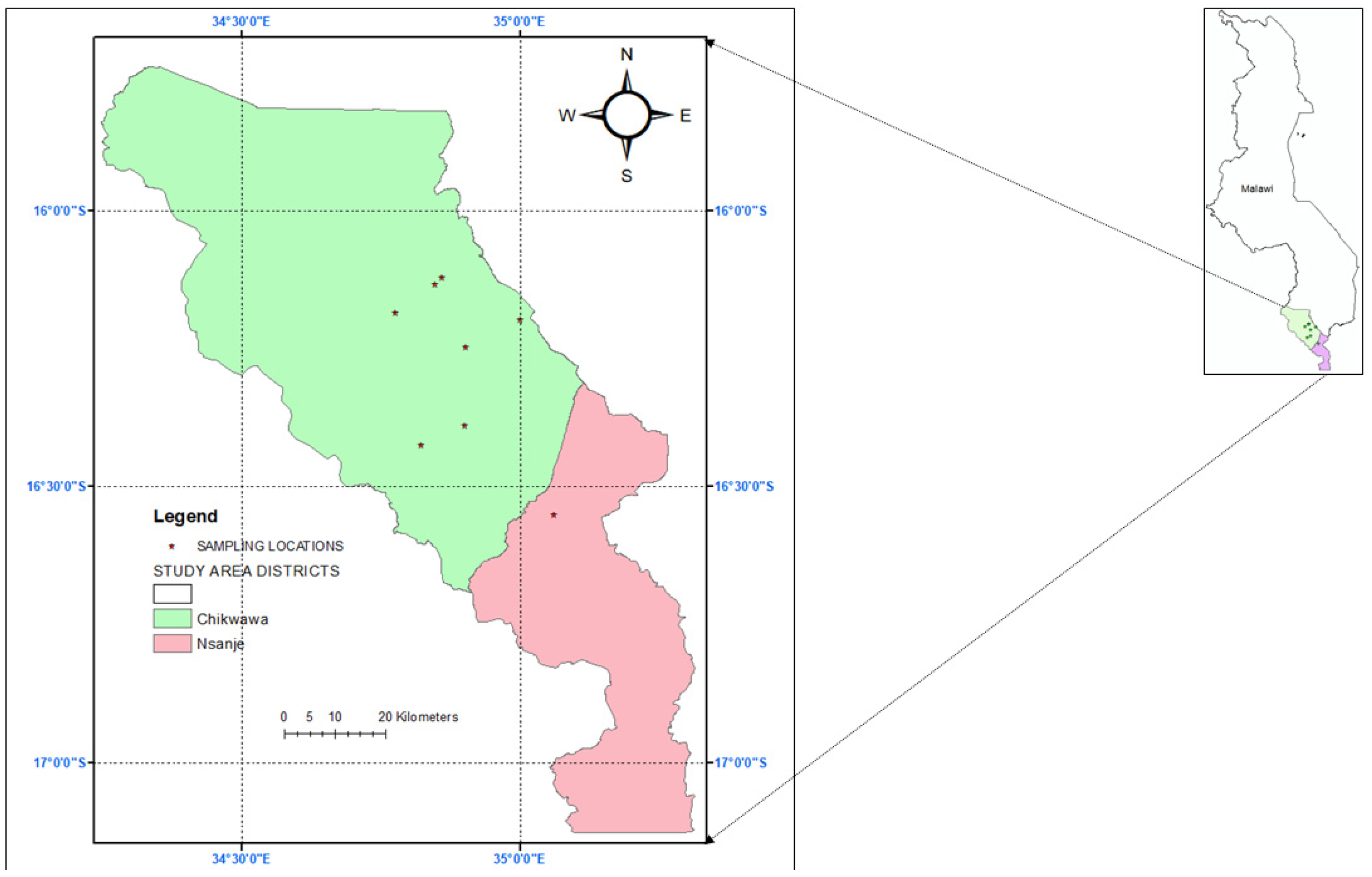
2.1. Survey Sites
2.2. Survey Methodology
2.3. Morphological and Molecular Identification
2.4. DNA Extraction and Amplification
2.5. DNA Sequencing
2.6. Sequence Analysis
3. Results
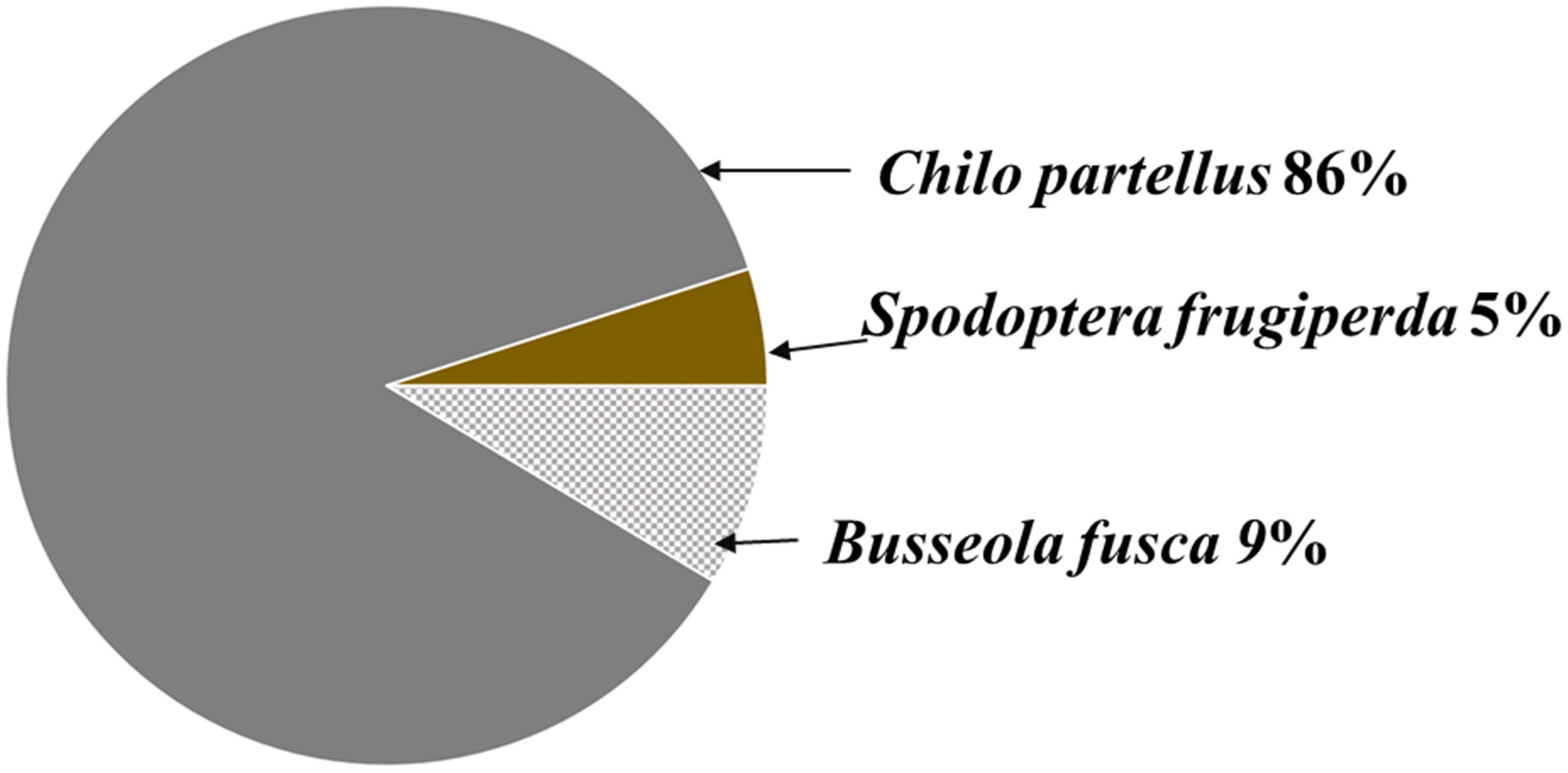
3.1. Occurrence of Busseola fusca, Chilo partellus, and Spodoptera frugiperda in Sugarcane Fields
3.1.1. Morphological Identification
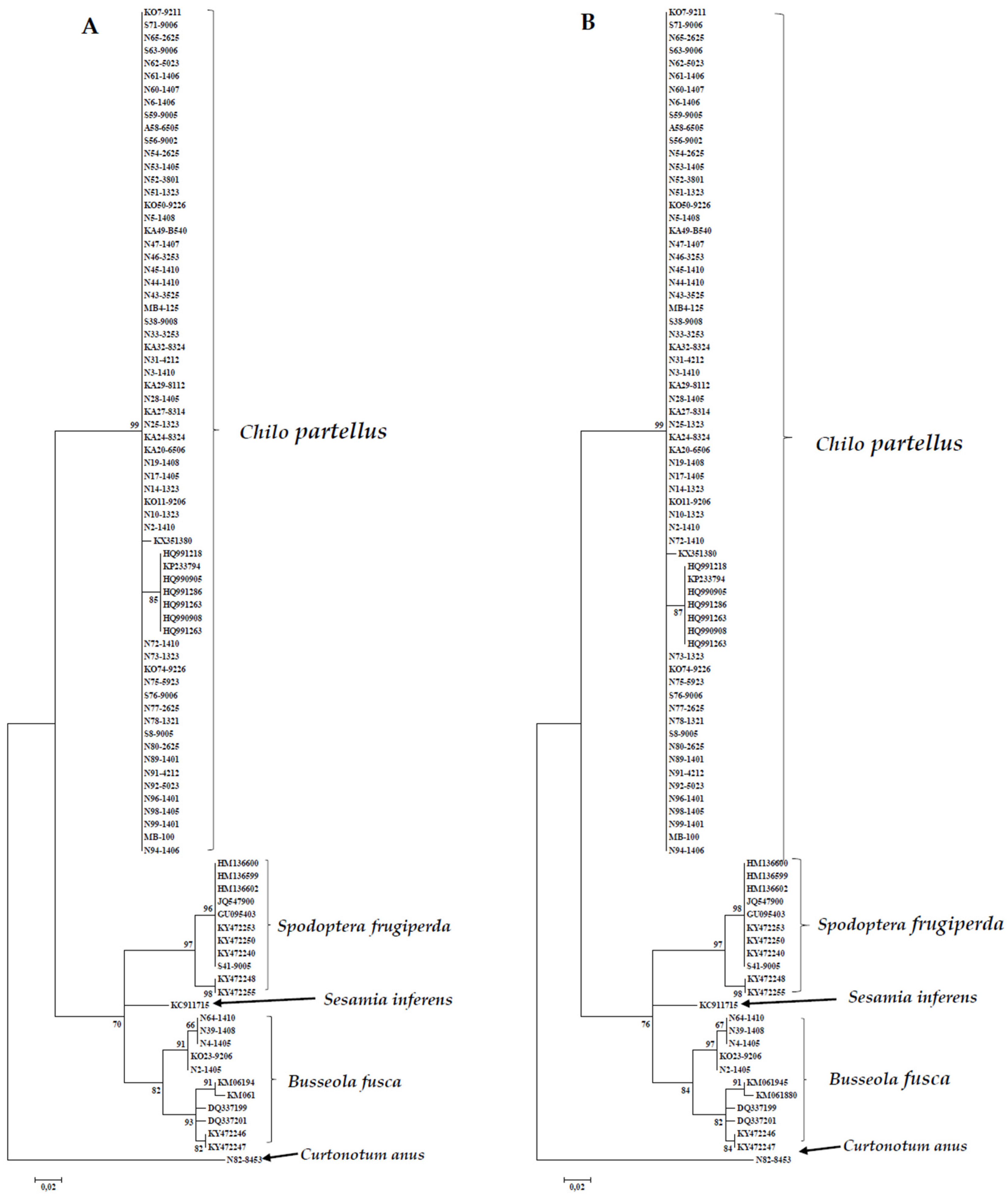
3.1.2. DNA Based Identification
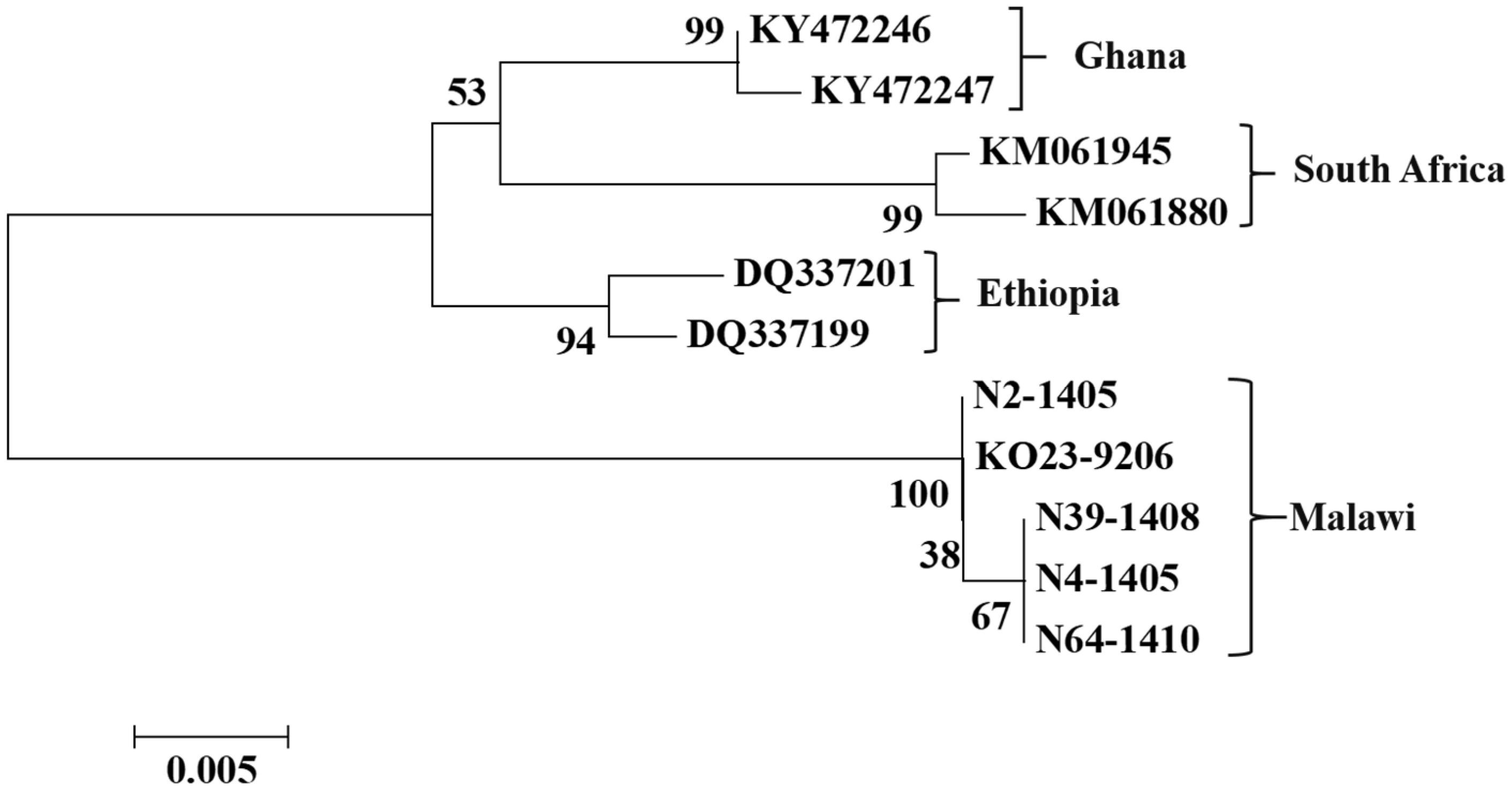
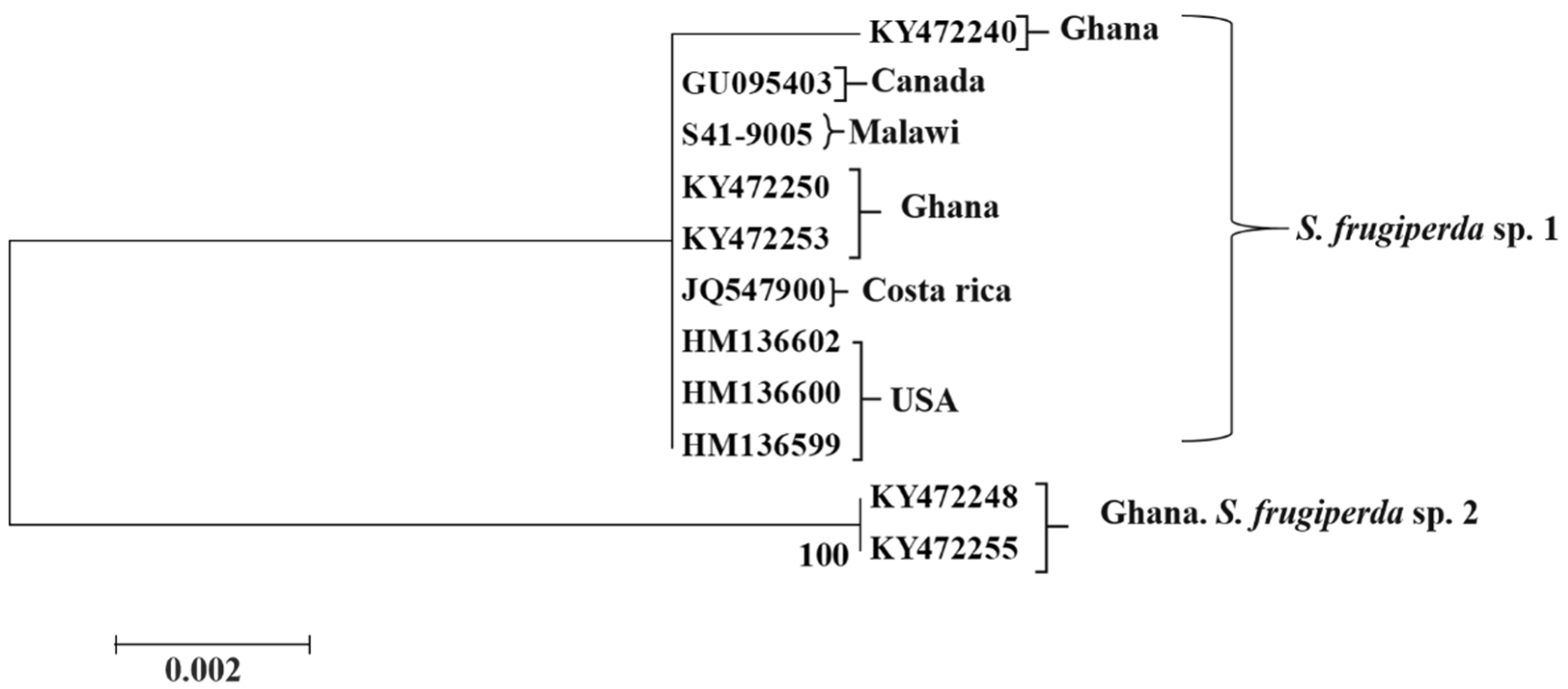
3.2. Sequence Analysis
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops: Sugar Cane. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 19 August 2017).
- OECD/FAO. OECD-FAO Agricultural Outlook 2015. Commodity Snapshots. 2015. Available online: http://dx.doi.org/10.1787/888933229199 (accessed on 24 July 2016).
- ILLOVO Sugar Malawi plc Annual Report. 2017. Available online: https://www.illovosugarafrica.com/UserContent/documents/Announcements/2017/Illovo-Sugar-(Malawi)-plc-Annual-Report-2017.pdf (accessed on 15 November 2017).
- Agricane Malawi. Handbook for Sustainable Sugarcane Production in Malawi for the Sugarcane Smallholder Outgrowers’ Capacity Buildings Project; Solidaridad Southern Africa: Blantyre, Malawi, 2011; pp. 3–10. [Google Scholar]
- Moolman, J.; Van den Berg, J.; Conlong, D.; Cugala, D.; Siebert, S.; Le Ru, B. Species diversity and distribution of lepidopteran stem borers in South Africa and Mozambique. J. Appl. Entomol. 2014, 138, 52–66. [Google Scholar] [CrossRef]
- Assefa, Y.; Conlong, D.E.; Van den Berg, J.; Mitchell, A. Distribution of sugar cane stem borers and their natural enemies in small-scale farmers’ fields, adjacent margins and wetlands of Ethiopia. Int. J. Pest Manag. 2010, 56, 233–241. [Google Scholar] [CrossRef]
- Plantwise. African Sugarcane Borer (Eldana saccharina). 2018. Available online: https://www.plantwise.org/KnowledgeBank/PWMap.aspx?speciesID=15469&dsID=20672&loc=global (accessed on 30 May 2018).
- Kfir, R.; Overholt, W.A.; Khan, Z.R.; Polaszek, A. Biology and management of economically important lepidopteran cereal stem borers in Africa. Annu. Rev. Entomol. 2002, 47, 701–731. [Google Scholar] [CrossRef] [PubMed]
- Overholt, W.A.; Maes, K.V.N.; Goebel, F.R. Field Guide to the Stemborer Larvae of Maize, Sorghum and Sugarcane in Eastern and Southern Africa; ICIPE Science Press: Nairobi, Kenya, 2001; pp. 3–7. ISBN 929064132X. [Google Scholar]
- Kfir, R. 1998. Maize and grain sorghum: Southern Africa. In African Cereal Stem Borers: Economic Importance, Taxonomy, Natural Enemies and Control; Polaszek, A., Ed.; CABI: Wallingford, UK, 1998; pp. 29–37, 530. ISBN 9780851991757. [Google Scholar]
- Van den Berg, J.; van Rensburg, J.B.J. Infestation and injury levels of stem borers in relation to yield potential of grain sorghum. S. Afr. J. Plant Soil. 1991, 8, 127–131. [Google Scholar] [CrossRef]
- Ong’amo, G.O.; Le Rü, B.P.; Dupas, S.; Moya, P.; Calatayud, P.; Silvain, J. Distribution, pest status and agro-climatic preferences of lepidopteran stem borers of maize in Kenya. Ann. Entomol. Soc. Fr. 2006, 42, 171–177. [Google Scholar] [CrossRef]
- Conlong, D.E. Indigenous African parasitoids of Eldana saccharina (Lepidoptera: Pyralidae). Proc. S. Afr. Sugar Technol. Assoc. 2000, 74, 201–211. [Google Scholar]
- Conlong, D.E.; Cugala, D. The use of classical and augmentation biological control for the south–east Asian borer Chilo sacchariphagus Bojer (Lepidoptera: Crambidae) in Mozambican sugarcane. In Proceedings of the Third International Symposium on Biological Control of Arthropods, Christchurch, New Zealand, 8–13 February 2009; Mason, P.G., Gillespie, D.R., Vincent, C., Eds.; USDA Forest Service: Morgantown, WV, USA, 2008. [Google Scholar]
- Mutamiswa, R.; Chidawanyika, F.; Nyamukondiwa, C. Dominance of spotted stemborer Chilo partellus Swinhoe (Lepidoptera: Crambidae) over indigenous stemborer species in Africa’s changing climates: Ecological and thermal biology perspectives. Agri. Forest Entomol. 2017, 115, 1–11. [Google Scholar] [CrossRef]
- FAO. Briefing Note on FAO Actions on Fall Armyworm in Africa. FAO Briefing Note on FAW. Available online: http://www.fao.org/food-chain-crisis/how-we-work/plant-protection/fallarmyworm/en/ (accessed on 5 December 2017).
- Barman, A.K.; Joyce, A.L.; Torres, R.; Higbee, B.S. Assessing genetic diversity in four stink bug species, Chinavia hilaris, Chlorochroa uhleri, Chlorochroa sayi, and Thyanta pallidovirens (Hemiptera: Pentatomidae), using DNA barcode. J. Econ. Entomol. 2017, 110, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.; Dlamini, T. Determining genetic variations in Busseola fusca Fuller (Lepidoptera: Noctuidae) and Chilo partellus Swinhoe (Lepidoptera: Crambidae) from Swaziland and South Africa through sequences of the mtDNA Cytochrome Oxidase Sub Unit I (COI) gene. Int. J. Adv. Res. Biol. Sci. 2016, 3, 208–213. [Google Scholar]
- Sezonlin, M.; Dupas, S.; Le Ru, B.; Faure, N.; Le Gall, P.; Silvain, J.-F. Phylogeographic pattern and regional evolutionary history of the maize stalk borer Busseola fusca (Fuller) (Lepidoptera: Noctuidae) in sub-Saharan Africa. Ann. Soc. Entomol. 2006, 42, 339–351. [Google Scholar] [CrossRef]
- Sezonlin, M.; Dupas, S.; Le Ru, B.; Le Gall, P.; Moyal, P.; Calatayud, P.-A.; Giffard, I.; Faure, N.; Silvain, J.-F. Phylogeography and population genetics of cereal stem borer Busseola fusca (Lepidoptera, Noctuidae) in sub-Saharan Africa. Mol. Ecol. 2006, 15, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Sezonlin, M.; Ndema, R.; Georgen, G.; Le Ru, B.; Dupas, S.; Silvain, J.-F. Genetic structure and origin of Busseola fusca populations in Cameroon. Entomol. Exp. Appl. 2012, 145, 143–152. [Google Scholar] [CrossRef]
- Calatayud, P.-A.; Gitau, C.; Calatayud, S.; Dupas, S.; Le Ru, B.; Silvain, J.-F. Variability in the reproductive biology and in resistance against Cotesia sesamiae among two Busseola fusca populations. J. Appl. Biol. 2011, 135, 423–429. [Google Scholar] [CrossRef]
- Kfir, R. Parasitoids of the African stemborer, Busseola fusca (Lepidoptera: Noctuidae) in South Africa. Bull. Entomol. Res. 1995, 85, 369–377. [Google Scholar] [CrossRef]
- Assefa, Y.; Mitchell, B.P.; Le rü, B.; Conlong, D.E. genetics of Eldana saccharina walker (Lepidoptera: Pyralidae) and the implications for management using biocontrol. Comm. Appl. Biol. Sci 2010, 75, 423–432. [Google Scholar]
- Assefa, Y.; Mitchell, A.; Conlong, D.E. Phylogeography of Eldana saccharina Walker (Lepidoptera: Pyralidae). Ann. Soc. Entomol. Fr. 2006, 42, 331–338. [Google Scholar] [CrossRef]
- Chinsinga, B. The Green Belt Initiative, politics and sugar Production in Malawi. J. South. Afr. Studies 2017, 43, 501–515. [Google Scholar] [CrossRef]
- Meijerman, L.; Ulenberg, S.A. Identification of African stemborer larvae (Lepidoptera: Noctuidae, Pyralidae) based on morphology. Bull. Entomol. Res. 1996, 86, 567–578. [Google Scholar] [CrossRef]
- FAO. The Fall Armyworm (Spodoptera frugiperda): Identification, Biology and Ecology. 2017. Available online: www.plantwise.org/fallarmyworm (accessed on 3 September 2017).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Marine Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 24, 4876–4882. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 16,111–120. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A Software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Cock, M.J.W.; Beseh, P.K.; Buddie, A.G.; Cafá, G.; Crozier, J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO) of the United Nations. Fall Armyworm Trapping. FAW Guidance Note 3. 2018. Available online: http://www.fao.org/3/i8322en/I8322EN.pdf (accessed on 14 February 2018).
- Baker, T.C. Use of pheromones in IPM. In Integrated Pest Management; Radcliffe, E.B., Hutchison, W.D., Cancelado, R.E., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 273–285. ISBN 9780123985293. [Google Scholar]
- Peterson, B.; Bezuidenhout, C.C.; Van den Berg, J. Cytochrome c oxidase I and cytochrome b gene sequences indicate low genetic diversity in South African Busseola fusca (Lepidoptera: Noctuidae) from maize. Afr. Entomol. 2016, 24, 518–523. [Google Scholar] [CrossRef]
- Assefa, Y.; Mitchell, A.; Conlong, D.E.; Moyal, P. DNA identification of Busseola (Lepidoptera: Noctuidae) larvae in Ethiopian sugarcane. Afr. Entomol. 2007, 15, 375–379. [Google Scholar] [CrossRef]
- Assefa, Y.; Mitchell, A.; Conlong, D.E.; Muirhead, K. A. Establishment of Cotesia flavipes (Hymenoptera: Braconidae) in sugarcane fields of Ethiopia and origin of founding population. J. Econ. Entomol. 2007, 101, 686–691. [Google Scholar] [CrossRef]
- Keller, I.; Largiader, C.R. Recent habitat fragmentation caused by major roads leads to reduction of gene flow and loss of genetic variability in ground beetles. Proc. Biol. Sci. 2003, 270, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M; Nwanze, K. Busseola fusca (FuU.er), the African Maize Stem Borer: A Handbook of Information; Information Bulletin 33; International Crops Research Institute for the Semi-arid Tropics: Patancheru, India, 1992; p. 84. [Google Scholar]
- Joyce, A.L.; White, W.H.; Nuessly, G.S.; Solis, M.A.; Scheffer, S.J; Lewis, M.L.; Medina, R.F. Geographic population structure of the sugarcane borer, Diatraea saccharalis (F.) (Lepidoptera: Crambidae), in the Southern United States. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Mitchell, A.; Conlong, D.E. Phylogeography of Eldana saccharina Walker (Lepidoptera: Pyralidae). In Annales de la Sociètè Entomologique de France; Taylor & Francis Group: Abingdon, UK, 2006; Volume 42, pp. 331–337. [Google Scholar]
| Family | Genus | Species | Accession No. |
|---|---|---|---|
| Noctuidae | Busseola | Fusca | KY472246, KY472247, KM061945, KM061880, DQ337201, DQ337199 |
| Spodoptera | frugiperda | KY472240, KY472248, KY472250, KY472253, KY472255, GU095403 JQ547900, HM136602 HM136600, HM136599 | |
| Sesamia | inferens | KC911715 | |
| Crambidae | Chilo | partellus | KX351380, HQ991218 KP233794, HQ990905 HQ991286, HQ991263 HQ990908, HQ991263 |
| Species | No. of Individuals (n) | No. of Polymorphic Sites (S) | No. of Parsimony Informative Sites (PI) | No. of Haplotypes | Haplotype Diversity (Hd) | Nucleotide Diversity (π) | Intraspecific Divergence (mean) |
|---|---|---|---|---|---|---|---|
| B. fusca | 11 | 40 | 36 | 8 | 0.9273 | 0.036 | 0.037 |
| C. partellus | 70 | 3 | 2 | 3 | 0.220 | 0.003 | 0.003 |
| S. frugiperda | 11 | 9 | 8 | 3 | 0.473 | 0.005 | 0.009 |
| Species | Haplotype | No. | Individuals |
|---|---|---|---|
| B. fusca | H-1 H-2 H-3 H-4 H-5 H-6 H-7 H-8 | 3 2 1 1 1 1 1 1 | N4-1405, N64-1410, N39-1408 KO23-9206, N2-1405 KY472246 KY472247 KM061945 KM061880 DQ337201 DQ337199 |
| S. frugiperda | H-1 H-2 H-3 | 8 1 2 | S41-9005, KY472250, KY472253, GU095403, JQ547900, HM136602, HM136600, HM136599 KY472240 KY472248, KY472255 |
| C. partellus | H-1 | 1 | KX351380 |
| H-2 | 7 | HQ991218, KP233794, HQ990905 HQ991286, HQ991263, HQ990908, HQ991263 | |
| H-3 | 58 | N2-1410, N10-1323, KO11-9206, N14-1323, N17-1405, N19-1408, KA20-6506, KA24-8324, N25-1323, KA27-8314, N28-1405, KA29-8112, N3-1410, N31-4212, KA32-8324, N33-3253, S38-9008, MB4-125, N43-3525, N44-1410, N45-1410, N46-3253, N47-1407, KA49-B540, N5-1408, KO50-9226, N51-1323, N52-3801, N53-1405, N54-2625, S56-9002, A58-6505, S59-9005, N6-1406, N60-1407, N61-1406, N62-5023, S63-9006, N65-2625, KO7-9211, S71-9006, N72-1410, N73-1323, KO74-9226, N75-5923, S76-9006, N77-2625, N78-1321, S8-9005, N80-2625, N89-1401, N91-4212, N92-5023, N96-1401, N98-1405, N99-1401, MB100, N94-1406 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasambala Donga, T.; Meadow, R. Determination of Genetic Diversity in Chilo partellus, Busseola fusca, and Spodoptera frugiperda Infesting Sugarcane in Southern Malawi Using DNA Barcodes. Insects 2018, 9, 74. https://doi.org/10.3390/insects9030074
Kasambala Donga T, Meadow R. Determination of Genetic Diversity in Chilo partellus, Busseola fusca, and Spodoptera frugiperda Infesting Sugarcane in Southern Malawi Using DNA Barcodes. Insects. 2018; 9(3):74. https://doi.org/10.3390/insects9030074
Chicago/Turabian StyleKasambala Donga, Trust, and Richard Meadow. 2018. "Determination of Genetic Diversity in Chilo partellus, Busseola fusca, and Spodoptera frugiperda Infesting Sugarcane in Southern Malawi Using DNA Barcodes" Insects 9, no. 3: 74. https://doi.org/10.3390/insects9030074
APA StyleKasambala Donga, T., & Meadow, R. (2018). Determination of Genetic Diversity in Chilo partellus, Busseola fusca, and Spodoptera frugiperda Infesting Sugarcane in Southern Malawi Using DNA Barcodes. Insects, 9(3), 74. https://doi.org/10.3390/insects9030074




